Chemical Insect Attractants Produced by Flowers of Impatiens spp. (Balsaminaceae) and List of Floral Visitors
Abstract
:1. Introduction
2. Results
2.1. GC-MS Analysis
2.2. Flower Visitors
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Field Observations of Insect Activity
4.3. GC/MS Analysis of Nectar Composition
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fischer, E.; Kubitzki, K. (Eds.) Balsaminaceae. In The Families and Genera of Vascular Plants VI; Springer: Berlin, Germany, 2004; pp. 20–25. [Google Scholar]
- Grey-Wilson, C. Introduction to the genus Impatiens. In Impatiens of Africa: Morphology, Pollination and Pollinators, Phytogeography, Hybridisation, Keys and a Systematic Treatment of All African Species, 6th ed.; Balkema, A.A., Ed.; Royal Botanic Gardens, Kew: Richmond, UK, 1980; p. 3. [Google Scholar]
- Utami, N.; Shimizu, T. Seed morphology and classification of Impatiens (Balsaminaceae). Blumea-Biodivers. Evol. Biogeogr. Plants 2005, 50, 447–456. [Google Scholar] [CrossRef]
- Pyšek, P.; Lambdon, P.W.; Arianoutsou, M.; Kühn, I.; Pino, J.; Winter, M. Alien vascular plants of Europe. In Handbook of Alien Species in Europe; Invading Nature—Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2009; Volume 3, pp. 43–61. [Google Scholar] [CrossRef]
- Schmitz, U.; Dericks, G. Spread of alien invasive Impatiens balfourii in Europe and its temperature, light and soil moisture demands. Flora Morphol. Distrib. Funct. Ecol. 2010, 205, 772–776. [Google Scholar] [CrossRef]
- Rewicz, A.; Myśliwy, M.; Rewicz, T.; Adamowski, W.; Kolanowska, M. Contradictory effect of climate change on American and European populations of Impatiens capensis Meerb. —Is this herb a global threat? Sci. Total Environ. 2022, 850, 157959. [Google Scholar]
- Najberek, K.; Solarz, W.; Pusz, W.; Patejuk, K.; Olejniczak, P. Two sides of the same coin: Does alien Impatiens balfourii fall into an ecological trap after releasing from enemies? Environ. Exp. Bot. 2020, 176, 104103. [Google Scholar] [CrossRef]
- Ruckli, R.; Rusterholz, H.-P.; Baur, B. Invasion of Impatiens glandulifera affects terrestrial gastropods by altering microclimate. Acta Oecologica 2013, 47, 16–23. [Google Scholar] [CrossRef]
- Kiełtyk, P.; Delimat, A. Impact of the alien plant Impatiens glandulifera on species diversity invaded vegetation in the northern foothills of the Tatra Mountains, Central Europe. Plant Ecol. 2019, 220, 1–12. [Google Scholar] [CrossRef]
- Myśliwy, M. Diversity and environmental variability of riparian tall herb fringe communities of the order Convolvuletalia sepium in Polish river valleys. Monogr. Bot. 2019, 108, 1–129. [Google Scholar] [CrossRef]
- Reczyńska, K.; Świerkosz, K.; Dajdok, Z. The spread of Impatiens parviflora DC. In Central European oak forests—Another stage of invasion? Acta Soc Bot Pol. 2015, 84, 401–411. [Google Scholar] [CrossRef]
- Vervoort, A.; Jacquemart, A.L. Habitat Overlap of the Invasive Impatiens parviflora DC with Its Native Congener I. noli-tangere L. Phytocoenologia 2012, 42, 249–257. [Google Scholar] [CrossRef]
- Najberek, K.; Kosior, A.; Solarz, W. Alien balsams, strawberries and their pollinators in a warmer world. BMC Plant Biol. 2021, 21, 500. [Google Scholar] [CrossRef] [PubMed]
- Najberek, K.; Patejuk, K.; Czeluśniak, I.; Solarz, W.; Hojniak, M.; Kaczmarek-Pieńczewska, A.; Jakubska-Busse, A. Biological Invasions Threaten Crops: Alien Himalayan Balsams Tempt Pollinators Away from Cultivated Tomatoes. NeoBiota 2023. in preparation. [Google Scholar]
- Adamowski, W.; Myśliwy, M.; Dajdok, Z. A Survey to Assess the Degree of Invasiveness of Impatiens capensis Meerb. in Poland, Based on a Protocol Harmonia+PL—Procedure for Negative Impact Risk Assessment for Invasive Alien Species and Potentially Invasive Alien Species in Poland. General Directorate of Environmental Protection. 2018. Available online: http://projekty.gdos.gov.pl/files/artykuly/127065/Impatiens-capensis_niecierpek-pomaranczowy_EN_icon.pdf (accessed on 30 October 2023).
