Can CD133 Be Regarded as a Prognostic Biomarker in Oncology: Pros and Cons
Abstract
1. Introduction
2. Putative Explanation of the Cause-and-Effect Relationship between CD133 Expression and More Malignant Phenotype of Tumor Cells
2.1. Increased Proliferation
2.2. Increased Chemo- and Radioresistance
2.3. Increased Invasion and Metastasis
2.4. CD133 as a Target of Therapies Aimed to Eliminate the Most Malignant Cells
3. Association of CD133 with Cancer Progression and Poor Prognosis
4. Possible Reasons for the Discrepancies in the Data on the Association of CD133 with the Disease Severity
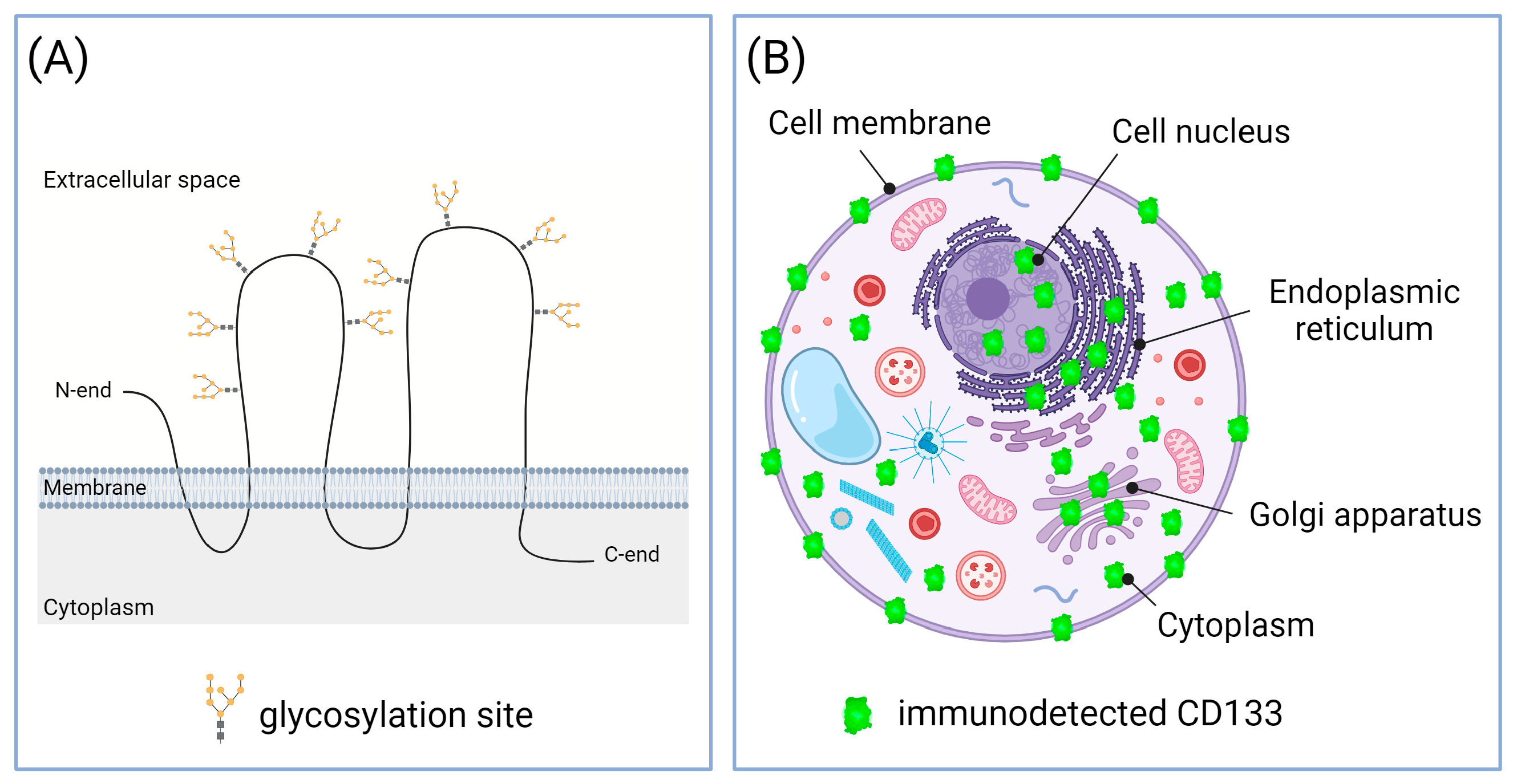
4.1. Different CD133 Immunodetection Techniques
4.2. Some CD133-Positive Cells Present within Tumors Are Normal, Benign Cells
4.3. Subcellular Localization of CD133 May Vary at Different Stages of Carcinogenesis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Immervoll, H.; Hoem, D.; Sakariassen, P.Ø.; Steffensen, O.J.; Molven, A. Expression of the “Stem Cell Marker” CD133 in Pancreas and Pancreatic Ductal Adenocarcinomas. BMC Cancer 2008, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.B.; Pehar, M.; Nixon, A.M.L.; Williams, R.A.; Uetrecht, A.C.; Puglielli, L.; Moffat, J. Post-Translational Regulation of CD133 by ATase1/ATase2-Mediated Lysine Acetylation. J. Mol. Biol. 2014, 426, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- Miraglia, S.; Godfrey, W.; Yin, A.H.; Atkins, K.; Warnke, R.; Holden, J.T.; Bray, R.A.; Waller, E.K.; Buck, D.W. A Novel Five-Transmembrane Hematopoietic Stem Cell Antigen: Isolation, Characterization, and Molecular Cloning. Blood 1997, 90, 5013–5021. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.H.; Miraglia, S.; Zanjani, E.D.; Almeida-Porada, G.; Ogawa, M.; Leary, A.G.; Olweus, J.; Kearney, J.; Buck, D.W. AC133, a Novel Marker for Human Hematopoietic Stem and Progenitor Cells. Blood 1997, 90, 5002–5012. [Google Scholar] [CrossRef] [PubMed]
- Weigmann, A.; Corbeil, D.; Hellwig, A.; Huttner, W.B. Prominin, a Novel Microvilli-Specific Polytopic Membrane Protein of the Apical Surface of Epithelial Cells, Is Targeted to Plasmalemmal Protrusions of Non-Epithelial Cells. Proc. Natl. Acad. Sci. USA 1997, 94, 12425–12430. [Google Scholar] [CrossRef] [PubMed]
- Fargeas, C.A.; Florek, M.; Huttner, W.B.; Corbeil, D. Characterization of Prominin-2, a New Member of the Prominin Family of Pentaspan Membrane Glycoproteins. J. Biol. Chem. 2003, 278, 8586–8596. [Google Scholar] [CrossRef] [PubMed]
- Shmelkov, S.V.; St Clair, R.; Lyden, D.; Rafii, S. AC133/CD133/Prominin-1. Int. J. Biochem. Cell Biol. 2005, 37, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Karbanová, J.; Lorico, A.; Bornhäuser, M.; Corbeil, D.; Fargeas, C.A. Prominin-1/CD133: Lipid Raft Association, Detergent Resistance, and Immunodetection. Stem Cells Transl. Med. 2017, 7, 155–160. [Google Scholar] [CrossRef]
- Nunukova, A.; Neradil, J.; Skoda, J.; Jaros, J.; Hampl, A.; Sterba, J.; Veselska, R. Atypical Nuclear Localization of CD133 Plasma Membrane Glycoprotein in Rhabdomyosarcoma Cell Lines. Int. J. Mol. Med. 2015, 36, 65–72. [Google Scholar] [CrossRef][Green Version]
- Cantile, M.; Collina, F.; D’Aiuto, M.; Rinaldo, M.; Pirozzi, G.; Borsellino, C.; Franco, R.; Botti, G.; Di Bonito, M. Nuclear Localization of Cancer Stem Cell Marker CD133 in Triple-Negative Breast Cancer: A Case Report. Tumori 2013, 99, e245–e250. [Google Scholar] [CrossRef]
- Pietrus, M.; Pitynski, K.; Waligora, M.; Milian-Ciesielska, K.; Bialon, M.; Ludwin, A.; Skrzypek, K. CD133 Expression in the Nucleus Is Associated with Endometrial Carcinoma Staging and Tumor Angioinvasion. J. Clin. Med. 2021, 10, 2144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, S.; Xie, L.; Cui, C.; Xing, Y.; Liu, C.; Cao, B.; Yang, F.; Li, Y.; Chen, X.; et al. Mutation of N-Linked Glycosylation at Asn548 in CD133 Decreases Its Ability to Promote Hepatoma Cell Growth. Oncotarget 2015, 6, 20650–20660. [Google Scholar] [CrossRef][Green Version]
- Kania, G.; Corbeil, D.; Fuchs, J.; Tarasov, K.V.; Blyszczuk, P.; Huttner, W.B.; Boheler, K.R.; Wobus, A.M. Somatic Stem Cell Marker Prominin-1/CD133 Is Expressed in Embryonic Stem Cell-Derived Progenitors. Stem Cells Dayt. Ohio 2005, 23, 791–804. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Corbeil, D.; Röper, K.; Hellwig, A.; Tavian, M.; Miraglia, S.; Watt, S.M.; Simmons, P.J.; Peault, B.; Buck, D.W.; Huttner, W.B. The Human AC133 Hematopoietic Stem Cell Antigen Is Also Expressed in Epithelial Cells and Targeted to Plasma Membrane Protrusions. J. Biol. Chem. 2000, 275, 5512–5520. [Google Scholar] [CrossRef] [PubMed]
- Peichev, M.; Naiyer, A.J.; Pereira, D.; Zhu, Z.; Lane, W.J.; Williams, M.; Oz, M.C.; Hicklin, D.J.; Witte, L.; Moore, M.A.; et al. Expression of VEGFR-2 and AC133 by Circulating Human CD34(+) Cells Identifies a Population of Functional Endothelial Precursors. Blood 2000, 95, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Richardson, G.D.; Robson, C.N.; Lang, S.H.; Neal, D.E.; Maitland, N.J.; Collins, A.T. CD133, a Novel Marker for Human Prostatic Epithelial Stem Cells. J. Cell Sci. 2004, 117, 3539–3545. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Buck, D.W.; He, D.; Reitsma, M.J.; Masek, M.; Phan, T.V.; Tsukamoto, A.S.; Gage, F.H.; Weissman, I.L. Direct Isolation of Human Central Nervous System Stem Cells. Proc. Natl. Acad. Sci. USA 2000, 97, 14720–14725. [Google Scholar] [CrossRef] [PubMed]
- Torrente, Y.; Belicchi, M.; Sampaolesi, M.; Pisati, F.; Meregalli, M.; D’Antona, G.; Tonlorenzi, R.; Porretti, L.; Gavina, M.; Mamchaoui, K.; et al. Human Circulating AC133(+) Stem Cells Restore Dystrophin Expression and Ameliorate Function in Dystrophic Skeletal Muscle. J. Clin. Investig. 2004, 114, 182–195. [Google Scholar] [CrossRef]
- Kordes, C.; Sawitza, I.; Müller-Marbach, A.; Ale-Agha, N.; Keitel, V.; Klonowski-Stumpe, H.; Häussinger, D. CD133+ Hepatic Stellate Cells Are Progenitor Cells. Biochem. Biophys. Res. Commun. 2007, 352, 410–417. [Google Scholar] [CrossRef]
- Karbanová, J.; Missol-Kolka, E.; Fonseca, A.-V.; Lorra, C.; Janich, P.; Hollerová, H.; Jászai, J.; Ehrmann, J.; Kolář, Z.; Liebers, C.; et al. The Stem Cell Marker CD133 (Prominin-1) Is Expressed in Various Human Glandular Epithelia. J. Histochem. Cytochem. 2008, 56, 977–993. [Google Scholar] [CrossRef]
- Karbanová, J.; Laco, J.; Marzesco, A.-M.; Janich, P.; Voborníková, M.; Mokrý, J.; Fargeas, C.A.; Huttner, W.B.; Corbeil, D. Human Prominin-1 (CD133) Is Detected in Both Neoplastic and Non-Neoplastic Salivary Gland Diseases and Released into Saliva in a Ubiquitinated Form. PLoS ONE 2014, 9, e98927. [Google Scholar] [CrossRef] [PubMed]
- Maw, M.A.; Corbeil, D.; Koch, J.; Hellwig, A.; Wilson-Wheeler, J.C.; Bridges, R.J.; Kumaramanickavel, G.; John, S.; Nancarrow, D.; Röper, K.; et al. A Frameshift Mutation in Prominin (Mouse)-like 1 Causes Human Retinal Degeneration. Hum. Mol. Genet. 2000, 9, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Jászai, J.; Fargeas, C.A.; Florek, M.; Huttner, W.B.; Corbeil, D. Focus on Molecules: Prominin-1 (CD133). Exp. Eye Res. 2007, 85, 585–586. [Google Scholar] [CrossRef] [PubMed]
