Dysregulated Hippo Signaling Pathway and YAP Activation in Atopic Dermatitis: Insights from Clinical and Animal Studies
Abstract
:1. Introduction
2. Results
2.1. YAP Is Overexpressed in AD Patients
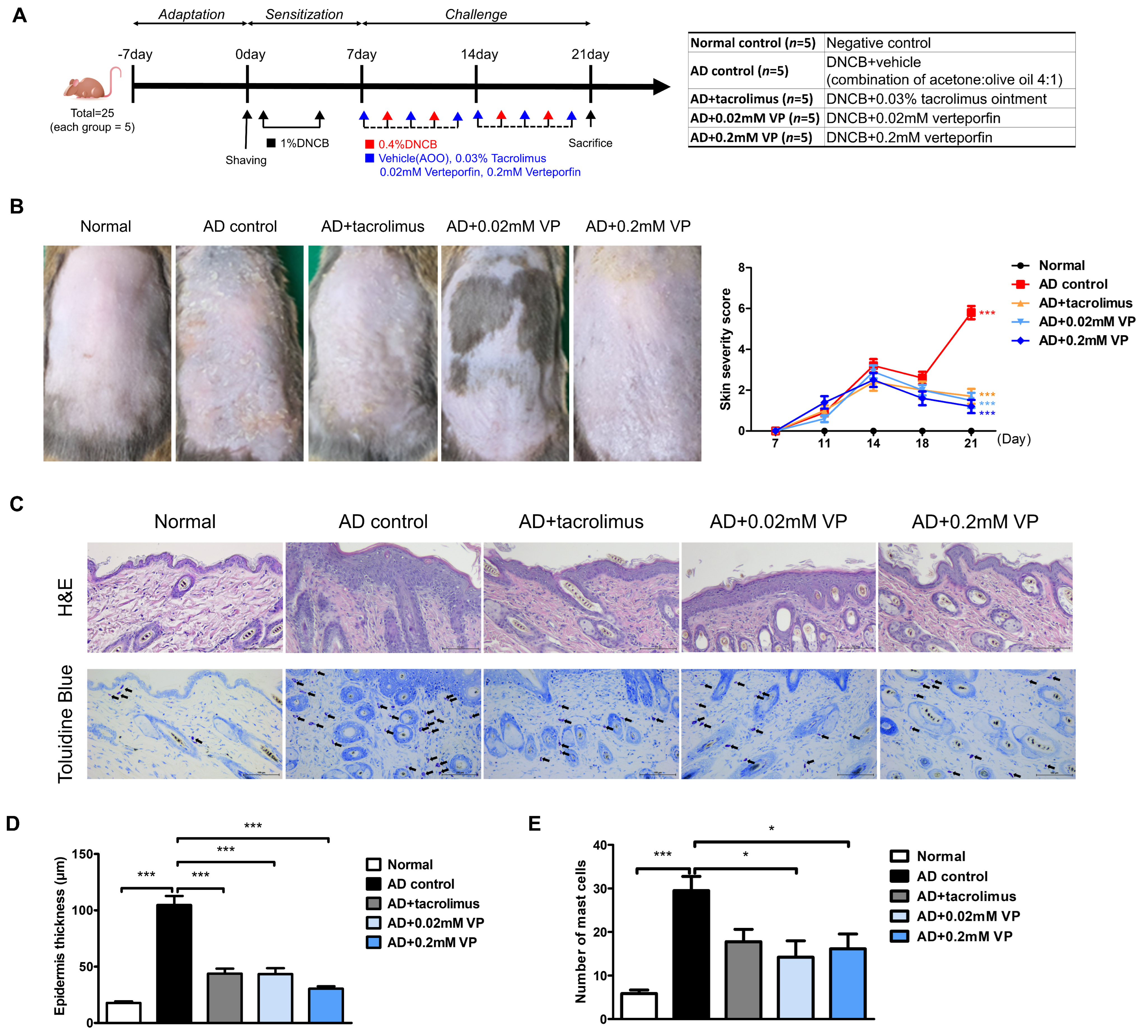
2.2. YAP Inhibitor Attenuates the Symptoms of AD in 1-chloro-2,4-dinitrobenzene-Induced Mice
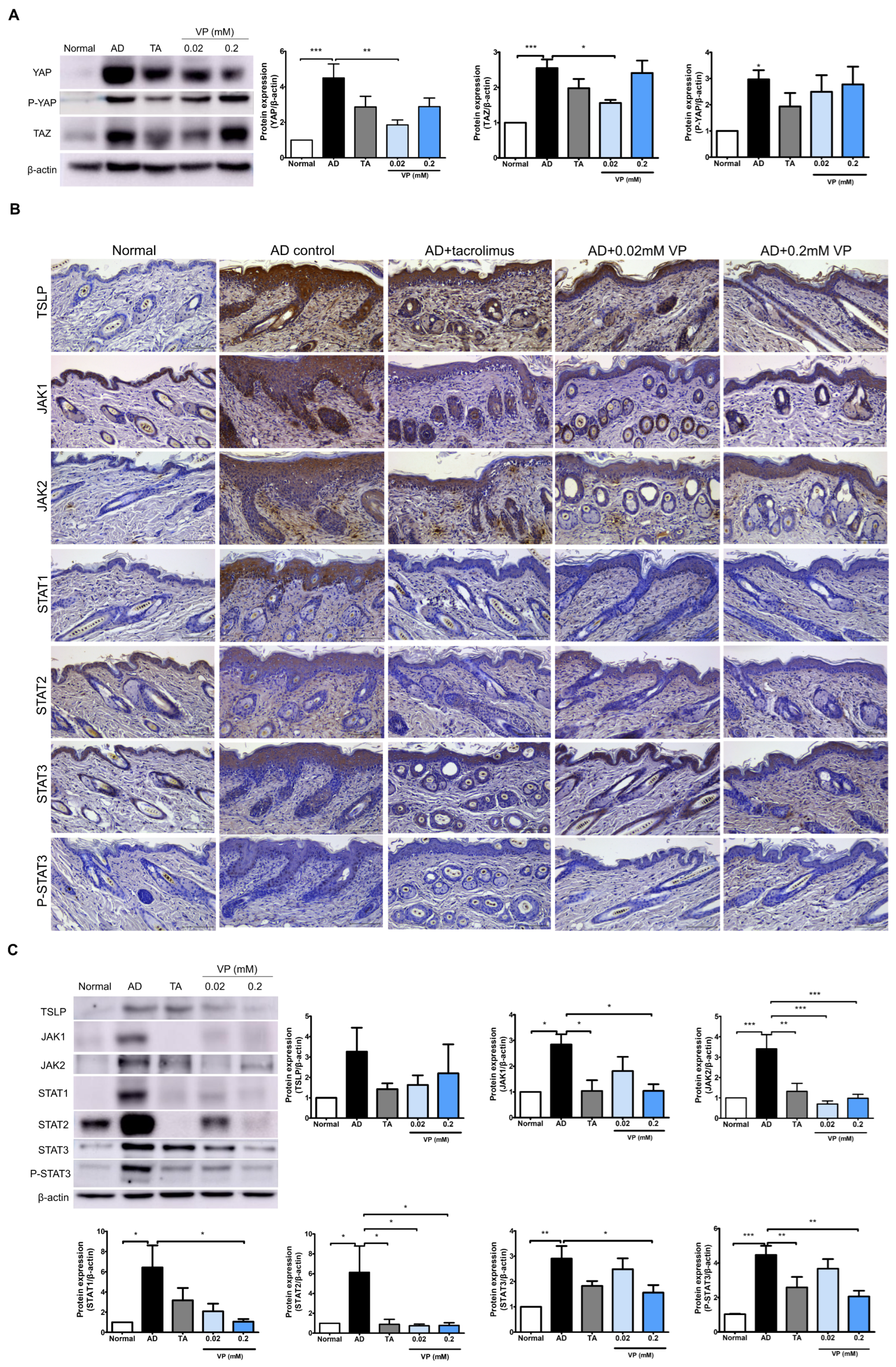
2.3. YAP Inhibitor Modulates Inflammation-Related Signaling and Improves It at the mRNA Level
2.4. YAP Inhibitors Relieve AD by Blocking Inflammatory Factors and the JAK-STAT Pathway through Inhibition of YAP Protein
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Animal Study
4.3. Evaluation of Skin Lesions
4.4. Quantitative Real-Time PCR in Dorsal Skin Tissue
4.5. Immunohistochemical Analysis
4.6. Western Blot
4.7. Digital Analysis of Immunohistochemistry Images
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| YAP | Yes-associated protein |
| TAZ | Transcription activator with a PDZ-binding motif |
| JAK | Janus kinase |
| STAT | Signal transducer and activator of transcription |
| AD | Atopic dermatitis |
| DNCB | 1-chloro-2,4-dinitrobenzene |
References
- Kwang Hoon, L.; Choi, E.H.; Park, C.O. Practical Insights into Atopic Dermatitis; Springer: Singapore, 2021. [Google Scholar]
- Narla, S.; Silverberg, J.I.; Simpson, E.L. Management of inadequate response and adverse effects to dupilumab in atopic dermatitis. J. Am. Acad. Dermatol. 2022, 86, 628–636. [Google Scholar] [CrossRef]
- Hemrajani, C.; Negi, P.; Parashar, A.; Gupta, G.; Jha, N.K.; Singh, S.K.; Chellappan, D.K.; Dua, K. Overcoming drug delivery barriers and challenges in topical therapy of atopic dermatitis: A nanotechnological perspective. Biomed. Pharmacother. 2022, 147, 112633. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya, R.; Paller, A.S. JAK inhibitors in the treatment of atopic dermatitis. J. Allergy Clin. Immunol. 2021, 148, 927–940. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, S.; Dupont, S.; Cordenonsi, M. The biology of YAP/TAZ: Hippo signaling and beyond. Physiol. Rev. 2014, 94, 1287–1312. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.T.; Guerrero-Castilla, A.; Cano, M.; Muñoz, M.F.; Ayala, A.; Argüelles, S. Dysregulation of the Hippo pathway signaling in aging and cancer. Pharmacol. Res. 2019, 143, 151–165. [Google Scholar] [CrossRef]
