Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications
Abstract
:1. Introduction
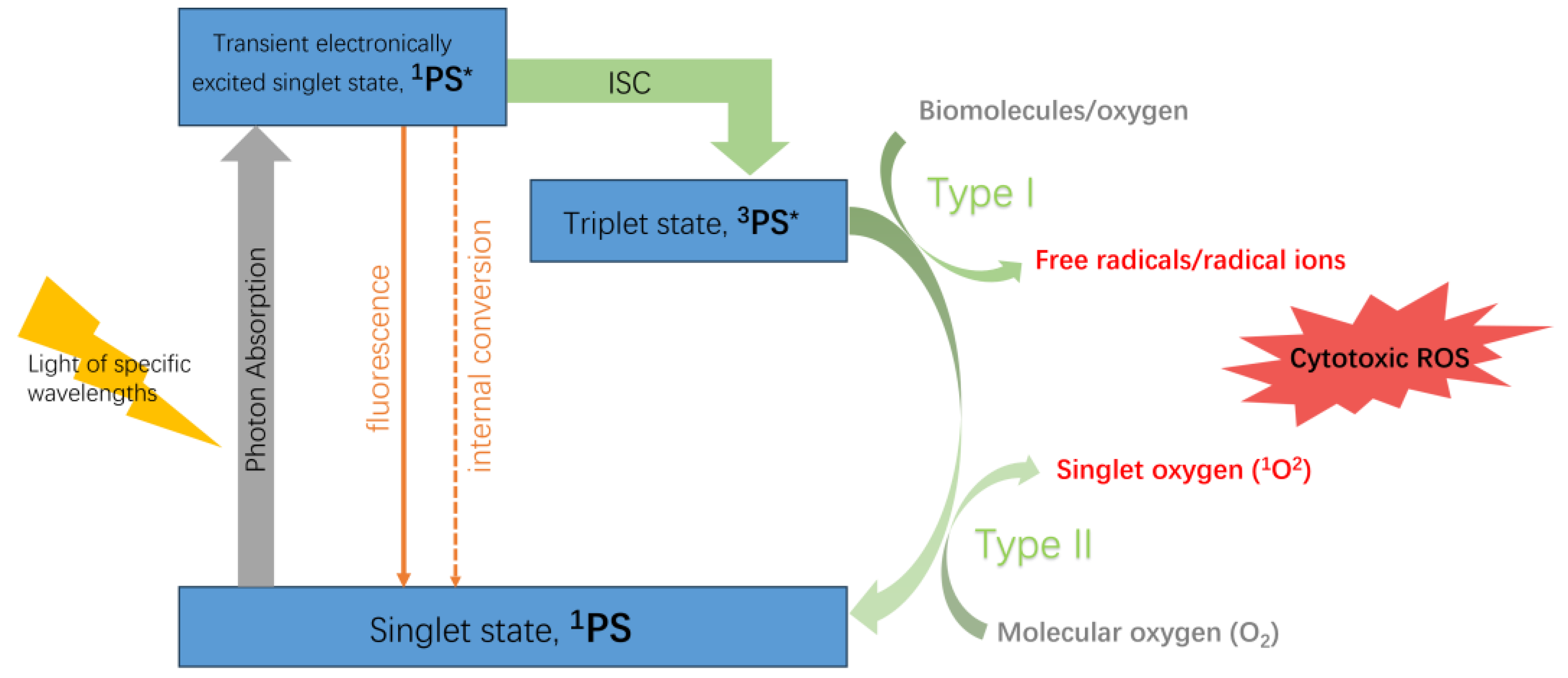
2. HPPH-Based Third-Generation Photodynamic Agents
2.1. Methods of Modification
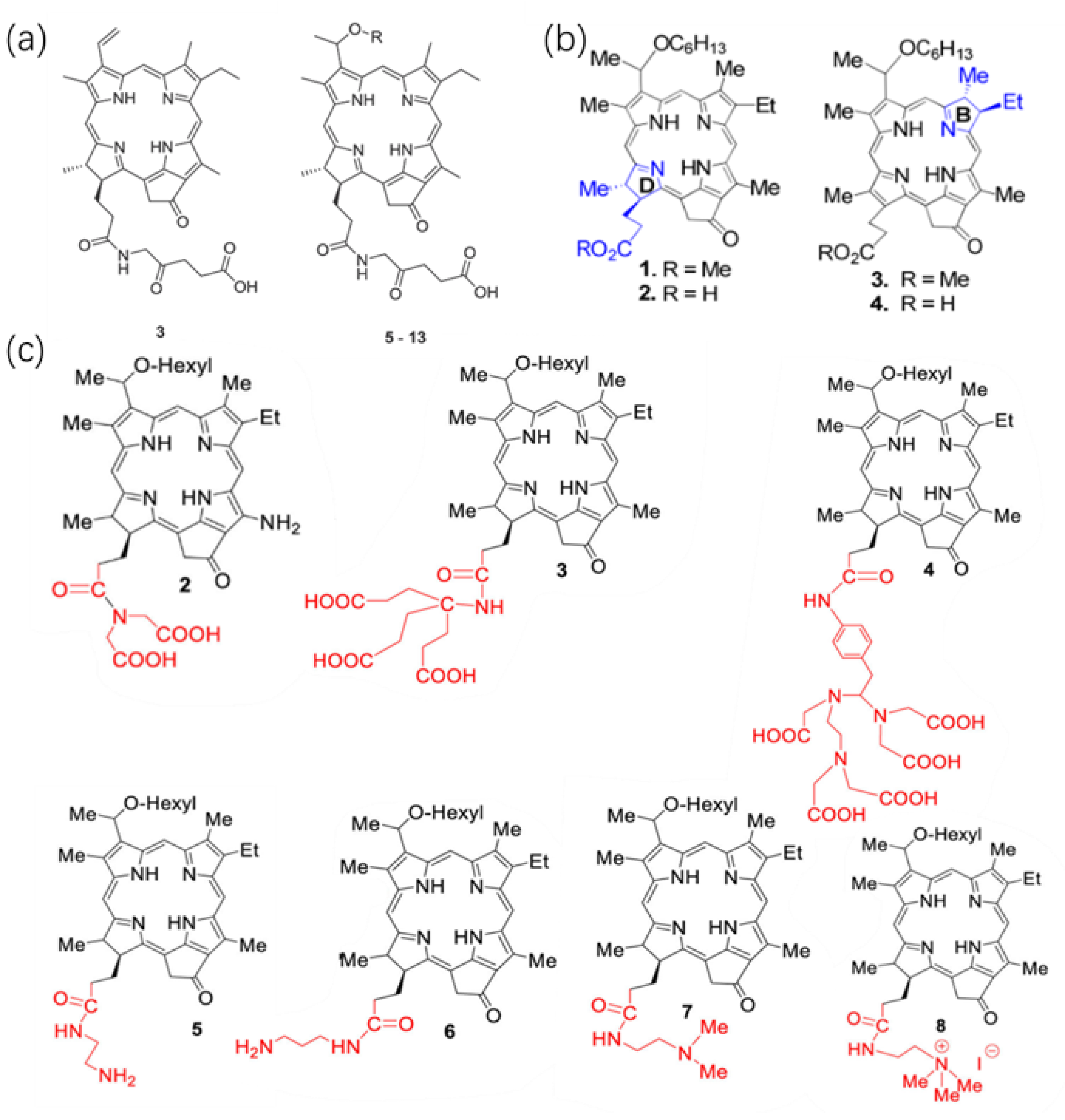
2.1.1. Direct Modification of The Basal Structure of HPPH
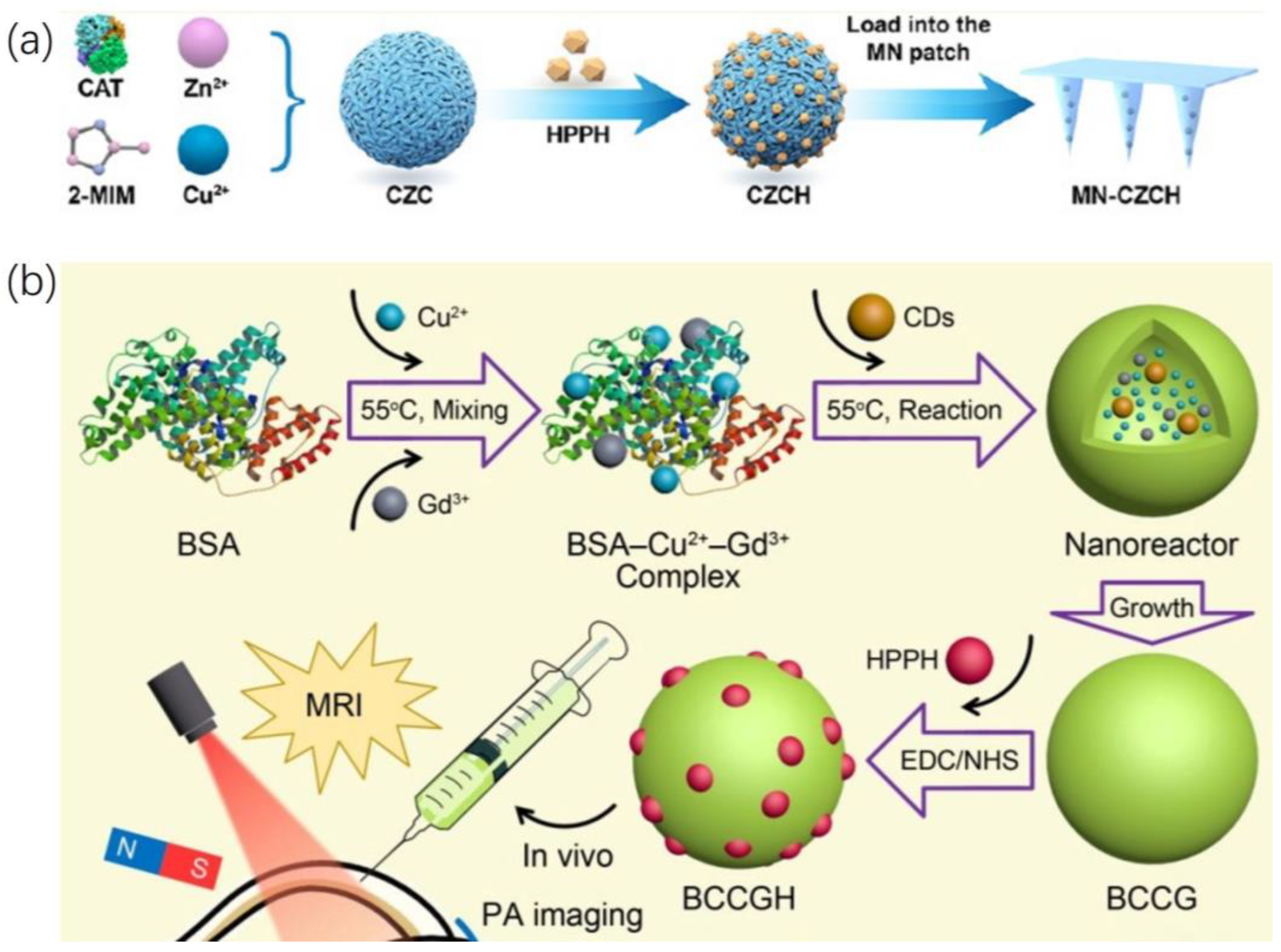
2.1.2. Loading HPPH into a Functional Carrier
2.2. Combinatorial Treatment of HPPH and Other Materials
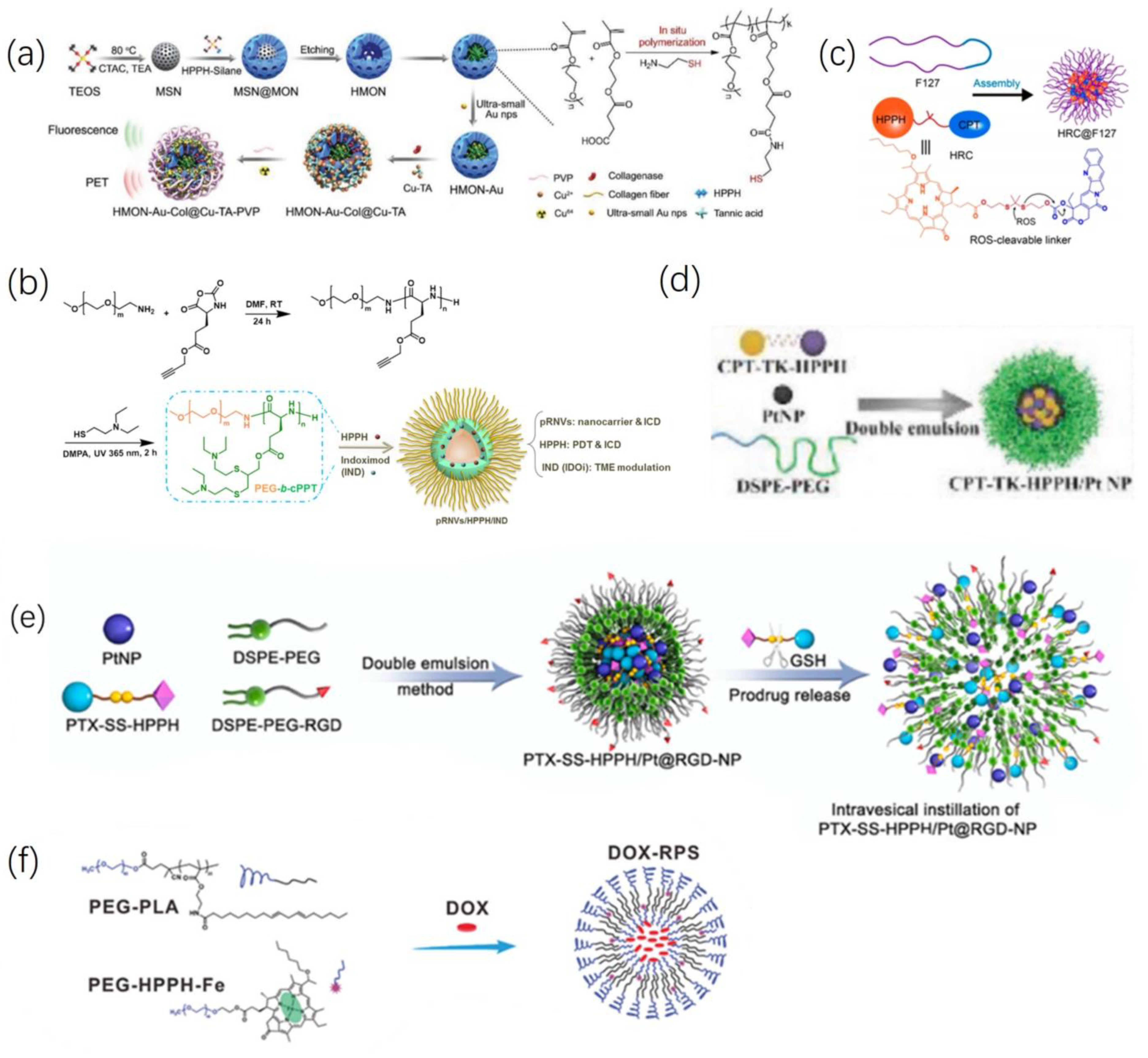
2.3. Combined Tumor Imaging and Treatment
2.3.1. Fluorescence Imaging
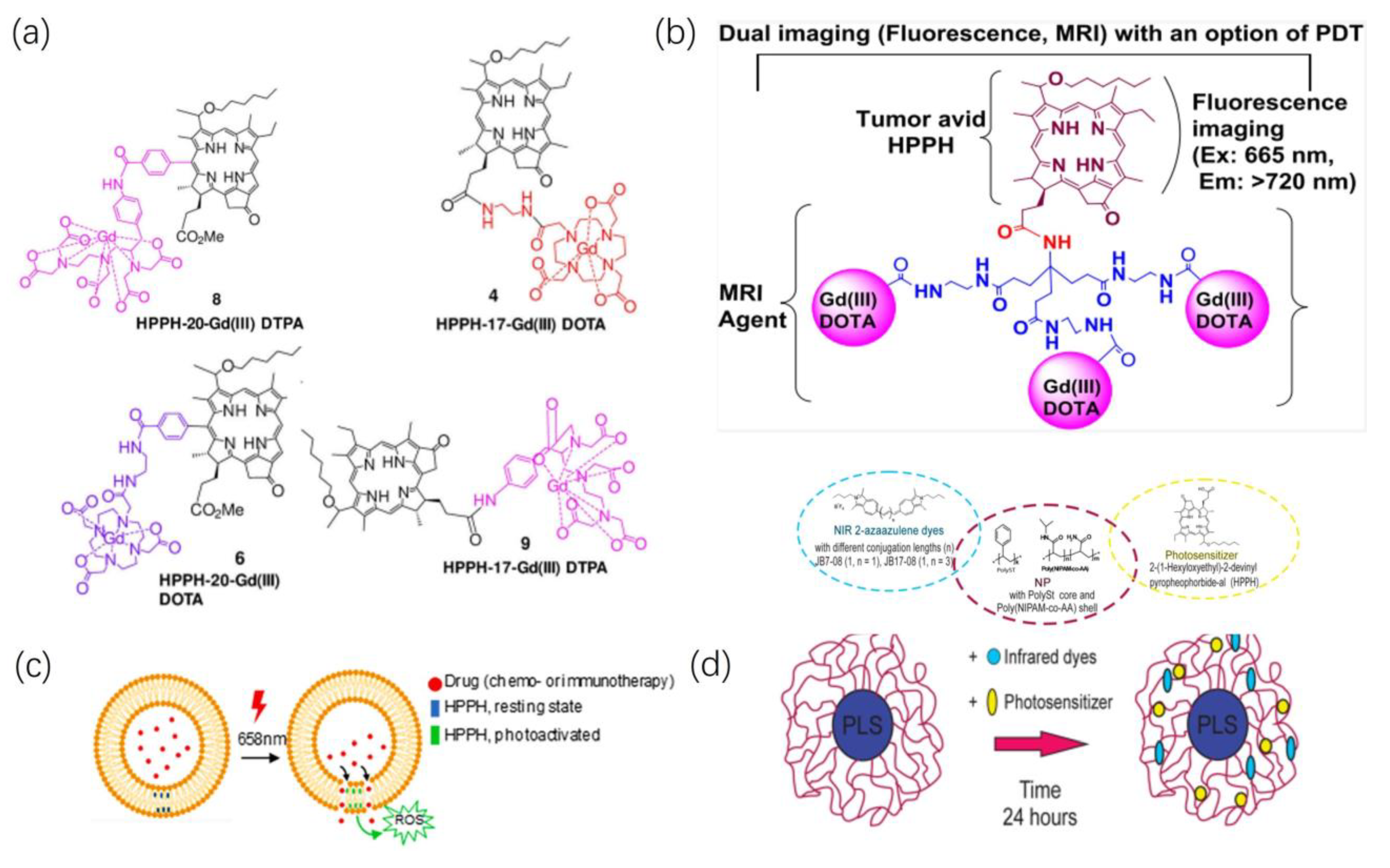
2.3.2. Magnetic Resonance Imaging
3. Conclusions
4. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dabrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-N.; Hsu, R.; Chen, H.; Wong, T.-W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef] [PubMed]
- Ackroyd, R.; Kelty, C.; Brown, N.; Reed, M. The History of Photodetection and Photodynamic Therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Mitton, D.; Ackroyd, R. A brief overview of photodynamic therapy in Europe. Photodiagnosis Photodyn. Ther. 2008, 5, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Sarbadhikary, P.; George, B.P.; Abrahamse, H. Recent Advances in Photosensitizers as Multifunctional Theranostic Agents for Imaging-Guided Photodynamic Therapy of Cancer. Theranostics 2021, 11, 9054–9088. [Google Scholar] [CrossRef]
- Felsher, D.W. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 8, 380–387. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Q.; Wang, W.; Shao, J.; Huang, W.; Dong, X. Type I Photosensitizers Revitalizing Photodynamic Oncotherapy. Small 2021, 17, 2006742. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef]
- Calixto, G.; Bernegossi, J.; de Freitas, L.; Fontana, C.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review. Molecules 2016, 21, 342. [Google Scholar] [CrossRef]