- Pellacani, D.; Oldridge, E.E.; Collins, A.T.; Maitland, N.J. Prominin-1 (CD133) Expression in the Prostate and Prostate Cancer: A Marker for Quiescent Stem Cells. Adv. Exp. Med. Biol. 2013, 777, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Bertolini, G.; Roz, L.; Perego, P.; Tortoreto, M.; Fontanella, E.; Gatti, L.; Pratesi, G.; Fabbri, A.; Andriani, F.; Tinelli, S.; et al. Highly Tumorigenic Lung Cancer CD133+ Cells Display Stem-like Features and Are Spared by Cisplatin Treatment. Proc. Natl. Acad. Sci. USA 2009, 106, 16281–16286. [Google Scholar] [CrossRef] [PubMed]
- Eramo, A.; Lotti, F.; Sette, G.; Pilozzi, E.; Biffoni, M.; Di Virgilio, A.; Conticello, C.; Ruco, L.; Peschle, C.; De Maria, R. Identification and Expansion of the Tumorigenic Lung Cancer Stem Cell Population. Cell Death Differ. 2008, 15, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Bellio, C.; DiGloria, C.; Foster, R.; James, K.; Konstantinopoulos, P.A.; Growdon, W.B.; Rueda, B.R. PARP Inhibition Induces Enrichment of DNA Repair-Proficient CD133 and CD117 Positive Ovarian Cancer Stem Cells. Mol. Cancer Res. MCR 2019, 17, 431–445. [Google Scholar] [CrossRef]
- Joseph, C.; Arshad, M.; Kurozomi, S.; Althobiti, M.; Miligy, I.M.; Al-Izzi, S.; Toss, M.S.; Goh, F.Q.; Johnston, S.J.; Martin, S.G.; et al. Overexpression of the Cancer Stem Cell Marker CD133 Confers a Poor Prognosis in Invasive Breast Cancer. Breast Cancer Res. Treat. 2019, 174, 387–399. [Google Scholar] [CrossRef]
- Shang, C.; Lang, B.; Meng, L.-R. Blocking NOTCH Pathway Can Enhance the Effect of EGFR Inhibitor through Targeting CD133+ Endometrial Cancer Cells. Cancer Biol. Ther. 2018, 19, 113–119. [Google Scholar] [CrossRef]
- Saeednejad Zanjani, L.; Madjd, Z.; Abolhasani, M.; Andersson, Y.; Rasti, A.; Shariftabrizi, A.; Asgari, M. Cytoplasmic Expression of CD133 Stemness Marker Is Associated with Tumor Aggressiveness in Clear Cell Renal Cell Carcinoma. Exp. Mol. Pathol. 2017, 103, 218–228. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Liu, W.; Ai, Z. CD133 Promotes the Self-Renewal Capacity of Thyroid Cancer Stem Cells through Activation of Glutamate Aspartate Transporter SLC1A3 Expression. Biochem. Biophys. Res. Commun. 2019, 511, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Rizvi, S.Z.; Lal, N.; Gupta, V.; Srivastav, A.N.; Musa, O. Expression of CD44 and CD133 Stem Cell Markers in Squamous Cell Carcinoma of Esophagus. Indian J. Pathol. Microbiol. 2021, 64, 472–478. [Google Scholar] [CrossRef]
- Feitosa, N.P.P.; Pereira, V.B.M.; Silva, B.G.B.; Queroz, A.V.F.; Rodrigues, B.J.; Costa, M.L.V.; Alencar, C.H.; Lima-Júnior, R.C.P.; Wong, D.V.T.; Frota, C.C.; et al. Cancerous and Non-Neoplastic Stem Cells in the Stomach Similarly Express CD44 and CD133. Acta Histochem. 2021, 123, 151787. [Google Scholar] [CrossRef] [PubMed]
- Wattanawongdon, W.; Bathpho, T.S.; Tongtawee, T. Co-Expression of LGR5 and CD133 Cancer Stem Cell Predicts a Poor Prognosis in Patients With Gastric Cancer. Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2021, 32, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Sheng, W.-Q.; Du, X. CD133: A Cancer Stem Cells Marker, Is Used in Colorectal Cancers. World J. Gastroenterol. 2013, 19, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Li, J.; Hu, C.; Chen, X.; Yao, M.; Yan, M.; Jiang, G.; Ge, C.; Xie, H.; Wan, D.; et al. CD133 Positive Hepatocellular Carcinoma Cells Possess High Capacity for Tumorigenicity. Int. J. Cancer 2007, 120, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Piao, L.S.; Hur, W.; Kim, T.-K.; Hong, S.W.; Kim, S.W.; Choi, J.E.; Sung, P.S.; Song, M.J.; Lee, B.-C.; Hwang, D.; et al. CD133+ Liver Cancer Stem Cells Modulate Radioresistance in Human Hepatocellular Carcinoma. Cancer Lett. 2012, 315, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, C.; Xing, Y.; Zhen, J.; Ai, Z. CD133 Promotes Gallbladder Carcinoma Cell Migration through Activating Akt Phosphorylation. Oncotarget 2016, 7, 17751–17759. [Google Scholar] [CrossRef]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma Stem Cells Promote Radioresistance by Preferential Activation of the DNA Damage Response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef]
- Maier, A.D.; Mirian, C.; Bartek, J.; Juhler, M.; Bartkova, J.; Broholm, H.; Mathiesen, T.I. Expression of the Stem Cell Marker CD133 in Malignant Meningioma. Clin. Neuropathol. 2021, 40, 151–159. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, J.; Wu, M.; Zhou, Y. Expression of CD133 Protein in Osteosarcoma and Its Relationship with the Clinicopathological Features and Prognosis. J. Cancer Res. Ther. 2018, 14, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Madjd, Z.; Erfani, E.; Gheytanchi, E.; Moradi-Lakeh, M.; Shariftabrizi, A.; Asadi-Lari, M. Expression of CD133 Cancer Stem Cell Marker in Melanoma: A Systematic Review and Meta-Analysis. Int. J. Biol. Markers 2016, 31, e118–e125. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.X.; Hawk, N.V.; Chen, W.; Coupar, J.; Lee, S.K.; Petersen, D.W.; Meltzer, P.S.; Montemarano, A.; Braun, M.; Chen, Z.; et al. Targeting Notch1 and IKKα Enhanced NF-κB Activation in CD133+ Skin Cancer Stem Cells. Mol. Cancer Ther. 2018, 17, 2034–2048. [Google Scholar] [CrossRef] [PubMed]
- Taussig, D.C.; Pearce, D.J.; Simpson, C.; Rohatiner, A.Z.; Lister, T.A.; Kelly, G.; Luongo, J.L.; Danet-Desnoyers, G.-A.H.; Bonnet, D. Hematopoietic Stem Cells Express Multiple Myeloid Markers: Implications for the Origin and Targeted Therapy of Acute Myeloid Leukemia. Blood 2005, 106, 4086–4092. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.J.; Abass-Shereef, J.; Walton, K.; Goodell, L.; Aviv, H.; Strair, R.K.; Budak-Alpdogan, T. Cobblestone-Area Forming Cells Derived from Patients with Mantle Cell Lymphoma Are Enriched for CD133+ Tumor-Initiating Cells. PLoS ONE 2014, 9, e91042. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, C.A.; Pollett, A.; Gallinger, S.; Dick, J.E. A Human Colon Cancer Cell Capable of Initiating Tumour Growth in Immunodeficient Mice. Nature 2007, 445, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.C.; Huber, S.L.; Herrler, T.; Aicher, A.; Ellwart, J.W.; Guba, M.; Bruns, C.J.; Heeschen, C. Distinct Populations of Cancer Stem Cells Determine Tumor Growth and Metastatic Activity in Human Pancreatic Cancer. Cell Stem Cell 2007, 1, 313–323. [Google Scholar] [CrossRef]
- Nagata, H.; Ishihara, S.; Kishikawa, J.; Sonoda, H.; Murono, K.; Emoto, S.; Kaneko, M.; Sasaki, K.; Otani, K.; Nishikawa, T.; et al. CD133 Expression Predicts Post-Operative Recurrence in Patients with Colon Cancer with Peritoneal Metastasis. Int. J. Oncol. 2018, 52, 721–732. [Google Scholar] [CrossRef]
- Huang, M.; Zhu, H.; Feng, J.; Ni, S.; Huang, J. High CD133 Expression in the Nucleus and Cytoplasm Predicts Poor Prognosis in Non-Small Cell Lung Cancer. Dis. Markers 2015, 2015, 986095. [Google Scholar] [CrossRef]