- Guo, P.; Wang, Z.; Zhou, Z.; Tai, Y.; Zhang, A.; Wei, W.; Wang, Q. Immuno-hippo: Research progress of the hippo pathway in autoimmune disease. Immunol. Lett. 2021, 230, 11–20. [Google Scholar] [CrossRef] [PubMed]
- D’Addario, I.; Abbruzzese, C.; Lo Iacono, M.; Teson, M.; Golisano, O.; Barone, V. Overexpression of YAP1 induces immortalization of normal human keratinocytes by blocking clonal evolution. Histochem. Cell Biol. 2010, 134, 265–276. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Jia, J.; Mo, X.; Yan, F.; Liu, J.; Ye, S.; Zhang, Y.; Lin, Y.; Li, H.; Chen, D. Role of YAP-related T cell imbalance and epidermal keratinocyte dysfunction in the pathogenesis of atopic dermatitis. J. Dermatol. Sci. 2021, 101, 164–173. [Google Scholar] [CrossRef]
- Jia, J.; Li, C.; Yang, J.; Wang, X.; Li, R.; Luo, S.; Li, Z.; Liu, J.; Liu, Z.; Zheng, Y. Yes-associated protein promotes the abnormal proliferation of psoriatic keratinocytes via an amphiregulin dependent pathway. Sci. Rep. 2018, 8, 14513. [Google Scholar] [CrossRef]
- He, J.; Bao, Q.; Zhang, Y.; Liu, M.; Lv, H.; Liu, Y.; Yao, L.; Li, B.; Zhang, C.; He, S.; et al. Yes-Associated Protein Promotes Angiogenesis via Signal Transducer and Activator of Transcription 3 in Endothelial Cells. Circ. Res. 2018, 122, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Deng, F. YAP regulates intestinal epithelial cell proliferation through activation of STAT3 in DSS-induced colitis and associated cancer. J. Cent. South Univ. Med. Sci. 2022, 47, 1637–1645. [Google Scholar]
- Wang, Y.; Wang, L.; Wise, J.T.F.; Shi, X.; Chen, Z. Verteporfin inhibits lipopolysaccharide-induced inflammation by multiple functions in RAW 264.7 cells. Toxicol. Appl. Pharmacol. 2020, 387, 114852. [Google Scholar] [CrossRef] [PubMed]
- Osama, M.; Essibayi, M.A.; Osama, M.; Ibrahim, I.A.; Nasr Mostafa, M.; Şakir Ekşi, M. The impact of interaction between verteporfin and yes-associated protein 1/transcriptional coactivator with PDZ-binding motif-TEA domain pathway on the progression of isocitrate dehydrogenase wild-type glioblastoma. J. Cent. Nerv. Syst. Dis. 2023, 15, 11795735231195760. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; He, R.; Oyoshi, M.; Geha, R.S. Animal models of atopic dermatitis. J. Investig. Dermatol. 2009, 129, 31–40. [Google Scholar] [CrossRef]