- Li, G.; Wang, Q.; Liu, J.; Wu, M.; Ji, H.; Qin, Y.; Zhou, X.; Wu, L. Innovative strategies for enhanced tumor photodynamic therapy. J. Mater. Chem. B 2021, 9, 7347–7370. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, R.; Lee, J.; Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Bagnato, V.S.; Cuenca, R.; Downie, G.H.; Sibata, C.H. The future of photodynamic therapy in oncology. Future Med. 2006, 19, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarthi, U.; Giribabu, L. Photodynamic Therapy: Past, Present and Future. Chem. Rec. 2017, 17, 775–802. [Google Scholar] [CrossRef]
- Mfouo-Tynga, I.S.; Dias, L.D.; Inada, N.M.; Kurachi, C. Features of third generation photosensitizers used in anticancer photodynamic therapy: Review. Photodiagnosis Photodyn. Ther. 2021, 34, 102091. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Naito, K.; Tsutsumi, J. Photosensitization of animals by the viscera of abalones, haliotis spp. Jpn. J. Med. Sci. Biol. 1962, 4, 136–140. [Google Scholar] [CrossRef]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef]
- Bellnier, D.A.; Greco, W.R.; Loewen, G.M.; Nava, H.; Oseroff, A.R.; Pandey, R.K.; Tsuchida, T.; Dougherty, T.J. Loewen Population pharmacokinetics of the photodynamic therapy agent 2-[1-hexyloxyethyl]-2-devinyl pyropheophorbide-a in cancer patients. Am. Assoc. Cancer Res. 2003, 8, 1806–1813. [Google Scholar]
- Chen, L.; Zhang, X.; Cao, Q.; Wu, Y.; Zhang, T.; Tong, H.; Wang, X.; Yang, J. Development and application of a physiologically based pharmacokinetic model for HPPH in rats and extrapolate to humans. Eur. J. Pharm. Sci. 2019, 129, 68–78. [Google Scholar] [CrossRef]
- Gao, Y.H.; Zhu, X.X.; Zhu, W.; Wu, D.; Chen, D.Y.; Yan, Y.J.; Wu, X.F.; O’Shea, D.F.; Chen, Z.L. Synthesis and evaluation of novel chlorophyll a derivatives as potent photosensitizers for photodynamic therapy. Eur. J. Med. Chem. 2020, 187, 111959. [Google Scholar] [CrossRef]
- Saenz, C.; Cheruku, R.R.; Ohulchanskyy, T.Y.; Joshi, P.; Tabaczynski, W.A.; Missert, J.R.; Chen, Y.; Pera, P.; Tracy, E.; Marko, A.; et al. Structural and Epimeric Isomers of HPPH [3-Devinyl 3-{1-(1-hexyloxy) ethyl}pyropheophorbide-a]: Effects on Uptake and Photodynamic Therapy of Cancer. ACS Chem. Biol. 2017, 12, 933–946. [Google Scholar] [CrossRef]