- Park, E.K.; Lee, J.C.; Park, J.W.; Bang, S.Y.; Yi, S.A.; Kim, B.K.; Park, J.H.; Kwon, S.H.; You, J.S.; Nam, S.W.; et al. Transcriptional Repression of Cancer Stem Cell Marker CD133 by Tumor Suppressor P53. Cell Death Dis. 2015, 6, e1964. [Google Scholar] [CrossRef]
- Liu, T.T.; Li, X.F.; Wang, L.; Yang, J.L. CD133 Expressionand Clinicopathologic Significance in Benign and Malignant Breast Lesions. Cancer Biomark. Sect. Dis. Markers 2020, 28, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Kostovski, O.; Antovic, S.; Trajkovski, G.; Kostovska, I.; Jovanovic, R.; Jankulovski, N. High Expression of CD133—Stem Cell Marker for Prediction of Clinically Agressive Type of Colorectal Cancer. Pol. Przegl. Chir. 2020, 92, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a Cancer Stem Cell in Human Brain Tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of Human Brain Tumour Initiating Cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Atashzar, M.R.; Baharlou, R.; Karami, J.; Abdollahi, H.; Rezaei, R.; Pourramezan, F.; Zoljalali Moghaddam, S.H. Cancer Stem Cells: A Review from Origin to Therapeutic Implications. J. Cell. Physiol. 2020, 235, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-S.; Huang, P.-I.; Chang, Y.-L.; Tzao, C.; Chen, Y.-W.; Shih, H.-C.; Hung, S.-C.; Chen, Y.-C.; Tseng, L.-M.; Chiou, S.-H. Cucurbitacin I Inhibits Tumorigenic Ability and Enhances Radiochemosensitivity in Nonsmall Cell Lung Cancer-Derived CD133-Positive Cells. Cancer 2011, 117, 2970–2985. [Google Scholar] [CrossRef] [PubMed]
- Curley, M.D.; Therrien, V.A.; Cummings, C.L.; Sergent, P.A.; Koulouris, C.R.; Friel, A.M.; Roberts, D.J.; Seiden, M.V.; Scadden, D.T.; Rueda, B.R.; et al. CD133 Expression Defines a Tumor Initiating Cell Population in Primary Human Ovarian Cancer. Stem Cells Dayt. Ohio 2009, 27, 2875–2883. [Google Scholar] [CrossRef]
- Attia, S.; Atwan, N.; Arafa, M.; Shahin, R.A. Expression of CD133 as a Cancer Stem Cell Marker in Invasive Gastric Carcinoma. Pathologica 2019, 111, 18–23. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and Expansion of Human Colon-Cancer-Initiating Cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef]
- Shi, C.-J.; Gao, J.; Wang, M.; Wang, X.; Tian, R.; Zhu, F.; Shen, M.; Qin, R.-Y. CD133+ Gallbladder Carcinoma Cells Exhibit Self-Renewal Ability and Tumorigenicity. World J. Gastroenterol. WJG 2011, 17, 2965–2971. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Hu, F.-W.; Yu, C.-C.; Tsai, L.-L.; Yu, C.-H.; Wu, B.-C.; Chen, Y.-W.; Huang, P.-I.; Lo, W.-L. Epithelial-Mesenchymal Transition Transcription Factor ZEB1/ZEB2 Co-Expression Predicts Poor Prognosis and Maintains Tumor-Initiating Properties in Head and Neck Cancer. Oral Oncol. 2013, 49, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Bayin, N.S.; Modrek, A.S.; Dietrich, A.; Lebowitz, J.; Abel, T.; Song, H.-R.; Schober, M.; Zagzag, D.; Buchholz, C.J.; Chao, M.V.; et al. Selective Lentiviral Gene Delivery to CD133-Expressing Human Glioblastoma Stem Cells. PLoS ONE 2014, 9, e116114. [Google Scholar] [CrossRef] [PubMed]
- Suvà, M.-L.; Riggi, N.; Stehle, J.-C.; Baumer, K.; Tercier, S.; Joseph, J.-M.; Suvà, D.; Clément, V.; Provero, P.; Cironi, L.; et al. Identification of Cancer Stem Cells in Ewing’s Sarcoma. Cancer Res. 2009, 69, 1776–1781. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kaidina, A.M.; Chiang, J.-H.; Yarygin, K.N.; Lupatov, A.Y. Cancer Stem Cell Molecular Markers Verified in Vivo. Biochem. Mosc. Suppl. Ser. B Biomed. Chem. 2017, 11, 43–54. [Google Scholar] [CrossRef]
- Suvorov, R.E.; Kim, Y.S.; Gisina, A.M.; Chiang, J.H.; Yarygin, K.N.; Lupatov, A.Y. Surface Molecular Markers of Cancer Stem Cells: Computation Analysis of Full-Text Scientific Articles. Bull. Exp. Biol. Med. 2018, 166, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, C.; Liu, X.; Fang, F.; Liu, S.; Liao, X.; Tao, S.; Mai, H. Characterisation of a Subpopulation of CD133+ Cancer Stem Cells from Chinese Patients with Oral Squamous Cell Carcinoma. Sci. Rep. 2020, 10, 8875. [Google Scholar] [CrossRef] [PubMed]
- Hur, W.; Ryu, J.Y.; Kim, H.U.; Hong, S.W.; Lee, E.B.; Lee, S.Y.; Yoon, S.K. Systems Approach to Characterize the Metabolism of Liver Cancer Stem Cells Expressing CD133. Sci. Rep. 2017, 7, 45557. [Google Scholar] [CrossRef]
- Gisina, A.M.; Kim, Y.S.; Potashnikova, D.M.; Tvorogova, A.V.; Yarygin, K.N.; Lupatov, A.Y. Proliferative Activity of Colorectal Cancer Cells with Different Levels of CD133 Expression. Bull. Exp. Biol. Med. 2019, 167, 541–545. [Google Scholar] [CrossRef]
- Kim, Y.S.; Potashnikova, D.M.; Gisina, A.M.; Kholodenko, I.V.; Kopylov, A.T.; Tikhonova, O.V.; Kurbatov, L.K.; Saidova, A.A.; Tvorogova, A.V.; Kholodenko, R.V.; et al. TRIM28 Is a Novel Regulator of CD133 Expression Associated with Cancer Stem Cell Phenotype. Int. J. Mol. Sci. 2022, 23, 9874. [Google Scholar] [CrossRef]
- Tirino, V.; Desiderio, V.; d’Aquino, R.; Francesco, F.D.; Pirozzi, G.; Galderisi, U.; Cavaliere, C.; Rosa, A.D.; Papaccio, G. Detection and Characterization of CD133+ Cancer Stem Cells in Human Solid Tumours. PLoS ONE 2008, 3, e3469. [Google Scholar] [CrossRef]
- Barrantes-Freer, A.; Renovanz, M.; Eich, M.; Braukmann, A.; Sprang, B.; Spirin, P.; Pardo, L.A.; Giese, A.; Kim, E.L. CD133 Expression Is Not Synonymous to Immunoreactivity for AC133 and Fluctuates throughout the Cell Cycle in Glioma Stem-Like Cells. PLoS ONE 2015, 10, e0130519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, Y.; Kong, W.; Falk, A.; Hu, J.; Zhou, L.; Pollard, S.; Smith, A. CD133 (Prominin) Negative Human Neural Stem Cells Are Clonogenic and Tripotent. PLoS ONE 2009, 4, e5498. [Google Scholar] [CrossRef] [PubMed]
- Mak, A.B.; Nixon, A.M.L.; Kittanakom, S.; Stewart, J.M.; Chen, G.I.; Curak, J.; Gingras, A.-C.; Mazitschek, R.; Neel, B.G.; Stagljar, I.; et al. Regulation of CD133 by HDAC6 Promotes β-Catenin Signaling to Suppress Cancer Cell Differentiation. Cell Rep. 2012, 2, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Bisson, I.; Prowse, D.M. WNT Signaling Regulates Self-Renewal and Differentiation of Prostate Cancer Cells with Stem Cell Characteristics. Cell Res. 2009, 19, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Sun, Q.; Wang, P.; Liu, M.; Xiong, S.; Luo, J.; Huang, H.; Du, Q.; Geller, D.A.; Cheng, B. Notch and Wnt/β-Catenin Signaling Pathway Play Important Roles in Activating Liver Cancer Stem Cells. Oncotarget 2016, 7, 5754–5768. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jiang, L.; Shi, Y.; Liu, B.; He, Y.; Shen, Q.; Jiang, X.; Nie, Z.; Pu, J.; Yang, C.; et al. Hypoxia-Induced GLT8D1 Promotes Glioma Stem Cell Maintenance by Inhibiting CD133 Degradation through N-Linked Glycosylation. Cell Death Differ. 2022, 29, 1834–1849. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, P.; Chen, T.; Gao, S.; Wu, Z.; Wang, X.; Li, J.; Marjani, S.L.; Costa, J.; Weissman, S.M.; et al. Colorectal Cancer Stem Cell States Uncovered by Simultaneous Single-Cell Analysis of Transcriptome and Telomeres. Adv. Sci. 2021, 8, 2004320. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Luo, Z.; Dong, L.; Tan, Y.; Yang, J.; Feng, G.; Wu, M.; Li, Z.; Wang, H. CD133/Prominin-1-Mediated Autophagy and Glucose Uptake Beneficial for Hepatoma Cell Survival. PLoS ONE 2013, 8, e56878. [Google Scholar] [CrossRef]