- Ebina-Shibuya, R.; Leonard, W.J. Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol. 2023, 23, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Chieosilapatham, P.; Kiatsurayanon, C.; Umehara, Y.; Trujillo-Paez, J.V.; Peng, G.; Yue, H.; Nguyen, L.T.H.; Niyonsaba, F. Keratinocytes: Innate immune cells in atopic dermatitis. Clin. Exp. Immunol. 2021, 204, 296–309. [Google Scholar] [CrossRef]
- Yoo, J.; Omori, M.; Gyarmati, D.; Zhou, B.; Aye, T.; Brewer, A.; Comeau, M.R.; Campbell, D.J.; Ziegler, S.F. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005, 202, 541–549. [Google Scholar] [CrossRef]
- Seltmann, J.; Roesner, L.M.; von Hesler, F.W.; Wittmann, M.; Werfel, T. IL-33 impacts on the skin barrier by downregulating the expression of filaggrin. J. Allergy Clin. Immunol. 2015, 135, 1659–1661.e4. [Google Scholar] [CrossRef]
- Cole, C.; Kroboth, K.; Schurch, N.J.; Sandilands, A.; Sherstnev, A.; O’Regan, G.M.; Watson, R.M.; McLean, W.H.; Barton, G.J.; Irvine, A.D.; et al. Filaggrin-stratified transcriptomic analysis of pediatric skin identifies mechanistic pathways in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2014, 134, 82–91. [Google Scholar] [CrossRef]
- Palmer, C.N.; Irvine, A.D.; Terron-Kwiatkowski, A.; Zhao, Y.; Liao, H.; Lee, S.P.; Goudie, D.R.; Sandilands, A.; Campbell, L.E.; Smith, F.J.; et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nat. Genet. 2006, 38, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Konishi, H.; Tsutsui, H.; Murakami, T.; Yumikura-Futatsugi, S.; Yamanaka, K.; Tanaka, M.; Iwakura, Y.; Suzuki, N.; Takeda, K.; Akira, S.; et al. IL-18 contributes to the spontaneous development of atopic dermatitis-like inflammatory skin lesion independently of IgE/stat6 under specific pathogen-free conditions. Proc. Natl. Acad. Sci. USA 2002, 99, 11340–11345. [Google Scholar] [CrossRef] [PubMed]
- Elbediwy, A.; Vincent-Mistiaen, Z.I.; Spencer-Dene, B.; Stone, R.K.; Boeing, S.; Wculek, S.K.; Cordero, J.; Tan, E.H.; Ridgway, R.; Brunton, V.G.; et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 2016, 143, 1674–1687. [Google Scholar] [PubMed]