- Saenz, C.; Ethirajan, M.; Tracy, E.C.; Bowman, M.-J.; Cacaccio, J.; Ohulchanskyy, T.; Baumann, H.; Pandey, R.K. Charged groups on pyropheophorbide-based photosensitizers dictate uptake by tumor cells and photodynamic therapy efficacy. J. Photochem. Photobiol. B Biol. 2022, 227, 112375. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Shen, X.; He, Z.; Lu, Y. The Role of Photodynamic Therapy in Triggering Cell Death and Facilitating Antitumor Immunology. Front. Oncol. 2022, 12, 863107. [Google Scholar] [CrossRef]
- Zenga, J.; Awan, M.; Hadi Razeghi Kondelaji, M.; Hansen, C.; Shafiee, S.; Frei, A.; Foeckler, J.; Kuehn, R.; Bruening, J.; Massey, B.; et al. Photoactivated HPPH-Liposomal therapy for the treatment of HPV-Negative head and neck cancer. Oral Oncol. 2023, 144, 106487. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.; Yang, H.; Cai, Y.; Sun, L.; Di, P.; Li, W.; Liu, G.; Tang, Y. ADMET-score—A comprehensive scoring function for evaluation of chemical drug-likeness. MedChemComm 2019, 10, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Fang, Y.; Zhao, Y.; Zhao, H.; Wang, Y.; Gu, Y.; Wu, F. Synthesis and in Vitro Photocytotoxicity of Coumarin Derivatives for One- and Two-Photon Excited Photodynamic Therapy. J. Med. Chem. 2013, 56, 5288–5294. [Google Scholar] [CrossRef]
- Zhu, W.; Gao, Y.-H.; Song, C.-H.; Lu, Z.-B.; Namulinda, T.; Han, Y.-P.; Yan, Y.-J.; Wang, L.-X.; Chen, Z.-L. Synthesis and evaluation of new 5-aminolevulinic acid derivatives as prodrugs of protoporphyrin for photodynamic therapy. Photochem. Photobiol. Sci. 2020, 16, 1623–1630. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Zhou, L.; Fang, K.; Zhou, L.; Lin, Y.; Zhou, J.; Wei, S. Synthesis of novel octa-cationic and non-ionic 1,2-ethanediamine substituted zinc (Ⅱ) phthalocyanines and their in vitro anti-cancer activity comparison. Eur. J. Med. Chem. 2012, 58, 12–21. [Google Scholar] [CrossRef]
- Black, P.N.; DiRusso, C.C. Transmembrane Movement of Exogenous Long-Chain Fatty Acids: Proteins, Enzymes, and Vectorial Esterification. Microbiol. Mol. Biol. Rev. 2003, 67, 454–472. [Google Scholar] [CrossRef]
- Liu, W.; Baer, M.R.; Bowman, M.J.; Pera, P.; Zheng, X.; Morgan, J.; Pandey, R.A.; Oseroff, A.R. The Tyrosine Kinase Inhibitor Imatinib Mesylate Enhances the Efficacy of Photodynamic Therapy by Inhibiting ABCG2. Clin. Cancer Res. 2007, 13, 2463–2470. [Google Scholar] [CrossRef]