- Izumi, H.; Li, Y.; Shibaki, M.; Mori, D.; Yasunami, M.; Sato, S.; Matsunaga, H.; Mae, T.; Kodama, K.; Kamijo, T.; et al. Recycling Endosomal CD133 Functions as an Inhibitor of Autophagy at the Pericentrosomal Region. Sci. Rep. 2019, 9, 2236. [Google Scholar] [CrossRef]
- Izumi, H.; Kaneko, Y.; Nakagawara, A. Molecular Regulation of Autophagy and Asymmetric Cell Division by Cancer Stem Cell Marker CD133. Cells 2023, 12, 819. [Google Scholar] [CrossRef]
- Izumi, H.; Li, Y.; Yasunami, M.; Sato, S.; Mae, T.; Kaneko, Y.; Nakagawara, A. Asymmetric Pericentrosomal CD133 Endosomes Induce the Unequal Autophagic Activity During Cytokinesis in CD133-Positive Human Neuroblastoma Cells. Stem Cells Dayt. Ohio 2022, 40, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Lupatov, A.Y.; Yarygin, K.N. Telomeres and Telomerase in the Control of Stem Cells. Biomedicines 2022, 10, 2335. [Google Scholar] [CrossRef] [PubMed]
- Post, Y.; Clevers, H. Defining Adult Stem Cell Function at Its Simplest: The Ability to Replace Lost Cells through Mitosis. Cell Stem Cell 2019, 25, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Grosse-Gehling, P.; Fargeas, C.A.; Dittfeld, C.; Garbe, Y.; Alison, M.R.; Corbeil, D.; Kunz-Schughart, L.A. CD133 as a Biomarker for Putative Cancer Stem Cells in Solid Tumours: Limitations, Problems and Challenges. J. Pathol. 2013, 229, 355–378. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, Z.; Mansoori, B.; Mohammadi, A.; Kazemi, T.; Mokhtarzadeh, A.; Shanehbandi, D.; Hemmat, N.; Derakhshani, A.; Brunetti, O.; Safaei, S.; et al. The Combination Effect of Prominin1 (CD133) Suppression and Oxaliplatin Treatment in Colorectal Cancer Therapy. Biomed. Pharmacother. 2021, 137, 111364. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yuan, X.; Zeng, Z.; Tunici, P.; Ng, H.; Abdulkadir, I.R.; Lu, L.; Irvin, D.; Black, K.L.; Yu, J.S. Analysis of Gene Expression and Chemoresistance of CD133+ Cancer Stem Cells in Glioblastoma. Mol. Cancer 2006, 5, 67. [Google Scholar] [CrossRef]
- Kholodenko, I.V.; Kim, Y.S.; Gisina, A.M.; Lupatov, A.Y.; Kholodenko, R.V.; Yarygin, K.N. Analysis of the Correlation between CD133 Expression on Human Colorectal Adenocarcinoma Cells HT-29 and Their Resistance to Chemotherapeutic Drugs. Bull. Exp. Biol. Med. 2021, 171, 156–163. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, Y.; Ye, G.; Zhao, Y.; Wu, J. Effects of CD133 Expression on Chemotherapy and Drug Sensitivity of Adenoid Cystic Carcinoma. Mol. Med. Rep. 2022, 25, 1–11. [Google Scholar] [CrossRef]
- Frank, N.Y.; Margaryan, A.; Huang, Y.; Schatton, T.; Waaga-Gasser, A.M.; Gasser, M.; Sayegh, M.H.; Sadee, W.; Frank, M.H. ABCB5-Mediated Doxorubicin Transport and Chemoresistance in Human Malignant Melanoma. Cancer Res. 2005, 65, 4320–4333. [Google Scholar] [CrossRef]
- Wei, Y.; Jiang, Y.; Zou, F.; Liu, Y.; Wang, S.; Xu, N.; Xu, W.; Cui, C.; Xing, Y.; Liu, Y.; et al. Activation of PI3K/Akt Pathway by CD133-P85 Interaction Promotes Tumorigenic Capacity of Glioma Stem Cells. Proc. Natl. Acad. Sci. USA 2013, 110, 6829–6834. [Google Scholar] [CrossRef]
- Mori, Y.; Takeuchi, A.; Miyagawa, K.; Yoda, H.; Soda, H.; Nabeya, Y.; Watanabe, N.; Ozaki, T.; Shimozato, O. CD133 Prevents Colon Cancer Cell Death Induced by Serum Deprivation through Activation of Akt-Mediated Protein Synthesis and Inhibition of Apoptosis. FEBS Open Bio 2021, 11, 1382–1394. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.M.E.; Alamodi, A.; Wahl, R.U.; Grada, Z.; Shareef, M.A.; Hassan, S.-Y.; Murad, F.; Hassan, S.-L.; Santourlidis, S.; Gomez, C.R.; et al. Melanoma Stem Cell Maintenance and Chemo-Resistance Are Mediated by CD133 Signal to PI3K-Dependent Pathways. Oncogene 2020, 39, 5468–5478. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Xing, T.; Yang, Z.; Dudek, R.; Lu, Q.; Chen, Y.-H. Epithelial Mesenchymal Transition in Embryonic Development, Tissue Repair and Cancer: A Comprehensive Overview. J. Clin. Med. 2017, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-Mesenchymal Transition in Cancer: A Historical Overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.W. Control of Invasion by Epithelial-to-Mesenchymal Transition Programs during Metastasis. J. Clin. Med. 2019, 8, 646. [Google Scholar] [CrossRef] [PubMed]
- Saltanatpour, Z.; Johari, B.; Alizadeh, A.; Lotfinia, M.; Majidzadeh-A, K.; Nikbin, B.; Kadivar, M. Enrichment of Cancer Stem-like Cells by the Induction of Epithelial-Mesenchymal Transition Using Lentiviral Vector Carrying E-Cadherin shRNA in HT29 Cell Line. J. Cell. Physiol. 2019, 234, 22935–22946. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cho, M.-Y.; Lee, S.; Jang, M.; Park, J.; Park, R. CRISPR-Cas9 Mediated CD133 Knockout Inhibits Colon Cancer Invasion through Reduced Epithelial-Mesenchymal Transition. PLoS ONE 2019, 14, e0220860. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Miyazaki, Y.; Tsukasa, K.; Matsubara, S.; Yoshimitsu, M.; Takao, S. CD133 Facilitates Epithelial-Mesenchymal Transition through Interaction with the ERK Pathway in Pancreatic Cancer Metastasis. Mol. Cancer 2014, 13, 15. [Google Scholar] [CrossRef]
- Ding, Q.; Yoshimitsu, M.; Kuwahata, T.; Maeda, K.; Hayashi, T.; Obara, T.; Miyazaki, Y.; Matsubara, S.; Natsugoe, S.; Takao, S. Establishment of a Highly Migratory Subclone Reveals That CD133 Contributes to Migration and Invasion through Epithelial-Mesenchymal Transition in Pancreatic Cancer. Hum. Cell 2012, 25, 1–8. [Google Scholar] [CrossRef]
- Moon, Y.; Kim, D.; Sohn, H.; Lim, W. Effect of CD133 Overexpression on the Epithelial-to-Mesenchymal Transition in Oral Cancer Cell Lines. Clin. Exp. Metastasis 2016, 33, 487–496. [Google Scholar] [CrossRef]
- Long, H.; Xiang, T.; Qi, W.; Huang, J.; Chen, J.; He, L.; Liang, Z.; Guo, B.; Li, Y.; Xie, R.; et al. CD133+ Ovarian Cancer Stem-like Cells Promote Non-Stem Cancer Cell Metastasis via CCL5 Induced Epithelial-Mesenchymal Transition. Oncotarget 2015, 6, 5846–5859. [Google Scholar] [CrossRef] [PubMed]
- Tirino, V.; Camerlingo, R.; Bifulco, K.; Irollo, E.; Montella, R.; Paino, F.; Sessa, G.; Carriero, M.V.; Normanno, N.; Rocco, G.; et al. TGF-Β1 Exposure Induces Epithelial to Mesenchymal Transition Both in CSCs and Non-CSCs of the A549 Cell Line, Leading to an Increase of Migration Ability in the CD133+ A549 Cell Fraction. Cell Death Dis. 2013, 4, e620. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhou, L.; Wu, S.; Song, W.; Cheng, Z.; Guo, B. Expressions of CD133, E-cadherin, and Snail in epithelial ovarian cancer and their clinicopathologic and prognostic implications. Nan Fang Yi Ke Da Xue Xue Bao 2015, 35, 1297–1302. [Google Scholar] [PubMed]
