Insight into the Binding of Argon to Cyclic Water Clusters from Symmetry-Adapted Perturbation Theory
Abstract
1. Introduction
2. Computational Details
3. Results and Discussion
3.1. Structures, Harmonic Vibrational Frequencies, and Relative Energies
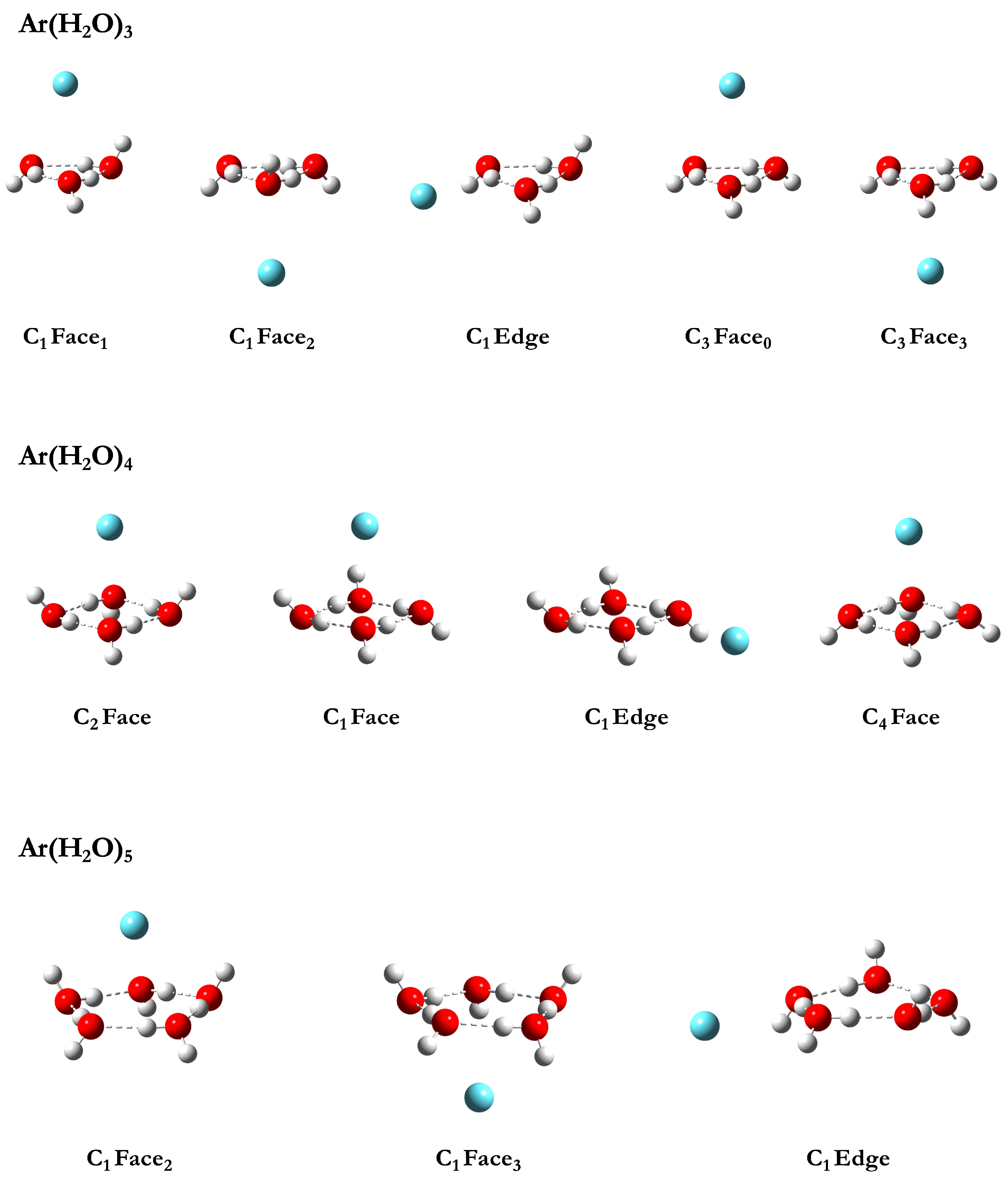
3.1.1. Ar(HO)
3.1.2. Ar(HO)
3.1.3. Ar(HO)
3.2. Binding and Interaction Energies
3.2.1. Binding Energies
3.2.2. Interaction Energies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, K.; Brown, M.G.; Carter, C.; Saykally, R.J.; Gregory, J.K.; Clary, D.C. Characterization of a cage form of the water hexamer. Nature 1996, 381, 501–503. [Google Scholar] [CrossRef]
- Liu, K.; Brown, M.G.; Saykally, R.J. Terahertz Laser Vibration-Rotation Tunneling Spectroscopy and Dipole Moment of a Cage Form of the Water Hexamer. J. Phys. Chem. A 1997, 101, 8995–9010. [Google Scholar] [CrossRef]
- Klots, T.D.; Ruoff, R.S.; Gutowsky, H.S. Rotational spectrum and structure of the linear CO2-HCN dimer: Dependence of isomer formation on carrier gas. J. Chem. Phys. 1989, 90, 4216–4221. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Klots, T.D.; Emilsson, T.; Gutowsky, H.S. Relaxation of conformers and isomers in seeded supersonic jets of inert gases. J. Chem. Phys. 1990, 93, 3142–3150. [Google Scholar] [CrossRef]
- Emilsson, T.; Germann, T.C.; Gutowsky, H.S. Kinetics of molecular association and relaxation in a pulsed supersonic expansion. J. Chem. Phys. 1992, 96, 8830–8839. [Google Scholar] [CrossRef]
- Steinbach, C.; Andersson, P.; Melzer, M.; Kazimirski, J.K.; Buck, U.; Buch, V. Detection of the book isomer from the OH-stretch spectroscopy of size selected water hexamers. Phys. Chem. Chem. Phys. 2004, 6, 3320–3324. [Google Scholar] [CrossRef]
- Pérez, C.; Muckle, M.T.; Zaleski, D.P.; Seifert, N.A.; Temelso, B.; Shields, G.C.; Kisiel, Z.; Pate, B.H. Structures of Cage, Prism, and Book Isomers of Water Hexamer from Broadband Rotational Spectroscopy. Science 2012, 336, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Lobsiger, S.; Seifert, N.A.; Zaleski, D.P.; Temelso, B.; Shields, G.C.; Kisiel, Z.; Pate, B.H. Broadband Fourier transform rotational spectroscopy for structure determination: The water heptamer. Chem. Phys. Lett. 2013, 571, 1–15. [Google Scholar] [CrossRef]
- Pérez, C.; Zaleski, D.P.; Seifert, N.A.; Temelso, B.; Shields, G.C.; Kisiel, Z.; Pate, B.H. Hydrogen Bond Cooperativity and the Three-Dimensional Structures of Water Nonamers and Decamers. Angew. Chem. 2014, 53, 14368–14372. [Google Scholar] [CrossRef]
- Otto, K.E.; Xue, Z.; Zielke, P.; Suhm, M.A. The Raman spectrum of isolated water clusters. Phys. Chem. Chem. Phys. 2014, 16, 9849–9858. [Google Scholar] [CrossRef]
- Richardson, J.O.; Pérez, C.; Lobsiger, S.; Reid, A.A.; Temelso, B.; Shields, G.C.; Kisiel, Z.; Wales, D.J.; Pate, B.H.; Althorpe, S.C. Concerted hydrogen-bond breaking by quantum tunneling in the water hexamer prism. Science 2016, 351, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Cole, W.T.S.; Farrell, J.D.; Wales, D.J.; Saykally, R.J. Structure and torsional dynamics of the water octamer from THz laser spectroscopy near 215 μm. Science 2016, 352, 1194–1197. [Google Scholar] [CrossRef]