- Li, Y.; He, G.; Fu, L.-H.; Younis, M.R.; He, T.; Chen, Y.; Lin, J.; Li, Z.; Huang, P. A Microneedle Patch with Self-Oxygenation and Glutathione Depletion for Repeatable Photodynamic Therapy. ACS Nano 2022, 16, 17298–17312. [Google Scholar] [CrossRef]
- Jamaledin, R.; Yiu, C.K.Y.; Zare, E.N.; Niu, L.N.; Vecchione, R.; Chen, G.; Gu, Z.; Tay, F.R.; Makvandi, P. Advances in Antimicrobial Microneedle Patches for Combating Infections. Adv. Mater. 2020, 32, e2002129. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.H.; Wan, Y.; Qi, C.; He, J.; Li, C.; Yang, C.; Xu, H.; Lin, J.; Huang, P. Nanocatalytic Theranostics with Glutathione Depletion and Enhanced Reactive Oxygen Species Generation for Efficient Cancer Therapy. Adv. Mater. 2021, 33, e2006892. [Google Scholar] [CrossRef]
- Wang, H.; Chao, Y.; Liu, J.; Zhu, W.; Wang, G.; Xu, L.; Liu, Z. Photosensitizer-crosslinked in-situ polymerization on catalase for tumor hypoxia modulation & enhanced photodynamic therapy. Biomaterials 2018, 181, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.-W.; Bao, Y.-W.; Zeng, J.; Wu, F.-G. Ultrasmall All-In-One Nanodots Formed via Carbon Dot-Mediated and Albumin-Based Synthesis: Multimodal Imaging-Guided and Mild Laser-Enhanced Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 42077–42087. [Google Scholar] [CrossRef] [PubMed]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal clearance of quantum dots. Nat. Biotechnol. 2007, 25, 1165–1170. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. 2018, 58, 946–956. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, S.-S.; Li, C.-X.; Xu, L.; Cheng, H.; Zhang, X.-Z. An Adenosine Triphosphate-Responsive Autocatalytic Fenton Nanoparticle for Tumor Ablation with Self-Supplied H2O2 and Acceleration of Fe(III)/Fe(II) Conversion. Nano Lett. 2018, 18, 7609–7618. [Google Scholar] [CrossRef]