- Matthews, J.; Beringen, R.; Boer, E.; Duistermaat, H.; Odé, B.; van Valkenburg, J.L.C.H.; van der Velde, G.; Leuven, R.S.E.W. Risks and Management of Non-Native Impatiens Species in the Netherlands; Radboud University: Nijmegen, The Netherlands; Naturalis Biodiversity Center: Leiden, The Netherlands, 2015; Available online: http://repository.ubn.ru.nl/handle/2066/149286 (accessed on 30 October 2023).
- Skálová, H.; Jarošík, V.; Dvořáčková, Š.; Pyšek, P. Effect of Intra- and Interspecific Competition on the Performance of Native and Invasive Species of Impatiens under Varying Levels of Shade and Moisture. PLoS ONE 2013, 8, e62842. [Google Scholar] [CrossRef]
- Lanza, J.; Smith, G.C.; Sack, S.; Cash, A. Variation in nectar volume and composition of Impatiens capensis at the individual, plant, and population levels. Oecologia 1995, 102, 113–119. [Google Scholar] [CrossRef]
- Ugoletti, P.; Stout, J.C.; Jones, M.B. Ecophysiological traits of invasive and non-invasive introduced Impatiens species. Biology and Environment. Biol. Environ. 2011, 111B, 143–156. [Google Scholar] [CrossRef]
- Najberek, K.; Olejniczak, P.; Berent, K.; Gąsienica-Staszeczek, M.; Solarz, W. The ability of seeds to float with water currents contributes to the invasion success of Impatiens balfourii and I. glandulifera. J. Plant Res. 2020, 133, 649–664. [Google Scholar] [CrossRef]
- Najberek, K.; Pusz, W.; Solarz, W.; Olejniczak, P. The seeds of success: Release from fungal attack on seeds may influence the invasiveness of alien Impatiens. Plant Ecol. 2018, 219, 1197–1207. [Google Scholar] [CrossRef]
- Raguso, R.A. Flowers as Sensory Billboards: Progress towards an Integrated Understanding of Floral Advertisement. Curr. Opin. Plant Biol. 2004, 7, 434–440. [Google Scholar] [CrossRef]
- Leonard, A.S.; Dornhaus, A.; Papaj, D.R. Why Are Floral Signals Complex? An Outline of Functional Hypotheses. In Evolution of Plant-Pollinator Relationships; Patiny, S., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 279–300. ISBN 978-1-139-01411-3. [Google Scholar]
- Najberek, K.; Solarz, W.; Wysoczański, W.; Węgrzyn, E.; Olejniczak, P. Flowers of Impatiens glandulifera as hubs for both pollinators and pathogens. NeoBiota 2023, 87, 1–26. [Google Scholar] [CrossRef]
- Descamps, C.; Quinet, M.; Baijot, A.; Jacquemart, A.-L. Temperature and water stress affect plant-pollinator interactions in Borago officinalis (Boraginaceae). Ecol. Evol. 2018, 8, 3443–3456. [Google Scholar] [CrossRef] [PubMed]
- Descamps, C.; Boubnan, N.; Jacquemart, A.-L.; Quinet, M. Growing and Flowering in a Changing Climate: Effects of Higher Temperatures and Drought Stress on the Bee-Pollinated Species Impatiens glandulifera Royle. Plants 2021, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P. Selection for pollination success and the mechanical fit of Impatiens flowers around bumblebee bodies. Biol. J. Linn. Soc. 1995, 55, 355–383. [Google Scholar] [CrossRef]
- Bell, G.; Lefebvre, L.; Giraldeau, L.-A.; Weary, D. Partial Preference of Insects for the Male Flowers of an Annual Herb. Oecologia 1984, 64, 287–294. [Google Scholar] [CrossRef]
- Law, J.H.; Regnier, F.E. Pheromones. Annu. Rev. Biochem. 1971, 40, 533–548. [Google Scholar] [CrossRef]