- Schlegelmilch, K.; Mohseni, M.; Kirak, O.; Pruszak, J.; Rodriguez, J.R.; Zhou, D.; Kreger, B.T.; Vasioukhin, V.; Avruch, J.; Brummelkamp, T.R.; et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 2011, 144, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, J.; Nakanishi, S.; Makita, M.; Uesaka, M.; Yasugahira, Y.; Kobayashi, Y.; Nagayama, M.; Denda, S.; Denda, M. Mathematical-model-guided development of full-thickness epidermal equivalent. Sci. Rep. 2018, 8, 17999. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.C.; Moroishi, T.; Meng, Z.; Jeong, H.-S.; Plouffe, S.W.; Sekido, Y.; Han, J.; Park, H.W.; Guan, K.-L. Regulation of Hippo pathway transcription factor TEAD by p38 MAPK-induced cytoplasmic translocation. Nat. Cell Biol. 2017, 19, 996–1002. [Google Scholar] [CrossRef]
- Condurat, A.L.; Aminzadeh-Gohari, S.; Malnar, M.; Schider, N.; Opitz, L.; Thomas, R.; Menon, V.; Kofler, B.; Pruszak, J. Verteporfin-induced proteotoxicity impairs cell homeostasis and survival in neuroblastoma subtypes independent of YAP/TAZ expression. Sci. Rep. 2023, 13, 3760. [Google Scholar] [CrossRef]
- Yuan, Y.; Salinas Parra, N.; Chen, Q.; Iglesias-Bartolome, R. Oncogenic Hedgehog-Smoothened Signaling Depends on YAP1–TAZ/TEAD Transcription to Restrain Differentiation in Basal Cell Carcinoma. J. Investig. Dermatol. 2022, 142, 65–76.e7. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, X.; Liu, Y.; Singhal, M.; Gürkaşlar, C.; Valls, A.F.; Lei, Y.; Hu, W.; Schermann, G.; Adler, H.; et al. STAT3-YAP/TAZ signaling in endothelial cells promotes tumor angiogenesis. Sci. Signal. 2021, 14, eabj8393. [Google Scholar] [CrossRef]
- Lee, J.; Jung, Y.; Jeong, S.W.; Jeong, G.H.; Moon, G.T.; Kim, M. Inhibition of Hippo Signaling Improves Skin Lesions in a Rosacea-Like Mouse Model. Int. J. Mol. Sci. 2021, 22, 931. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Ferreira, S.; Guttman-Yassky, E.; Torres, T. Selective JAK1 Inhibitors for the Treatment of Atopic Dermatitis: Focus on Upadacitinib and Abrocitinib. Am. J. Clin. Dermatol. 2020, 21, 783–798. [Google Scholar] [CrossRef]