- Li, L.; Yang, Z.; Fan, W.; He, L.; Cui, C.; Zou, J.; Tang, W.; Jacobson, O.; Wang, Z.; Niu, G.; et al. In Situ Polymerized Hollow Mesoporous Organosilica Biocatalysis Nanoreactor for Enhancing ROS—Mediated Anticancer Therapy. Adv. Funct. Mater. 2019, 30, 1907716. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, F.; Deng, H.; Lin, L.; Wang, S.; Kang, F.; Yu, G.; Lau, J.; Tian, R.; Zhang, M.; et al. Smart Nanovesicle-Mediated Immunogenic Cell Death through Tumor Microenvironment Modulation for Effective Photodynamic Immunotherapy. ACS Nano 2019, 14, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Meng, M.; Bao, Y.; Zhang, C.; Gao, B.; Sa, R.; Luo, W. miR-1301/TRIAP1 Axis Participates in Epirubicin-Mediated Anti-Proliferation and Pro-Apoptosis in Osteosarcoma. Yonsei Med. J. 2019, 60, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Li, Y.; Tjong, S. Graphene Nanomaterials: Synthesis, Biocompatibility, and Cytotoxicity. Int. J. Mol. Sci. 2018, 19, 3564. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, J.D.; Flynn, N.H. Cell-penetrating peptides transport therapeutics into cells. Pharmacol. Ther. 2015, 154, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-F.; Wu, Y.-F.; Lan, T.-J.; Chen, Y.; Su, S.-H.; Zhang, X.; Gao, W.; Chen, J.; Guo, Y.; Zhu, J.; et al. Codelivery of Anticancer Drug and Photosensitizer by PEGylated Graphene Oxide and Cell Penetrating Peptide Enhanced Tumor-Suppressing Effect on Osteosarcoma. Front. Mol. Biosci. 2021, 7, 618896. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.; He, X.; Yu, Y.; Han, R.; Li, Y.; Yang, C.; Hu, D.; Qian, Z. Polymeric Nanoparticles with ROS—Responsive Prodrug and Platinum Nanozyme for Enhanced Chemophotodynamic Therapy of Colon Cancer. Adv. Sci. 2020, 7, 2001853. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Mu, J.; Jacobson, O.; Wang, Z.; He, L.; Zhang, F.; Yang, W.; Lin, Q.; Zhou, Z.; Ma, Y.; et al. Reactive Oxygen Species Activatable Heterodimeric Prodrug as Tumor-Selective Nanotheranostics. ACS Nano 2020, 14, 16875–16886. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.; He, X.; Han, R.; Yang, C.; Liu, T.; Yang, Y.; Liu, Q.; Qian, Z. RGD peptide modified platinum nanozyme Co-loaded glutathione-responsive prodrug nanoparticles for enhanced chemo-photodynamic bladder cancer therapy. Biomaterials 2023, 293, 121975. [Google Scholar] [CrossRef]