- Cardé, R.T.; Willis, M.A. Navigational Strategies Used by Insects to Find Distant, Wind-Borne Sources of Odor. J. Chem. Ecol. 2008, 34, 854–866. [Google Scholar] [CrossRef]
- Hoddle, M.S.; Robinson, L.; Morgan, D. Attraction of Thrips (Thysanoptera: Thripidae and Aeolothripidae) to Colored Sticky Cards in a California Avocado Orchard. Crop Prot. 2002, 21, 383–388. [Google Scholar] [CrossRef]
- Dethier, V.G.; Browne, B.L.; Smith, C.N. The Designation of Chemicals in Terms of the Responses They Elicit from Insects. J. Econ. Entomol. 1960, 53, 134–136. [Google Scholar] [CrossRef]
- Miller, J.R.; Siegert, P.Y.; Amimo, F.A.; Walker, E.D. Designation of Chemicals in Terms of the Locomotor Responses They Elicit from Insects: An Update of Dethier et al. (1960). J. Econ. Entomol. 2009, 102, 2056–2060. [Google Scholar] [CrossRef] [PubMed]
- Díaz, M.A.; Osorio, C.; Coy-Barrera, E.; Rodríguez, D. Semiochemicals Associated with the Western Flower Thrips Attraction: A Systematic Literature Review and Meta-Analysis. Insects 2023, 14, 269. [Google Scholar] [CrossRef]
- Cardé, R.T.; Millar, J.G. (Eds.) Advances in Insect Chemical Ecology; Cambridge University Press: New York, NY, USA, 2004; ISBN 9780521792752. [Google Scholar]
- Seigler, D.S. Plant Secondary Metabolism; Kuwar Academic Press: Dordrecht, The Netherlands; New York, NY, USA, 1998; pp. 51–55. [Google Scholar]
- Mitra, P.; Mobarak, S.H.; Debnath, R.; Barik, A. The role of Lathyrus sativus flower surface wax in short-range attraction and stimulant for nymph laying by an adult viviparous aphid. Bull. Entomol. Res. 2020, 110, 231–241. [Google Scholar] [CrossRef]
- Davidson, M.M.; Nielsen, M.-C.; Butler, R.C.; Castañé, C.; Alomar, O.; Riudavets, J.; Teulon, D.A.J. Can Semiochemicals Attract Both Western Flower Thrips and Their Anthocorid Predators? Entomol. Exp. Appl. 2015, 155, 54–63. [Google Scholar] [CrossRef]
- Jakubska-Busse, A.; Dziadas, M.; Gruss, I.; Kobyłka, M.J. Floral Volatile Organic Compounds and a List of Pollinators of Fallopia baldschuanica (Polygonaceae). Insects 2022, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Jacquemart, A.L.; Somme, L.; Colin, C.; Quinet, M. Floral biology and breeding system of Impatiens balfourii (Balsaminaceae): An exotic species in extension in temperate areas. Flora Morphol. Distrib. Funct. Ecol. 2015, 214, 70–75. [Google Scholar] [CrossRef]
- Rust, R.W. Pollination of Impatiens capensis: Pollinators and Nectar Robbers. J. Kans. Entomol. Soc. 1979, 52, 297–308. [Google Scholar]
- Vervoort, A.; Cawoy, V.; Jacquemart, A.L. Comparative reproductive biology in co-occurring invasive and native Impatiens species. Int. J. Plant Sci. 2011, 172, 366–377. [Google Scholar] [CrossRef]
- Csiszar, A.; Bartha, D. Small balsam (Impatiens parviflora DC.). In The Most Important Invasive Plants in Hungary; Botta-Dukat, Z., Balogh, L., Eds.; Institute of Ecology and Botany, Hungarian Academy of Sciences: Budapest, Hungary, 2008; pp. 139–149. [Google Scholar]
- Hatcher, P.E. Impatiens noli-tangere L. J. Ecol. 2003, 91, 147–167. [Google Scholar] [CrossRef]
- Tian, J.; Liu, K.; Hu, G. Pollination ecology and pollination system of Impatiens reptans (Balsaminaceae) endemic to China. Ann. Bot. 2004, 93, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Rust, R.W. Pollination in Impatiens capensis and Impatiens pallida (Balsaminaceae). Bull. Torrey Bot. Club 1977, 104, 361–367. [Google Scholar] [CrossRef]