- Thiel, M.V.; Becker, E.D.; Pimentel, G.C. Infrared Studies of Hydrogen Bonding of Water by the Matrix Isolation Technique. J. Chem. Phys. 1957, 27, 486–490. [Google Scholar] [CrossRef]
- Redington, R.L.; Milligan, D.E. Infrared Spectroscopic Evidence for the Rotation of the Water Molecule in Solid Argon. J. Chem. Phys. 1962, 37, 2162–2166. [Google Scholar] [CrossRef]
- Redington, R.L.; Milligan, D.E. Molecular Rotation and Ortho-Para Nuclear Spin Conversion of Water Suspended in Solid Ar, Kr and Xe. J. Chem. Phys. 1963, 39, 1276–1284. [Google Scholar] [CrossRef]
- Ayers, G.P.; Pullin, A.D.E. The i.r. spectra of matrix isolated water species-I. Assignment of bands to (H2O)2, (D2O)2 and HDO dimer species in argon matrices. Spectrochim. Acta Part A 1976, 32, 1629–1639. [Google Scholar] [CrossRef]
- Bentwood, R.M.; Barnes, A.J.; Orvill-Thomas, W.J. Studies of intermolecular interactions by matrix isolation vibrational spectroscopy: Self-association of water. J. Mol. Struct. 1980, 84, 391–404. [Google Scholar] [CrossRef]
- Engdahl, A.; Nelander, B. On the structure of the water trimer. A matrix isolation study. J. Chem. Phys. 1987, 86, 4831–4837. [Google Scholar] [CrossRef]
- Knözinger, E.; Schuller, W.; Langel, W. Structure and dynamics in pure and doped rare-gas matrices. Faraday Discuss. Chem. Soc. 1988, 86, 285–293. [Google Scholar] [CrossRef]
- Engdahl, A.; Nelander, B. Water in krypton matrices. J. Mol. Struct. 1989, 193, 101–109. [Google Scholar] [CrossRef]
- Forney, D.; Jacox, M.E.; Thompson, W.E. The Mid- and Near-Infrared Spectra of Water and Water Dimer Isolated in Solid Neon. J. Mol. Spectrosc. 1993, 157, 479–493. [Google Scholar] [CrossRef]
- Perchard, J.P. Anharmonicity and hydrogen bonding. III. Analysis of the near infrared spectrum of water trapped in argon matrix. Chem. Phys. 2001, 273, 217–233. [Google Scholar] [CrossRef]
- Michaut, X.; Vasserot, A.M.; Abouaf-Marguin, L. Temperature and time effects on the rovibrational structure of fundamentals of H2O trapped in solid argon: Hindered rotation and RTC satellite. Vib. Spectrosc. 2004, 34, 83–93. [Google Scholar] [CrossRef]
- Ceponkus, J.; Karlström, G.; Nelander, B. Intermolecular Vibrations of the Water Trimer, a Matrix Isolation Study. J. Phys. Chem. A 2005, 109, 7859–7864. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Yamada, K.M.T. Infrared spectra of the H2O-Kr and H2O-Xe complexes in argon matrices. Chem. Phys. Lett. 2005, 418, 323–327. [Google Scholar] [CrossRef]
- Coussan, S.; Roubin, P.; Perchard, J.P. Infrared induced isomerizations of water polymers trapped in nitrogen matrix. Chem. Phys. 2006, 324, 527–540. [Google Scholar] [CrossRef]