- Liu, H.; Mei, C.; Deng, X.; Lin, W.; He, L.; Chen, T. Rapid visualizing and pathological grading of bladder tumor tissues by simple nanodiagnostics. Biomaterials 2021, 264, 120434. [Google Scholar] [CrossRef]
- Wang, S.; Yu, G.; Yang, W.; Wang, Z.; Jacobson, O.; Tian, R.; Deng, H.; Lin, L.; Chen, X. Photodynamic—Chemodynamic Cascade Reactions for Efficient Drug Delivery and Enhanced Combination Therapy. Adv. Sci. 2021, 8, 2002927. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Li, J.; Peng, Z.-H.; Sheinin, Y.; Zhou, J.; Oupický, D. Tumor-Penetrating Nanoparticles for Enhanced Anticancer Activity of Combined Photodynamic and Hypoxia-Activated Therapy. ACS Nano 2017, 11, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Duan, J.-J.; Bian, X.-W.; Yu, S.-C. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Shao, N.; Weng, H.; Li, W.; Zhang, J.; Zhang, L.; Yang, L.; Ye, S. Correlation between epidermal growth factor receptor and tumor stem cell markers CD44/CD24 and their relationship with prognosis in breast invasive ductal carcinoma. Med. Oncol. 2014, 32, 275. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Lu, M.; Ming, L.; Chen, Y.; Cheng, K.; Zhou, J.; Jiang, S.; Lin, Z.; Chen, D. 89Zr-Labeled Multifunctional Liposomes Conjugate Chitosan for PET-Trackable Triple-Negative Breast Cancer Stem Cell Targeted Therapy. Int. J. Nanomed. 2020, 15, 9061–9074. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, R.; Yu, W.; Hu, C.; Zhang, Z.; Liu, D.; An, Y.; Wang, X.; He, C.; Liu, P.; et al. Chitosan oligosaccharide decorated liposomes combined with TH302 for photodynamic therapy in triple negative breast cancer. J. Nanobiotechnology 2021, 19, 147. [Google Scholar] [CrossRef]
- Ogbonna, S.J.; Hazama, H.; Awazu, K. Mass Spectrometric Analysis of the Photobleaching of Protoporphyrin IX Used in Photodynamic Diagnosis and Therapy of Cancer. Photochem. Photobiol. 2021, 97, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Haedicke, K.; Kozlova, D.; Gräfe, S.; Teichgräber, U.; Epple, M.; Hilger, I. Multifunctional calcium phosphate nanoparticles for combining near-infrared fluorescence imaging and photodynamic therapy. Acta Biomater. 2015, 14, 197–207. [Google Scholar] [CrossRef]