- Heinrich, B. Bumblebee Economics; Harvard University Press: Cambridge, MA, USA, 1979; p. 850. [Google Scholar]
- Kato, M.; Itino, I.; Hotta, M.; Abbas, I.; Okada, H. Flower visitors of 32 plant species in West Sumatra. Occas. Pap. Kagoshima Univ. Res. Cent. S. Pac. 1989, 16, 15–31. [Google Scholar]
- Ruchisansakun, S.; Tangtorwongsakul, P.; Cozien, R.J.; Smets, E.F.; van der Niet, T. Floral specialization for different pollinators and divergent use of the same pollinator among co-occurring Impatiens species (Balsaminaceae) from Southeast Asia. Bot. J. Linn. Soc. 2016, 181, 651–666. [Google Scholar] [CrossRef]
- Chittka, L.; Schürkens, S. Successful invasion of a floral market. Nature 2001, 411, 653. [Google Scholar] [CrossRef]
- Lobstein, A.; Brenne, X.; Feist, E.; Metz, N.; Weniger, B.; Anton, R. Quantitative determination of naphthoquinones of Impatiens species. Phytochem. Anal. 2001, 12, 202–205. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, S.; Wolfender, J.L.; Hakizamungu, E.; Hostettmann, K. An antifungal naphthoquinone, xanthones and secoiridoids from Swertia calycina. Planta Medica 1995, 61, 362–364. [Google Scholar] [CrossRef] [PubMed]
- Gniłun, Czyli Niegroźna Muszka w Barwach Bojowych. Available online: https://natura.wm.pl/382790,Gnilun-czyli-niegrozna-muszka-w-barwach-bojowych.html (accessed on 21 November 2023).
- Baer, B.; Maile, R.; Schmid-Hempel, P.; Morgan, E.D.; Jones, G.R. Chemistry of a mating plug in bumblebees. J. Chem. Ecol. 2000, 26, 1869–1875. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. 2019. Available online: https://www.pherobase.com/database/compound/compounds-index.php (accessed on 14 October 2023).
- Crewe, R.M.; Moritz, R.F.A.; Lattorff, H.M.G. Trapping pheromonal components with silicone rubber tubes: Fatty acid secretions in honeybees (Apis mellifera). Chemoecology 2004, 14, 77–79. [Google Scholar] [CrossRef]
- Villar, G.; Wolfson, M.D.; Hefetz, A.; Grozinger, C.M. Evaluating the role of drone-produced chemical signals in mediating social interactions in honey bees (Apis mellifera). J. Chem. Ecol. 2018, 44, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cahlíková, L.; Hovorka, O.; Ptácek, V.; Valterová, I. Exocrine gland secretions of virgin queens of five bumblebee species (Hymenoptera: Apidae, Bombini). Z. Für Naturforsch. C 2004, 59, 582–589. [Google Scholar] [CrossRef]
- Stökl, J.; Twele, R.; Erdmann, D.H.; Francke, W.; Ayasse, M. Comparison of the flower scent of the sexually deceptive orchid Ophrys iricolor and the female sex pheromone of its pollinator Andrena morio. Chemoecology 2008, 17, 231–233. [Google Scholar] [CrossRef]
- Appelgren, M.; Bergström, G.; Svensson, B.G.; Cederberg, B. Marking pheromones of Megabombus bumble bee males. Acta Chem. 1991, 45, 972–974. [Google Scholar] [CrossRef]
- Ndungu, N.N.; Kiatoko, N.; Masiga, D.K.; Raina, S.K.; Pirk, C.W.W.; Yusuf, A.A. Compounds extracted from heads of African stingless bees (Hypotrigona species) as a prospective taxonomic tool. Chemoecology 2018, 28, 51–60. [Google Scholar] [CrossRef]