- Cai, X.; Li, J.; Yuan, X.; Xiao, J.; Dooley, S.; Wan, X.; Weng, H.; Lu, L. CD133 Expression in Cancer Cells Predicts Poor Prognosis of Non-Mucin Producing Intrahepatic Cholangiocarcinoma. J. Transl. Med. 2018, 16, 50. [Google Scholar] [CrossRef]
- Sun, W.; Dou, J.; Zhang, L.; Qiao, L.; Shen, N.; Gao, W. Expression of CD133, E-Cadherin and WWOX in Colorectal Cancer and Related Analysis. Pak. J. Med. Sci. 2017, 33, 425–429. [Google Scholar] [CrossRef]
- Su, Y.-J.; Chang, Y.-W.; Lin, W.-H.; Liang, C.-L.; Lee, J.-L. An Aberrant Nuclear Localization of E-Cadherin Is a Potent Inhibitor of Wnt/β-Catenin-Elicited Promotion of the Cancer Stem Cell Phenotype. Oncogenesis 2015, 4, e157. [Google Scholar] [CrossRef] [PubMed]
- Gay, D.L.; Yang, C.-C.; Plikus, M.V.; Ito, M.; Rivera, C.; Treffeisen, E.; Doherty, L.; Spata, M.; Millar, S.E.; Cotsarelis, G. CD133 Expression Correlates with Membrane Beta-Catenin and E-Cadherin Loss from Human Hair Follicle Placodes during Morphogenesis. J. Investig. Dermatol. 2015, 135, 45–55. [Google Scholar] [CrossRef]
- Gisina, A.; Novikova, S.; Kim, Y.; Sidorov, D.; Bykasov, S.; Volchenko, N.; Kaprin, A.; Zgoda, V.; Yarygin, K.; Lupatov, A. CEACAM5 Overexpression Is a Reliable Characteristic of CD133-Positive Colorectal Cancer Stem Cells. Cancer Biomark. Sect. Dis. Markers 2021, 32, 85–98. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, C.; Liu, N. CEACAM5 Targeted by miR-498 Promotes Cell Proliferation, Migration and Epithelial to Mesenchymal Transition in Gastric Cancer. Transl. Oncol. 2022, 24, 101491. [Google Scholar] [CrossRef]
- Pospieszna, J.; Dams-Kozlowska, H.; Udomsak, W.; Murias, M.; Kucinska, M. Unmasking the Deceptive Nature of Cancer Stem Cells: The Role of CD133 in Revealing Their Secrets. Int. J. Mol. Sci. 2023, 24, 10910. [Google Scholar] [CrossRef]
- Ullah, M.; Pocard, M.; Mirshahi, M. CD133 Clinical Trials: Safety and Efficacy. J. Regen. Med. 2019, 8, 2. [Google Scholar] [CrossRef]
- Li, W.; Wang, Z.; Gao, T.; Sun, S.; Xu, M.; Pei, R. Selection of CD133-Targeted DNA Aptamers for the Efficient and Specific Therapy of Colorectal Cancer. J. Mater. Chem. B 2022, 10, 2057–2066. [Google Scholar] [CrossRef]
- Riegg, F.; Lutz, M.S.; Schmied, B.J.; Heitmann, J.S.; Queudeville, M.; Lang, P.; Jung, G.; Salih, H.R.; Märklin, M. An Fc-Optimized CD133 Antibody for Induction of NK Cell Reactivity against B Cell Acute Lymphoblastic Leukemia. Cancers 2021, 13, 1632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, M.; Wu, Z.; Tong, C.; Dai, H.; Guo, Y.; Liu, Y.; Huang, J.; Lv, H.; Luo, C.; et al. CD133-Directed CAR T Cells for Advanced Metastasis Malignancies: A Phase I Trial. Oncoimmunology 2018, 7, e1440169. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Sun, B.; Cai, H.; Xuan, Y. Simultaneously Target of Normal and Stem Cells-like Gastric Cancer Cells via Cisplatin and Anti-CD133 CAR-T Combination Therapy. Cancer Immunol. Immunother. CII 2021, 70, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Taromi, S.; Firat, E.; Simonis, A.; Braun, L.M.; Apostolova, P.; Elze, M.; Passlick, B.; Schumacher, A.; Lagies, S.; Frey, A.; et al. Enhanced AC133-Specific CAR T Cell Therapy Induces Durable Remissions in Mice with Metastatic Small Cell Lung Cancer. Cancer Lett. 2022, 538, 215697. [Google Scholar] [CrossRef] [PubMed]
- Vora, P.; Venugopal, C.; Salim, S.K.; Tatari, N.; Bakhshinyan, D.; Singh, M.; Seyfrid, M.; Upreti, D.; Rentas, S.; Wong, N.; et al. The Rational Development of CD133-Targeting Immunotherapies for Glioblastoma. Cell Stem Cell 2020, 26, 832–844.e6. [Google Scholar] [CrossRef]
- Xu, N.; Kang, Y.; Wang, W.; Zhou, J. The Prognostic Role of CD133 Expression in Patients with Osteosarcoma. Clin. Exp. Med. 2020, 20, 261–267. [Google Scholar] [CrossRef]
- Han, M.; Guo, L.; Zhang, Y.; Huang, B.; Chen, A.; Chen, W.; Liu, X.; Sun, S.; Wang, K.; Liu, A.; et al. Clinicopathological and Prognostic Significance of CD133 in Glioma Patients: A Meta-Analysis. Mol. Neurobiol. 2016, 53, 720–727. [Google Scholar] [CrossRef]
- Wu, B.; Sun, C.; Feng, F.; Ge, M.; Xia, L. Do Relevant Markers of Cancer Stem Cells CD133 and Nestin Indicate a Poor Prognosis in Glioma Patients? A Systematic Review and Meta-Analysis. J. Exp. Clin. Cancer Res. CR 2015, 34, 44. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, H.; Lv, S.; Yang, H. High CD133 Expression Is Associated with Worse Prognosis in Patients with Glioblastoma. Mol. Neurobiol. 2016, 53, 2354–2360. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yin, S.; Zhang, L.; Liu, W.; Chen, B.; Xing, H. Clinicopathological Characteristics and Prognostic Value of Cancer Stem Cell Marker CD133 in Breast Cancer: A Meta-Analysis. OncoTargets Ther. 2017, 10, 859–870. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, Y.; Peng, J.; Zhang, E.; Jiang, N.; Li, J.; Zhang, Q.; Zhang, X.; Niu, Y. CD133 Expression May Be Useful as a Prognostic Indicator in Colorectal Cancer, a Tool for Optimizing Therapy and Supportive Evidence for the Cancer Stem Cell Hypothesis: A Meta-Analysis. Oncotarget 2016, 7, 10023–10036. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Song, X.; Chen, Z.; Li, X.; Li, M.; Liu, H.; Li, J. CD133 Expression and the Prognosis of Colorectal Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e56380. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Mo, D.; Wu, J.; Ai, H.; Lu, Y. CD133 Expression Correlates with Clinicopathologic Features and Poor Prognosis of Colorectal Cancer Patients: An Updated Meta-Analysis of 37 Studies. Medicine 2018, 97, e10446. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Xu, J.; Zhang, J.; Huang, J. Prognostic Role of CD133 Expression in Colorectal Cancer: A Meta-Analysis. BMC Cancer 2012, 12, 573. [Google Scholar] [CrossRef] [PubMed]
- Kamil Mohammed Al-Mosawi, A.; Cheshomi, H.; Hosseinzadeh, A.; M Matin, M. Prognostic and Clinical Value of CD44 and CD133 in Esophageal Cancer: A Systematic Review and Meta-Analysis. Iran. J. Allergy Asthma Immunol. 2020, 19, 105–116. [Google Scholar] [CrossRef]
- Yiming, L.; Yunshan, G.; Bo, M.; Yu, Z.; Tao, W.; Gengfang, L.; Dexian, F.; Shiqian, C.; Jianli, J.; Juan, T.; et al. CD133 Overexpression Correlates with Clinicopathological Features of Gastric Cancer Patients and Its Impact on Survival: A Systematic Review and Meta-Analysis. Oncotarget 2015, 6, 42019–42027. [Google Scholar] [CrossRef]
- Lu, L.; Wu, M.; Sun, L.; Li, W.; Fu, W.; Zhang, X.; Liu, T. Clinicopathological and Prognostic Significance of Cancer Stem Cell Markers CD44 and CD133 in Patients with Gastric Cancer: A Comprehensive Meta-Analysis with 4729 Patients Involved. Medicine 2016, 95, e5163. [Google Scholar] [CrossRef]
- Fan, Z.; Li, M.; Chen, X.; Wang, J.; Liang, X.; Wang, H.; Wang, Z.; Cheng, B.; Xia, J. Prognostic Value of Cancer Stem Cell Markers in Head and Neck Squamous Cell Carcinoma: A Meta-Analysis. Sci. Rep. 2017, 7, 43008. [Google Scholar] [CrossRef]