- James, N.S.; Joshi, P.; Ohulchanskyy, T.Y.; Chen, Y.; Tabaczynski, W.; Durrani, F.; Shibata, M.; Pandey, R.K. Photosensitizer (PS)-cyanine dye (CD) conjugates: Impact of the linkers joining the PS and CD moieties and their orientation in tumor-uptake and photodynamic therapy (PDT). Eur. J. Med. Chem. 2016, 122, 770–785. [Google Scholar] [CrossRef]
- James, N.S.; Ohulchanskyy, T.Y.; Chen, Y.; Joshi, P.; Zheng, X.; Goswami, L.N.; Pandey, R.K. Comparative Tumor Imaging and PDT Efficacy of HPPH Conjugated in the Mono- and Di-Forms to Various Polymethine Cyanine Dyes: Part 2. Theranostics 2013, 3, 703–718. [Google Scholar] [CrossRef]
- Carr, J.A.; Valdez, T.A.; Bruns, O.T.; Bawendi, M.G. Using the shortwave infrared to image middle ear pathologies. Proc. Natl. Acad. Sci. USA 2016, 113, 9989–9994. [Google Scholar] [CrossRef]
- Chepurna, O.M.; Yakovliev, A.; Ziniuk, R.; Nikolaeva, O.A.; Levchenko, S.M.; Xu, H.; Losytskyy, M.Y.; Bricks, J.L.; Slominskii, Y.L.; Vretik, L.O.; et al. Core–shell polymeric nanoparticles co-loaded with photosensitizer and organic dye for photodynamic therapy guided by fluorescence imaging in near and short-wave infrared spectral regions. J. Nanobiotechnology 2020, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cheruku, R.R.; Dukh, M.; Tabaczynski, W.; Patel, N.J.; White, W.H.; Missert, J.R.; Spernyak, J.A.; Pandey, R.K. The Structures of Gd(III) Chelates Conjugated at the Periphery of 3-(1’-Hexyloxy)ethyl-3-devinylpyropheophorbide-a (HPPH) Have a Significant Impact on the Imaging and Therapy of Cancer. ChemMedChem 2020, 15, 2058–2070. [Google Scholar] [CrossRef]
- Cheruku, R.R.; Turowski, S.G.; Durrani, F.A.; Tabaczynski, W.A.; Cacaccio, J.; Missert, J.R.; Curtin, L.; Sexton, S.; Alberico, R.; Hendler, C.M.; et al. Tumor-Avid 3-(1’-Hexyloxy)ethyl-3-devinylpyrpyropheophorbide-a (HPPH)-3Gd(III)tetraxetan (DOTA) Conjugate Defines Primary Tumors and Metastases. J. Med. Chem. 2022, 65, 9267–9280. [Google Scholar] [CrossRef] [PubMed]