- Kamatou, G.P.; Viljoen, A.M. Linalool—A review of a biologically active compound of commercial importance. Nat. Prod. Commun. 2008, 3, 1934578X0800300727. [Google Scholar] [CrossRef]
- Arctander, S. Perfume and Flavor Chemicals (Aroma Chemicals); Allured Publishing Corporation: Carol Stream, IL, USA, 1994. [Google Scholar]
- Raguso, R.C.; Pichersky, E. New Perspectives in Pollination Biology: Floral Fragrances. A day in the life of a linalool molecule: Chemical communication in a plant-pollinator system. Part 1: Linalool biosynthesis in flowering plants. Plant Species Biol. 1999, 14, 95–120. [Google Scholar] [CrossRef]
- Phillips, T.W.; Parajulee, M.N.; Weaver, D.K. Toxicity of terpenes secreted by the predator Xylocovis flauipes (Reuter) to Tribolium castaneum (Herbst) and Ovy ZaephiIus surinamensis (L.). J. Stored Prod. Res. 1995, 31, 131–138. [Google Scholar] [CrossRef]
- Williams, I.H.; Pickett, J.A.; Martin, A.P. The Nasonov pheromone of the honeybee Apis mellifera L. (Hymenoptera, Apidae). Part II. Bioassay of the components using foragers. J. Chem. Ecol. 1981, 7, 225–237. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Danka, R.G.; Williams, J.L.; Rinderer, T.E. A bait station for survey and detection of honey bees. Apidologie 1990, 21, 287–292. [Google Scholar] [CrossRef]
- Goulson, D.; Stout, J.C.; Langley, J.; Hughes, W.O. Identity and function of scent marks deposited by foraging bumblebees. J. Chem. Ecol. 2000, 26, 2897–2911. [Google Scholar] [CrossRef]
- Keeling, C.I.; Slessor, K.N.; Higo, H.A.; Winston, M.L. New components of the honey bee (Apis mellifera L.) queen retinue pheromone. Proc. Natl. Acad. Sci. USA 2003, 100, 4486–4491. [Google Scholar] [CrossRef]
- Hiap, W.W.; Wee, S.L.; Tan, K.H.; Hee, A.K.-W. Phenylpropanoid sex pheromone component in hemolymph of male Carambola fruit fly, Bactrocera carambolae (Diptera: Tephritidae). Chemoecology 2019, 29, 25–34. [Google Scholar] [CrossRef]
- Niogret, J.; Epsky, N.D. Attraction of Ceratitis capitata (Diptera: Tephritidae) sterile males to essential oils: The importance of linalool. Environ. Entomol. 2018, 47, 1287–1292. [Google Scholar] [CrossRef]
- Heiduk, A.; Meve, U.; Menzel, F.; Haenni, J.P.; Tschirnhaus, M.V.; Dötterl, S.; Johnson, S.D. Fly Pollination of Kettle Trap Flowers of Riocreuxia torulosa (Ceropegieae-Anisotominae): A Generalized System of Floral Deception. Plants 2021, 10, 1564. [Google Scholar] [CrossRef] [PubMed]
- Jhumur, U.S.; Dötterl, S.; Jürgens, A. Floral odors of Silene otites: Their variability and attractiveness to mosquitoes. J. Chem. Ecol. 2008, 34, 14–25. [Google Scholar] [CrossRef]
- Almohamad, R.; Verheggen, F.J.; Francis, F.; Haubruge, E. Predatory hoverflies select their oviposition site according to aphid host plant and aphid species. Entomol. Exp. Appl. 2007, 125, 13–21. [Google Scholar] [CrossRef]
- Cui, L.L.; Francis, F.; Heuskin, S.; Lognay, G.; Liu, Y.J.; Dong, J.; Chen, J.L.; Song, X.M.; Liu, Y. The functional significance of E-β-Farnesene: Does it influence the populations of aphid natural enemies in the fields? Biol. Control. 2012, 60, 108–112. [Google Scholar] [CrossRef]
- Synergy Semiochemicals Corporation. Kairomones. Available online: https://semiochemical.com/kairomones/ (accessed on 27 October 2023).