- Cheng, B.; Yang, G.; Jiang, R.; Cheng, Y.; Yang, H.; Pei, L.; Qiu, X. Cancer Stem Cell Markers Predict a Poor Prognosis in Renal Cell Carcinoma: A Meta-Analysis. Oncotarget 2016, 7, 65862–65875. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Wu, J.-D.; Fang, M.-M.; Pu, L.-Y. Clinicopathological Significance and Prognostic Value of the Expression of the Cancer Stem Cell Marker CD133 in Hepatocellular Carcinoma: A Meta-Analysis. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 7623–7630. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-C.; Yang, J.-Y.; Yan, L.-N. Relevant Markers of Cancer Stem Cells Indicate a Poor Prognosis in Hepatocellular Carcinoma Patients: A Meta-Analysis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Shen, Y.; Nan, K.; Mi, B.; Wu, T.; Guo, J.; Li, M.; Lv, Y.; Guo, H. Association Between Expression of Cancer Stem Cell Markers and Poor Differentiation of Hepatocellular Carcinoma: A Meta-Analysis (PRISMA). Medicine 2015, 94, e1306. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Zeng, Z.; Bai, B.; Zhu, J.; Song, Z. The Prognostic Value of CSCs Biomarker CD133 in NSCLC: A Meta-Analysis. Oncotarget 2016, 7, 56526–56539. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Deng, J.; Zhou, J.; Zhou, Y.; Wang, S.; Zhou, J. The Prognostic Value of CD133 Expression in Non-Small Cell Lung Cancer: A Meta-Analysis. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2014, 35, 9769–9775. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Li, R.; Liu, Z.; Zhang, J.; Luo, R. Prognostic Value of Cancer Stem Cell Marker CD133 Expression in Non-Small Cell Lung Cancer: A Systematic Review. Int. J. Clin. Exp. Pathol. 2013, 6, 2644–2650. [Google Scholar]
- Wu, H.; Qi, X.; Yan, G.; Zhang, Q.; Xu, C.; Bian, X. Is CD133 Expression a Prognostic Biomarker of Non-Small-Cell Lung Cancer? A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e100168. [Google Scholar] [CrossRef]
- Saluja, T.S.; Ali, M.; Mishra, P.; Kumar, V.; Singh, S.K. Prognostic Value of Cancer Stem Cell Markers in Potentially Malignant Disorders of Oral Mucosa: A Meta-Analysis. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2019, 28, 144–153. [Google Scholar] [CrossRef]
- Tao, Y.; Li, H.; Huang, R.; Mo, D.; Zeng, T.; Fang, M.; Li, M. Clinicopathological and Prognostic Significance of Cancer Stem Cell Markers in Ovarian Cancer Patients: Evidence from 52 Studies. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2018, 46, 1716–1726. [Google Scholar] [CrossRef]
- Zhao, L.; Li, J.; Liu, M.; Zhou, H.; Zou, H.; Wei, Y.; Sun, K.; Li, G.; Li, S.; Pang, L. The Clinicopathological Parameters Significance of CD133 and Nestin in Epithelial Ovarian Cancer: A Meta-Analysis. Future Oncol. Lond. Engl. 2017, 13, 2555–2570. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Gu, J.; Zheng, L. Prognostic Value of Cancer Stem Cell Marker CD133 Expression in Pancreatic Ductal Adenocarcinoma (PDAC): A Systematic Review and Meta-Analysis. Int. J. Clin. Exp. Pathol. 2015, 8, 12084–12092. [Google Scholar] [PubMed]
- Kim, K.-J.; Lee, K.-H.; Kim, H.-S.; Moon, K.-S.; Jung, T.-Y.; Jung, S.; Lee, M.-C. The Presence of Stem Cell Marker-Expressing Cells Is Not Prognostically Significant in Glioblastomas. Neuropathol. Off. J. Jpn. Soc. Neuropathol. 2011, 31, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Melguizo, C.; Prados, J.; González, B.; Ortiz, R.; Concha, A.; Alvarez, P.J.; Madeddu, R.; Perazzoli, G.; Oliver, J.A.; López, R.; et al. MGMT Promoter Methylation Status and MGMT and CD133 Immunohistochemical Expression as Prognostic Markers in Glioblastoma Patients Treated with Temozolomide plus Radiotherapy. J. Transl. Med. 2012, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Schrøder, H.D.; Kristensen, B.W. CD133 Identifies Perivascular Niches in Grade II–IV Astrocytomas. J. Neurooncol. 2008, 90, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Kaida, T.; Fujiyama, Y.; Soeno, T.; Yokota, M.; Nakamoto, S.; Goto, T.; Watanabe, A.; Okuno, K.; Nie, Y.; Fujino, S.; et al. Less Demand on Stem Cell Marker-Positive Cancer Cells May Characterize Metastasis of Colon Cancer. PLoS ONE 2023, 18, e0277395. [Google Scholar] [CrossRef] [PubMed]
- Mia-Jan, K.; Jung, S.Y.; Kim, I.-Y.; Oh, S.S.; Choi, E.; Chang, S.J.; Kang, T.Y.; Cho, M.-Y. CD133 Expression Is Not an Independent Prognostic Factor in Stage II and III Colorectal Cancer but May Predict the Better Outcome in Patients with Adjuvant Therapy. BMC Cancer 2013, 13, 166. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, T.; Oshima, T.; Murayama, D.; Okamoto, S.; Matsui, A.I.; Yasukawa, M.; Matsubara, Y.; Toda, S.; Hiroshima, Y.; Aoyama, T.; et al. Clinical Significance of Cancer Stem Cell Markers in Primary and Metastatic Tissues in Patients With Breast Cancer. Anticancer Res. 2023, 43, 2145–2154. [Google Scholar] [CrossRef]
- Nakamura, M.; Kyo, S.; Zhang, B.; Zhang, X.; Mizumoto, Y.; Takakura, M.; Maida, Y.; Mori, N.; Hashimoto, M.; Ohno, S.; et al. Prognostic Impact of CD133 Expression as a Tumor-Initiating Cell Marker in Endometrial Cancer. Hum. Pathol. 2010, 41, 1516–1529. [Google Scholar] [CrossRef]
- Mancebo, G.; Sole-Sedeno, J.M.; Pino, O.; Miralpeix, E.; Mojal, S.; Garrigos, L.; Lloveras, B.; Navarro, P.; Gibert, J.; Lorenzo, M.; et al. Prognostic Impact of CD133 Expression in Endometrial Cancer Patients. Sci. Rep. 2017, 7, 7687. [Google Scholar] [CrossRef]
- Marzesco, A.-M.; Janich, P.; Wilsch-Bräuninger, M.; Dubreuil, V.; Langenfeld, K.; Corbeil, D.; Huttner, W.B. Release of Extracellular Membrane Particles Carrying the Stem Cell Marker Prominin-1 (CD133) from Neural Progenitors and Other Epithelial Cells. J. Cell Sci. 2005, 118, 2849–2858. [Google Scholar] [CrossRef] [PubMed]
- Janich, P.; Corbeil, D. GM1 and GM3 Gangliosides Highlight Distinct Lipid Microdomains within the Apical Domain of Epithelial Cells. FEBS Lett. 2007, 581, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Taïeb, N.; Maresca, M.; Guo, X.-J.; Garmy, N.; Fantini, J.; Yahi, N. The First Extracellular Domain of the Tumour Stem Cell Marker CD133 Contains an Antigenic Ganglioside-Binding Motif. Cancer Lett. 2009, 278, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Fargeas, C.A.; Karbanová, J.; Jászai, J.; Corbeil, D. CD133 and Membrane Microdomains: Old Facets for Future Hypotheses. World J. Gastroenterol. 2011, 17, 4149–4152. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The Mystery of Membrane Organization: Composition, Regulation and Roles of Lipid Rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Binnington, B.; Róg, T.; Vattulainen, I.; Grzybek, M.; Coskun, U.; Lingwood, C.A.; Simons, K. Cholesterol Modulates Glycolipid Conformation and Receptor Activity. Nat. Chem. Biol. 2011, 7, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Röper, K.; Corbeil, D.; Huttner, W.B. Retention of Prominin in Microvilli Reveals Distinct Cholesterol-Based Lipid Micro-Domains in the Apical Plasma Membrane. Nat. Cell Biol. 2000, 2, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Krenacs, L.; Krenacs, T.; Stelkovics, E.; Raffeld, M. Heat-Induced Antigen Retrieval for Immunohistochemical Reactions in Routinely Processed Paraffin Sections. Methods Mol. Biol. Clifton NJ 2010, 588, 103–119. [Google Scholar] [CrossRef]