- Mowen, K.A.; Glimcher, L.H. Signaling pathways in Th2 development. Immunol. Rev. 2004, 202, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.A.; Bennett, B.L.; Graham, N.M.; Pirozzi, G.; Stahl, N.; Yancopoulos, G.D. Targeting key proximal drivers of type 2 inflammation in disease. Nat. Rev. Drug Discov. 2016, 15, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Salas, A.; Hernandez-Rocha, C.; Duijvestein, M.; Faubion, W.; McGovern, D.; Vermeire, S.; Vetrano, S.; Vande Casteele, N. JAK-STAT pathway targeting for the treatment of inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Molfino, N.A.; Gossage, D.; Kolbeck, R.; Parker, J.M.; Geba, G.P. Molecular and clinical rationale for therapeutic targeting of interleukin-5 and its receptor. Clin. Exp. Allergy 2012, 42, 712–737. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, H.; Chan, L.S. The involvement of the JAK-STAT signaling pathway in chronic inflammatory skin disease atopic dermatitis. Jakstat 2013, 2, e24137. [Google Scholar] [CrossRef]
- Rochman, Y.; Kashyap, M.; Robinson, G.W.; Sakamoto, K.; Gomez-Rodriguez, J.; Wagner, K.U.; Leonard, W.J. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc. Natl. Acad. Sci. USA 2010, 107, 19455–19460. [Google Scholar] [CrossRef]
- Peng, Q.; Li, S.; Shi, X.; Guo, Y.; Hao, L.; Zhang, Z.; Ji, J.; Zhao, Y.; Li, C.; Xue, Y.; et al. Dihydroartemisinin broke the tumor immunosuppressive microenvironment by inhibiting YAP1 expression to enhance anti-PD-1 efficacy. Phytother. Res. 2022, 37, 1740–1753. [Google Scholar] [CrossRef]
- Taniguchi, K.; Wu, L.-W.; Grivennikov, S.I.; de Jong, P.R.; Lian, I.; Yu, F.-X.; Wang, K.; Ho, S.B.; Boland, B.S.; Chang, J.T.; et al. A gp130–Src–YAP module links inflammation to epithelial regeneration. Nature 2015, 519, 57–62. [Google Scholar] [CrossRef]
- Symons, R.A.; Colella, F.; Collins, F.L.; Rafipay, A.J.; Kania, K.; McClure, J.J.; White, N.; Cunningham, I.; Ashraf, S.; Hay, E.; et al. Targeting the IL-6-Yap-Snail signalling axis in synovial fibroblasts ameliorates inflammatory arthritis. Ann. Rheum. Dis. 2022, 81, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Rasool, M. Underpinning IL-6 biology and emphasizing selective JAK blockade as the potential alternate therapeutic intervention for rheumatoid arthritis. Life Sci. 2022, 298, 120516. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Pratt, H.; Gao, M.; Wei, F.; Weng, Z.; Struhl, K. YAP and TAZ are transcriptional co-activators of AP-1 proteins and STAT3 during breast cellular transformation. eLife 2021, 10, e67312. [Google Scholar] [CrossRef]