| No | Name | Chemical Formula | CAS No | IPar | IGlan | INol | ICap |
|---|---|---|---|---|---|---|---|
| Oxygen-containing compounds | |||||||
| 1 | heptan-2-one | C7H14O | 110-43-0 | A | - | - | - |
| 2 | phenylmethanol | C7H8O | 100-51-6 | A | B | - | A |
| 3 | phenylethanol | C8H10O | 60-12-8 | - | B | - | A |
| 4 | nonan-2-one | C9H18O | 821-55-6 | A | A | B | B |
| 5 | pelargonaldehyde (nonanal) | C9H18O | 124-19-6 | A | - | B | B |
| 6 | p-vinylguaiacol (2-methoxy-4-vinylphenol) | C9H10O2 | 7786-61-0 | A | A | - | A |
| 7 | conipheryl alcohol | C10H12O3 | 458-35-5 | - | B | - | - |
| 8 | geraniol | C10H18O | 106-24-1 | B | B | - | A |
| 9 | linalool (2,6-dimethyl-2,7-octadien-6-ol) | C10H18O | 78-70-6 | A | A | - | A |
| 10 | linalool oxide (trans-tetrahydro-2,2,6-trimethyl-6-vinyl-2H-pyran-3-ol) | C10H18O2 | 39028-58-5 | A | - | - | - |
| 11 | 8-hydroxylinalool (2,6-dimethyl-2,7-octadiene-1,6-diol) | C10H18O2 | 64142-78-5 | A | - | - | - |
| 12 | ethyl 4-ethoxybenzoate | C11H14O3 | 23676-09-7 | A | - | A | B |
| 13 | 1-heptadecanal | C17H34O | 629-90-3 | - | - | - | B |
| 14 | heptadecan-2-one | C17H34O | 2922-51-2 | - | - | - | B |
| 15 | octadecanal | C18H36O | 638-66-4 | A | A | - | A |
| 16 | nonadecan-2-one | C19H38O | 629-66-3 | A | A | B | A |
| 17 | eicosanal | C20H40O | 2400-66-0 | A | B | - | B |
| 18 | 1-eicosanol | C20H40O | 629-96-9 | - | - | - | A |
| 19 | 1-docosanal | C22H46O | 57402-36-5 | A | B | A | A |
| 20 | 1-docosanol | C22H46O | 661-19-8 | A | A | - | A |
| 21 | 1-tetracosanol | C24H50O | 506-51-4 | - | - | - | A |
| 22 | 1-tetracosanal | C24H48O | 57866-08-7 | B | - | A | B |
| 23 | 1-pentacosanol | C25H52O | 26040-98-2 | A | B | - | A |
| 24 | 1-hexacosanal | C26H52O | 26627-85-0 | - | - | A | A |
| 25 | 1-hexacosanol | C26H54O | 506-52-5 | - | - | A | - |
| 26 | 1-heptacosanol | C27H56O | 2004-39-9 | A | A | A | A |
| 27 | 1-octacosanal | C28H56O | 22725-64-0 | - | - | A | A |
| 28 | 1-octacosanol | C28H58O | 557-61-9 | A | A | A | A |
| Fatty acids and their esters | |||||||
| 29 | decanoic (capric) acid | C10H20O2 | 334-48-5 | - | B | - | - |
| 30 | tetradecanoic (myristic) acid | C12H28O2 | 544-63-8 | B | A | - | - |