- Yerushalmi, R.; Woods, R.; Ravdin, P.M.; Hayes, M.M.; Gelmon, K.A. Ki67 in Breast Cancer: Prognostic and Predictive Potential. Lancet Oncol. 2010, 11, 174–183. [Google Scholar] [CrossRef]
- Royce, M.; Osgood, C.; Mulkey, F.; Bloomquist, E.; Pierce, W.F.; Roy, A.; Kalavar, S.; Ghosh, S.; Philip, R.; Rizvi, F.; et al. FDA Approval Summary: Abemaciclib With Endocrine Therapy for High-Risk Early Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1155–1162. [Google Scholar] [CrossRef]
- Kandyala, R.; Raghavendra, S.P.C.; Rajasekharan, S.T. Xylene: An Overview of Its Health Hazards and Preventive Measures. J. Oral Maxillofac. Pathol. JOMFP 2010, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Kemper, K.; Sprick, M.R.; de Bree, M.; Scopelliti, A.; Vermeulen, L.; Hoek, M.; Zeilstra, J.; Pals, S.T.; Mehmet, H.; Stassi, G.; et al. The AC133 Epitope, but Not the CD133 Protein, Is Lost upon Cancer Stem Cell Differentiation. Cancer Res. 2010, 70, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, S.K.; Christensen, K.G.; Jensen, S.S.; Kristensen, B.W. Inconsistent Immunohistochemical Expression Patterns of Four Different CD133 Antibody Clones in Glioblastoma. J. Histochem. Cytochem. 2011, 59, 391–407. [Google Scholar] [CrossRef] [PubMed]
- Ghani, S.; Yarian, F.; Bandehpour, M.; Kazemi, B. An In-Silico Approach and Experimental Analysis Combination: Two Strategies for Selecting the Third Extracellular Domain (D-EC3) of Human CD133 Marker as a Target for Detection of Cancer Stem Cells. Iran. J. Pharm. Res. IJPR 2021, 20, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, Y.; Li, Y.; Li, W.; Zheng, X.; Xia, H.; Mao, Q. Detection of CD133 Expression in U87 Glioblastoma Cells Using a Novel Anti-CD133 Monoclonal Antibody. Oncol. Lett. 2015, 9, 2603–2608. [Google Scholar] [CrossRef] [PubMed]
- Glumac, P.M.; Forster, C.L.; Zhou, H.; Murugan, P.; Gupta, S.; LeBeau, A.M. The Identification of a Novel Antibody for CD133 Using Human Antibody Phage Display. Prostate 2018, 78, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Glumac, P.M.; Gallant, J.P.; Shapovalova, M.; Li, Y.; Murugan, P.; Gupta, S.; Coleman, I.M.; Nelson, P.S.; Dehm, S.M.; LeBeau, A.M. Exploitation of CD133 for the Targeted Imaging of Lethal Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 1054–1064. [Google Scholar] [CrossRef]
- Itai, S.; Fujii, Y.; Nakamura, T.; Chang, Y.-W.; Yanaka, M.; Saidoh, N.; Handa, S.; Suzuki, H.; Harada, H.; Yamada, S.; et al. Establishment of CMab-43, a Sensitive and Specific Anti-CD133 Monoclonal Antibody, for Immunohistochemistry. Monoclon. Antibodies Immunodiagn. Immunother. 2017, 36, 231–235. [Google Scholar] [CrossRef]
- Swaminathan, S.K.; Olin, M.R.; Forster, C.L.; Cruz, K.S.S.; Panyam, J.; Ohlfest, J.R. Identification of a Novel Monoclonal Antibody Recognizing CD133. J. Immunol. Methods 2010, 361, 110–115. [Google Scholar] [CrossRef]
- Mak, A.B.; Blakely, K.M.; Williams, R.A.; Penttilä, P.-A.; Shukalyuk, A.I.; Osman, K.T.; Kasimer, D.; Ketela, T.; Moffat, J. CD133 Protein N-Glycosylation Processing Contributes to Cell Surface Recognition of the Primitive Cell Marker AC133 Epitope. J. Biol. Chem. 2011, 286, 41046–41056. [Google Scholar] [CrossRef]
- Platet, N.; Liu, S.Y.; Atifi, M.E.; Oliver, L.; Vallette, F.M.; Berger, F.; Wion, D. Influence of Oxygen Tension on CD133 Phenotype in Human Glioma Cell Cultures. Cancer Lett. 2007, 258, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Griguer, C.E.; Oliva, C.R.; Gobin, E.; Marcorelles, P.; Benos, D.J.; Lancaster, J.R.; Gillespie, G.Y. CD133 Is a Marker of Bioenergetic Stress in Human Glioma. PLoS ONE 2008, 3, e3655. [Google Scholar] [CrossRef] [PubMed]
- McCord, A.M.; Jamal, M.; Shankavaram, U.T.; Lang, F.F.; Camphausen, K.; Tofilon, P.J. Physiologic Oxygen Concentration Enhances the Stem-like Properties of CD133+ Human Glioblastoma Cells in Vitro. Mol. Cancer Res. MCR 2009, 7, 489–497. [Google Scholar] [CrossRef]
- Campos, B.; Zeng, L.; Daotrong, P.H.; Eckstein, V.; Unterberg, A.; Mairbäurl, H.; Herold-Mende, C. Expression and Regulation of AC133 and CD133 in Glioblastoma. Glia 2011, 59, 1974–1986. [Google Scholar] [CrossRef] [PubMed]
- Bar, E.E.; Lin, A.; Mahairaki, V.; Matsui, W.; Eberhart, C.G. Hypoxia Increases the Expression of Stem-Cell Markers and Promotes Clonogenicity in Glioblastoma Neurospheres. Am. J. Pathol. 2010, 177, 1491–1502. [Google Scholar] [CrossRef] [PubMed]
- Lehnus, K.S.; Donovan, L.K.; Huang, X.; Zhao, N.; Warr, T.J.; Pilkington, G.J.; An, Q. CD133 Glycosylation Is Enhanced by Hypoxia in Cultured Glioma Stem Cells. Int. J. Oncol. 2013, 42, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Donovan, L.K.; Potter, N.E.; Warr, T.; Pilkington, G.J. A Prominin-1-Rich Pediatric Glioblastoma: Biologic Behavior Is Determined by Oxygen Tension-Modulated CD133 Expression but Not Accompanied by Underlying Molecular Profiles. Transl. Oncol. 2012, 5, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Shmelkov, S.V.; Jun, L.; St Clair, R.; McGarrigle, D.; Derderian, C.A.; Usenko, J.K.; Costa, C.; Zhang, F.; Guo, X.; Rafii, S. Alternative Promoters Regulate Transcription of the Gene That Encodes Stem Cell Surface Protein AC133. Blood 2004, 103, 2055–2061. [Google Scholar] [CrossRef]
- Fargeas, C.A.; Joester, A.; Missol-Kolka, E.; Hellwig, A.; Huttner, W.B.; Corbeil, D. Identification of Novel Prominin-1/CD133 Splice Variants with Alternative C-Termini and Their Expression in Epididymis and Testis. J. Cell Sci. 2004, 117, 4301–4311. [Google Scholar] [CrossRef]
- Pötgens, A.; Schmitz, U.; Kaufmann, P.; Frank, H. Monoclonal Antibody CD133-2 (AC141) against Hematopoietic Stem Cell Antigen CD133 Shows Crossreactivity with Cytokeratin 18. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2002, 50, 1131–1134. [Google Scholar] [CrossRef]
- Sgambato, A.; Errico, F.; Caredda, E.; Puglisi, M.A.; Cittadini, A. Divergent Expression of CD133 in Different Studies: The Need for a Consensus Panel? Int. J. Cancer 2011, 128, 2247–2249. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, E.; Gemei, M.; Mirabelli, P.; D’Alessio, F.; Di Noto, R.; Fortunato, G.; Del Vecchio, L. The Percentage of CD133+ Cells in Human Colorectal Cancer Cell Lines Is Influenced by Mycoplasma Hyorhinis Infection. BMC Cancer 2010, 10, 120. [Google Scholar] [CrossRef] [PubMed]
- Rebetz, J.; Tian, D.; Persson, A.; Widegren, B.; Salford, L.G.; Englund, E.; Gisselsson, D.; Fan, X. Glial Progenitor-like Phenotype in Low-Grade Glioma and Enhanced CD133-Expression and Neuronal Lineage Differentiation Potential in High-Grade Glioma. PLoS ONE 2008, 3, e1936. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zheng, Q.; Liu, Y.; Sun, L.; Han, P.; Wang, R.; Zhao, J.; Hu, S.; Zhao, X. Human CD133-Positive Hematopoietic Progenitor Cells Enhance the Malignancy of Breast Cancer Cells. BMC Cancer 2020, 20, 1158. [Google Scholar] [CrossRef]
- Liu, K.; Hao, M.; Ouyang, Y.; Zheng, J.; Chen, D. CD133+ Cancer Stem Cells Promoted by VEGF Accelerate the Recurrence of Hepatocellular Carcinoma. Sci. Rep. 2017, 7, 41499. [Google Scholar] [CrossRef]