- Ardeshirpour, Y.; Chernomordik, V.; Capala, J.; Hassan, M.; Zielinsky, R.; Griffiths, G.; Gandjbakhche, A. Using in vivo fluorescence imaging in personalized cancer diagnosis and therapy, an image and treat paradigm. Technol. Cancer Res. Treat. 2011, 10, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Borah, B.M.; Cacaccio, J.; Watson, R.; Pandey, R.K. Phototriggered Release of Tumor-Imaging and Therapy Agents from Lyophilized Multifunctional Polyacrylamide Nanoparticles. ACS Appl. Bio Mater. 2019, 2, 5663–5675. [Google Scholar] [CrossRef]
- Weżgowiec, J.; Kulbacka, J.; Saczko, J.; Rossowska, J.; Chodaczek, G.; Kotulska, M. Biological effects in photodynamic treatment combined with electropermeabilization in wild and drug resistant breast cancer cells. Bioelectrochemistry 2018, 123, 9–18. [Google Scholar] [CrossRef]
- Algorri, J.F.; Ochoa, M.; Roldán-Varona, P.; Rodríguez-Cobo, L.; López-Higuera, J.M. Light Technology for Efficient and Effective Photodynamic Therapy: A Critical Review. Cancers 2021, 13, 3484. [Google Scholar] [CrossRef]
- Tracy, E.C.; Bowman, M.-J.; Pandey, R.K.; Baumann, H. Tumor cell-specific retention of photosensitizers determines the outcome of photodynamic therapy for head and neck cancer. J. Photochem. Photobiol. B Biol. 2022, 234, 112513. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.; Jiang, Z.; Xiong, Y.; Xu, Z.; Yang, X.; Gu, D.; Ainiwaer, M.; Li, L.; Liu, J.; Chen, F. Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 17404. https://doi.org/10.3390/ijms242417404
Fan L, Jiang Z, Xiong Y, Xu Z, Yang X, Gu D, Ainiwaer M, Li L, Liu J, Chen F. Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications. International Journal of Molecular Sciences. 2023; 24(24):17404. https://doi.org/10.3390/ijms242417404
Chicago/Turabian StyleFan, Lixiao, Zheng Jiang, Yu Xiong, Zepeng Xu, Xin Yang, Deying Gu, Mailudan Ainiwaer, Leyu Li, Jun Liu, and Fei Chen. 2023. "Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications" International Journal of Molecular Sciences 24, no. 24: 17404. https://doi.org/10.3390/ijms242417404
APA StyleFan, L., Jiang, Z., Xiong, Y., Xu, Z., Yang, X., Gu, D., Ainiwaer, M., Li, L., Liu, J., & Chen, F. (2023). Recent Advances in the HPPH-Based Third-Generation Photodynamic Agents in Biomedical Applications. International Journal of Molecular Sciences, 24(24), 17404. https://doi.org/10.3390/ijms242417404