| 31 | dodecanoic (lauric) acid | C16H32O2 | 59154-43-7 | A | A | - | B |
| 32 | hexadecanoic (palmitic) acid | C16H32O2 | 57-10-3 | A | A | A | A |
| 33 | 9,12,15-octadecatrienoic (linolenic) acid | C18H30O2 | 463-40-1 | A | A | A | A |
| 34 | octadecanoic (stearic) acid | C18H36O2 | 57-11-4 | A | A | - | A |
| 35 | eicosanoic (arachic) acid | C20H40O2 | 506-30-9 | B | A | A | A |
| 36 | ethyl docosanoate | C24H48O2 | 5908-87-2 | - | A | - | B |
| 37 | methyl tetracosanoate | C25H50O2 | 2442-49-1 | - | - | A | B |
| Long-chain hydrocarbons | |||||||
| 38 | undecane | C11H24 | 1120-21-4 | A | A | A | A |
| 39 | dodecane | C12H26 | 112-40-3 | A | A | - | - |
| 40 | tetradecane | C14H30 | 629-59-4 | A | A | - | A |
| 41 | tetradec-1-ene | C14H28 | 1120-36-1 | B | A | - | B |
| 42 | farnesene | C15H24 | 18794-84-8 | A | B | B | - |
| 43 | hexadecane | C16H34 | 544-76-3 | A | A | B | A |
| 44 | heptadecane | C17H36 | 629-78-7 | A | A | A | A |
| 45 | octadecane | C18H38 | 593-45-3 | A | A | A | A |
| 46 | nonadecane | C19H40 | 629-92-5 | A | A | - | A |
| 47 | eicosane | C20H42 | 112-95-8 | A | A | - | A |
| 48 | neophytadiene | C28H38 | 504-96-1 | B | B | B | B |
| 49 | heneicosane | C21H44 | 629-94-7 | A | A | A | A |
| 50 | docosane | C22H46 | 629-97-0 | A | A | A | A |
| 51 | tricosane | C23H48 | 638-67-5 | A | A | A | A |
| 52 | pentacosane | C25H52 | 629-99-2 | A | A | A | A |
| 53 | pentacos-1-ene | C25H50 | 16980-85-1 | A | A | - | A |
| 54 | hexacosane | C26H54 | 630-01-3 | - | - | - | A |
| 55 | hexacos-1-ene | C26H52 | 18835-33-1 | - | A | A | B |
| 56 | heptacosane | C27H56 | 593-49-7 | A | B | A | A |
| 57 | heptacos-1-ene | C27H54 | 15306-27-1 | - | - | A | - |
| 58 | octacosane | C28H58 | 630-02-4 | - | - | - | A |
| 59 | nonacosane | C29H60 | 630-03-5 | A | A | A | A |
| 60 | triacontane | C30H62 | 638-68-6 | - | - | - | A |
| 61 | hentriacontane | C31H64 | 630-04-6 | A | A | A | A |
| Flower pigments | |||||||
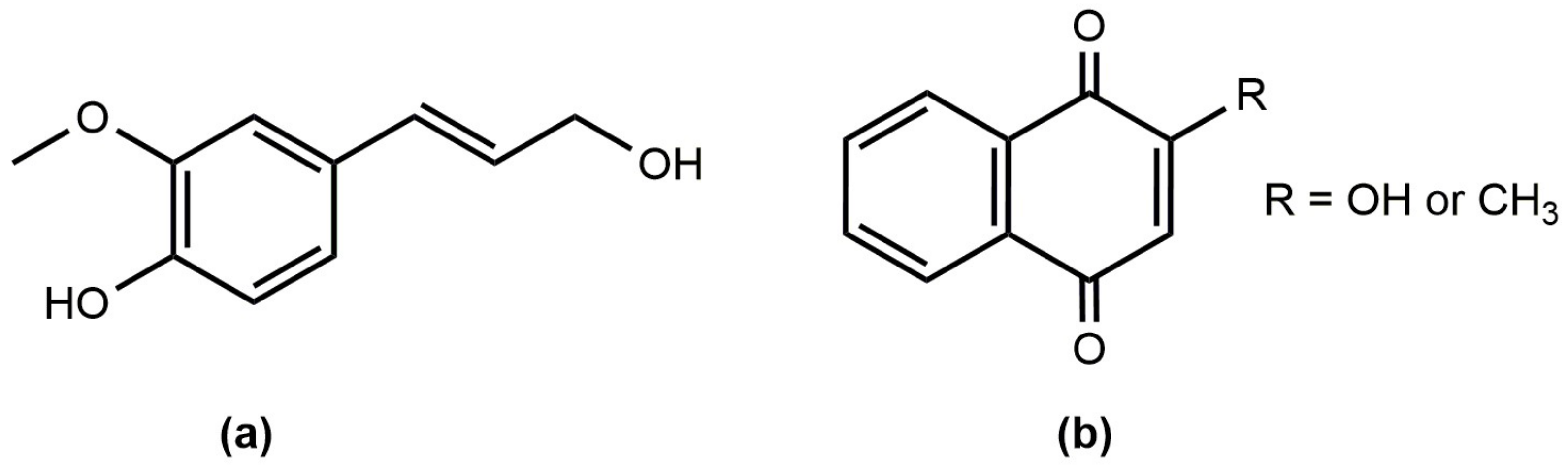
| 62 | 1,4-naphtalenedione 2-hydroxy (lawsone) | C10H6O3 | 83-72-7 | A | A | A | A |
| 63 | 1,4-naphtalenedione 2-metoxy | C11H8O3 | 2348-82-5 | - | A | A | - |