- Wang, S.-S.; Gao, X.-L.; Liu, X.; Gao, S.-Y.; Fan, Y.-L.; Jiang, Y.-P.; Ma, X.-R.; Jiang, J.; Feng, H.; Chen, Q.-M.; et al. CD133+ Cancer Stem-like Cells Promote Migration and Invasion of Salivary Adenoid Cystic Carcinoma by Inducing Vasculogenic Mimicry Formation. Oncotarget 2016, 7, 29051–29062. [Google Scholar] [CrossRef] [PubMed]
- Pallini, R.; Ricci-Vitiani, L.; Montano, N.; Mollinari, C.; Biffoni, M.; Cenci, T.; Pierconti, F.; Martini, M.; De Maria, R.; Larocca, L.M. Expression of the Stem Cell Marker CD133 in Recurrent Glioblastoma and Its Value for Prognosis. Cancer 2011, 117, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Pallini, R.; Ricci-Vitiani, L.; Banna, G.L.; Signore, M.; Lombardi, D.; Todaro, M.; Stassi, G.; Martini, M.; Maira, G.; Larocca, L.M.; et al. Cancer Stem Cell Analysis and Clinical Outcome in Patients with Glioblastoma Multiforme. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 8205–8212. [Google Scholar] [CrossRef]
- Greenfield, J.P.; Jin, D.K.; Young, L.M.; Christos, P.J.; Abrey, L.; Rafii, S.; Gutin, P.H. Surrogate Markers Predict Angiogenic Potential and Survival in Patients with Glioblastoma Multiforme. Neurosurgery 2009, 64, 819–826; discussion 826–827. [Google Scholar] [CrossRef]
- Pilati, P.; Mocellin, S.; Bertazza, L.; Galdi, F.; Briarava, M.; Mammano, E.; Tessari, E.; Zavagno, G.; Nitti, D. Prognostic Value of Putative Circulating Cancer Stem Cells in Patients Undergoing Hepatic Resection for Colorectal Liver Metastasis. Ann. Surg. Oncol. 2012, 19, 402–408. [Google Scholar] [CrossRef]
- Lin, E.H.; Hassan, M.; Li, Y.; Zhao, H.; Nooka, A.; Sorenson, E.; Xie, K.; Champlin, R.; Wu, X.; Li, D. Elevated Circulating Endothelial Progenitor Marker CD133 Messenger RNA Levels Predict Colon Cancer Recurrence. Cancer 2007, 110, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. The Involvement of Endothelial Progenitor Cells in Tumor Angiogenesis. J. Cell. Mol. Med. 2004, 8, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Florek, M.; Haase, M.; Marzesco, A.-M.; Freund, D.; Ehninger, G.; Huttner, W.B.; Corbeil, D. Prominin-1/CD133, a Neural and Hematopoietic Stem Cell Marker, Is Expressed in Adult Human Differentiated Cells and Certain Types of Kidney Cancer. Cell Tissue Res. 2005, 319, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Zenali, M.J.; Tan, D.; Li, W.; Dhingra, S.; Brown, R.E. Stemness Characteristics of Fibrolamellar Hepatocellular Carcinoma: Immunohistochemical Analysis with Comparisons to Conventional Hepatocellular Carcinoma. Ann. Clin. Lab. Sci. 2010, 40, 126–134. [Google Scholar] [PubMed]
- Yu, G.-F.; Lin, X.; Luo, R.-C.; Fang, W.-Y. Nuclear CD133 Expression Predicts Poor Prognosis for Hepatocellular Carcinoma. Int. J. Clin. Exp. Pathol. 2018, 11, 2092–2099. [Google Scholar] [PubMed]
- Chen, Y.-L.; Lin, P.-Y.; Ming, Y.-Z.; Huang, W.-C.; Chen, R.-F.; Chen, P.-M.; Chu, P.-Y. The Effects of the Location of Cancer Stem Cell Marker CD133 on the Prognosis of Hepatocellular Carcinoma Patients. BMC Cancer 2017, 17, 474. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Yeo, M.-K.; Seong, I.-O.; Kim, K.-H. Nuclear Expression of CD133 Is Associated with Good Prognosis in Patients with Colorectal Adenocarcinoma. Anticancer Res. 2018, 38, 4819–4826. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, C.; Poppleton, H.; Kocak, M.; Hogg, T.L.; Fuller, C.; Hamner, B.; Oh, E.Y.; Gaber, M.W.; Finklestein, D.; Allen, M.; et al. A Perivascular Niche for Brain Tumor Stem Cells. Cancer Cell 2007, 11, 69–82. [Google Scholar] [CrossRef]
- Iida, H.; Suzuki, M.; Goitsuka, R.; Ueno, H. Hypoxia Induces CD133 Expression in Human Lung Cancer Cells by Up-Regulation of OCT3/4 and SOX2. Int. J. Oncol. 2012, 40, 71–79. [Google Scholar] [CrossRef]




| Tumor Type | Reference | Analyzed Studies | Total Cancer Cases | CD133 Detection Method | Correlations * |
|---|---|---|---|---|---|
| Osteosarcoma | [118] | 7 | 498 | IHC | Yes (OS, stage, recurrence, mts) |
| Glioma | [119] | 21 | 1535 | IHC, WB, RT-PCR, DNA microarray | Yes (OS, PFS) |
| [120] | 11 | 740 | IHC, WB, RT-PCR | Yes (OS, PFS) | |
| Glioblastoma | [121] | 10 | 715 | IHC | Yes (OS, PFS) |
| Breast cancer | [122] | 13 | 1734 | Not reported | Yes (OS, DFS, DG, ER, PR, HER2, LN mts) No (age, tumor size) |
| Colorectal cancer | [123] | 28 | 4546 | IHC, PCR | Yes (OS, DFS) |
| [124] | 15 | 2297 | IHC | Yes (OS, DFS) No (histology type, LN mts, distant mts) | |
| [125] | 37 | 5397 | IHC | Yes (OS, DFS, distant mts, invasion) | |
| [126] | 12 | 3652 | IHC | Yes (OS) No (LN mts, DG) | |
| Esophageal cancer | [127] | 5 | 451 | IHC | No (OS) |
| Gastric cancer | [128] | 8 | 603 | IHC | Yes (OS, stage, mts) |
| [129] | 10 | 1569 | IHC | Yes (OS, stage, invasion, mts) | |
| Head and neck squamous cell carcinoma | [130] | 22 | 2143 | IHC | Yes (OS, DFS) |
| Renal cell carcinoma | [131] | 4 | 611 | IHC | Yes (CSS) No (OS, DFS, PFS) |
| Hepatocellular carcinoma | [132] | 21 | 2592 | IHC | Yes (OS, DFS, DG, stage) No (hepatitis, cirrhosis, α-fetoprotein level, tumor size, mts) |
| [133] | 10 | 890 | IHC, RT-PCR, WB | Yes (OS, DFS, DG, α-fetoprotein level) No (tumor size, hepatitis, cirrhosis) | |
| [134] | 15 | 1807 | IHC | Yes (DG) | |
| Non-small cell lung cancer | [135] | 32 | 3595 | IHC, RT-PCR | Yes (OS (only Asian patients), differentiation, LN mts) No (age, smoking status, stage, distant mts) |
| [136] | 13 | 1727 | IHC, RT-PCR | Yes (OS, DG) No (DFS, gender, smoking status, invasion, mts, DG) | |
| [137] | 11 | 1004 | IHC | Yes (OS, stage, DG) | |
| [138] | 23 | 2538 | IHC, PCR | Yes (OS, LN mts) No (DFS) | |
| Potentially malignant disorders of oral mucosa | [139] | 2 | 251 | IHC | Yes (risk of malignant transformation) |
| Ovarian cancer | [140] | 8 | 1129 | IHC | Yes (FIGO stage, DG) No (OS, DFS) |
| [141] | 17 | 1600 | IHC | Yes (FIGO stage, size, LN mts) | |
| Pancreatic ductal adenocarcinoma | [142] | 15 | 908 | IHC | Yes (OS, stage, DG, LN mts) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gisina, A.; Kim, Y.; Yarygin, K.; Lupatov, A. Can CD133 Be Regarded as a Prognostic Biomarker in Oncology: Pros and Cons. Int. J. Mol. Sci. 2023, 24, 17398. https://doi.org/10.3390/ijms242417398
Gisina A, Kim Y, Yarygin K, Lupatov A. Can CD133 Be Regarded as a Prognostic Biomarker in Oncology: Pros and Cons. International Journal of Molecular Sciences. 2023; 24(24):17398. https://doi.org/10.3390/ijms242417398
Chicago/Turabian StyleGisina, Alisa, Yan Kim, Konstantin Yarygin, and Alexey Lupatov. 2023. "Can CD133 Be Regarded as a Prognostic Biomarker in Oncology: Pros and Cons" International Journal of Molecular Sciences 24, no. 24: 17398. https://doi.org/10.3390/ijms242417398
APA StyleGisina, A., Kim, Y., Yarygin, K., & Lupatov, A. (2023). Can CD133 Be Regarded as a Prognostic Biomarker in Oncology: Pros and Cons. International Journal of Molecular Sciences, 24(24), 17398. https://doi.org/10.3390/ijms242417398