- Crowe, A.R.; Yue, W. Semi-quantitative Determination of Protein Expression using Immunohistochemistry Staining and Analysis: An Integrated Protocol. Bio Protoc. 2019, 9, e3465. [Google Scholar] [CrossRef]


| Target | Sequence (5′–3′) | |
|---|---|---|
| Tslp | Forward | AAAGGGGCTAAGTTCGAGCA |
| Reverse | AGGGCTTCTCTTGTTCTCCG | |
| Il1b | Forward | TGCCACCTTTTGACAGTGAT |
| Reverse | AGTGATACTGCCTGCCTGAA | |
| Il4 | Forward | TCTCGAATGTACCAGGAGCCATATC |
| Reverse | AGCACCTTGGAAGCCTACAGA | |
| Il6 | Forward | CCCCAATTTCCAATGCTCTCC |
| Reverse | AGGCATAACGCACTAGGTTT | |
| Il13 | Forward | CTGCTACCTCACTGTAGCCT |
| Reverse | TATTTCATGGCTGAGGGCTG | |
| Il17 | Forward | TCCACCGCAATGAAGACCCTGATA |
| Reverse | ACCAGCATCTTCTCGACCCTGAAA | |
| Il18 | Forward | AGGCATCCAGGACAAATCAG |
| Reverse | GGTGTACTCATCGTTGTGGG | |
| Il22 | Forward | CTTGTGCGATCTCTGATGGCT |
| Reverse | GCTGGAAGTTGGACACCTCA | |
| Il33 | Forward | TCCTGTCTGTATTGAGAAACCT |
| Reverse | CTTATGGTGAGG CCAGAACG | |
| Ifng | Forward | TGATTGCGGGGTTGTATCTG |
| Reverse | CTGTCTGGCCTGCTGTTAAA | |
| Actb | Forward | TGCTAGGAGCCAGAGCAGTA |
| Reverse | AGTGTGACGTTGACATCCGT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, G.H.; Lee, J.H. Dysregulated Hippo Signaling Pathway and YAP Activation in Atopic Dermatitis: Insights from Clinical and Animal Studies. Int. J. Mol. Sci. 2023, 24, 17322. https://doi.org/10.3390/ijms242417322
Jeong GH, Lee JH. Dysregulated Hippo Signaling Pathway and YAP Activation in Atopic Dermatitis: Insights from Clinical and Animal Studies. International Journal of Molecular Sciences. 2023; 24(24):17322. https://doi.org/10.3390/ijms242417322
Chicago/Turabian StyleJeong, Ga Hee, and Ji Hyun Lee. 2023. "Dysregulated Hippo Signaling Pathway and YAP Activation in Atopic Dermatitis: Insights from Clinical and Animal Studies" International Journal of Molecular Sciences 24, no. 24: 17322. https://doi.org/10.3390/ijms242417322
APA StyleJeong, G. H., & Lee, J. H. (2023). Dysregulated Hippo Signaling Pathway and YAP Activation in Atopic Dermatitis: Insights from Clinical and Animal Studies. International Journal of Molecular Sciences, 24(24), 17322. https://doi.org/10.3390/ijms242417322