| Insect Order | Family | Species | Flower Visitation Rate | Type of Floral Reward | |||
|---|---|---|---|---|---|---|---|
| IPar | IGlan | INol | ICap | ||||
| Hymenoptera | Apidae | Apis mellifera | C | A | B | A | n, p |
| Bombus sp. | C | A | B | A | n, p | ||
| Bombus hortorum | - | - | B | C | n, p | ||
| Bombus hypnorum | - | B | - | - | n, p | ||
| Bombus lucorum-complex (including B. lucorum, B. cryptarum and B. magnus) | - | B | - | - | n, p | ||
| Bombus pascuorum | C | A | B | A | n, p | ||
| Bombus terrestris | - | B | B | - | n, p | ||
| Vespidae | Vespula vulgaris | - | C | - | B | n | |
| Halictidae | Halictus sp. | - | - | - | C | p | |
| Lasioglossum sp. | - | B | C | C | n | ||
| Diptera | Muscidae | Musca domestica | - | - | C | - | p |
| Syrphidae | Melanostoma sp. | C | - | C | - | n *, p | |
| Eupeodes corollae | A | B | - | - | n *, p | ||
| Episyrphus balteatus | A | B | B | B | n *, p | ||
| Helophilus trivittatus | - | C | - | - | p | ||
| Sphaerophoria scripta | C | - | C | - | n *, p | ||
| Syrphus ribesii | B | - | B | - | n *, p | ||
| Lepidoptera | Sphingidae | Macroglossum stellatarum | - | C | - | - | n |
| Coleoptera | Coccinellidae | Coccinella septempunctata | C | C | - | - | n |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubska-Busse, A.; Czeluśniak, I.; Hojniak, M.; Myśliwy, M.; Najberek, K. Chemical Insect Attractants Produced by Flowers of Impatiens spp. (Balsaminaceae) and List of Floral Visitors. Int. J. Mol. Sci. 2023, 24, 17259. https://doi.org/10.3390/ijms242417259
Jakubska-Busse A, Czeluśniak I, Hojniak M, Myśliwy M, Najberek K. Chemical Insect Attractants Produced by Flowers of Impatiens spp. (Balsaminaceae) and List of Floral Visitors. International Journal of Molecular Sciences. 2023; 24(24):17259. https://doi.org/10.3390/ijms242417259
Chicago/Turabian StyleJakubska-Busse, Anna, Izabela Czeluśniak, Marek Hojniak, Monika Myśliwy, and Kamil Najberek. 2023. "Chemical Insect Attractants Produced by Flowers of Impatiens spp. (Balsaminaceae) and List of Floral Visitors" International Journal of Molecular Sciences 24, no. 24: 17259. https://doi.org/10.3390/ijms242417259