- Abouaf-Marguin, L.; Vasserot, A.M.; Pardanaud, C.; Michaut, X. Nuclear spin conversion of water diluted in solid argon at 4.2 K: Environment and atmospheric impurities effects. Chem. Phys. Lett. 2007, 447, 232–235. [Google Scholar] [CrossRef]
- Pardanaud, C.; Vasserot, A.M.; Michaut, X.; Abouaf-Marguin, L. Observation of nuclear spin species conversion inside the 1593 cm-1 structure of H2O trapped in argon matrices: Nitrogen impurities and the H2O:N2 complex. J. Mol. Struct. 2008, 873, 181–190. [Google Scholar] [CrossRef]
- Ceponkus, J.; Uvdal, P.; Nelander, B. Acceptor switching and axial rotation of the water dimer in matrices, observed by infrared spectroscopy. J. Chem. Phys. 2010, 133, 074301. [Google Scholar] [CrossRef]
- Ceponkus, J.; Uvdal, P.; Nelander, B. Water Tetramer, Pentamer, and Hexamer in Inert Matrices. J. Phys. Chem. A 2012, 116, 4842–4850. [Google Scholar] [CrossRef]
- Ceponkus, J.; Engdahl, A.; Uvdal, P.; Nelander, B. Structure and dynamics of small water clusters, trapped in inert matrices. Chem. Phys. Lett. 2013, 581, 1–9. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Cole, W.T.S.; Saykally, R.J. The water dimer I: Experimental characterization. Chem. Phys. Lett. 2015, 633, 13–26. [Google Scholar] [CrossRef]
- Pogorelov, V.Y.; Doroshenko, I.Y. Vibrational spectra of water clusters, trapped in low temperature matrices. Low Temp. Phys. 2016, 42, 1163–1166. [Google Scholar] [CrossRef]
- Oswald, S.; Suhm, M.A.; Coussan, S. Incremental NH stretching downshift through stepwise nitrogen complexation of pyrrole: A combined jet expansion and matrix isolation study. Phys. Chem. Chem. Phys. 2019, 21, 1277. [Google Scholar] [CrossRef] [PubMed]
- Oswald, S.; Coussan, S. Chloroform-nitrogen aggregates: Upshifted CH and downshifted CCl stretching vibrations observed by matrix isolation and jet expansion infrared spectroscopy. Low Temp. Phys. 2019, 45, 639–648. [Google Scholar] [CrossRef]
- Nauta, K.; Miller, R.E. Formation of Cyclic Water Hexamer in Liquid Helium: Smallest Piece of Ice. Science 2000, 287, 293–295. [Google Scholar] [CrossRef]
- Burnham, C.J.; Xantheas, S.S.; Miller, M.A.; Applegate, B.E.; Miller, R.E. The formation of cyclic water complexes by sequential ring insertion: Experiment and theory. J. Chem. Phys. 2002, 117, 1109–1122. [Google Scholar] [CrossRef]
- Lindsay, C.M.; Douberly, G.E.; Miller, R.E. Rotational and vibrational dynamics of H2O and HDO in helium nanodroplets. J. Mol. Struct. 2006, 786, 96–104. [Google Scholar] [CrossRef]
- Kuyanov-Prozument, K.; Choi, M.Y.; Vilesov, A.F. Spectrum and infrared intensities of OH-stretching bands of water dimers. J. Chem. Phys. 2010, 132, 014304. [Google Scholar] [CrossRef]
- Schwan, R.; Kaufmann, M.; Leicht, D.; Schwaab, G.; Havenith, M. Infrared spectroscopy of the ν2 band of the water monomer and small water clusters (H2O)n=2,3,4 in helium droplets. Phys. Chem. Chem. Phys. 2016, 18, 24063–24069. [Google Scholar] [CrossRef]
- Douberly, G.E.; Miller, R.E.; Xantheas, S.S. Formation of Exotic Networks of Water Clusters in Helium Droplets Facilitated by the Presence of Neon Atoms. J. Am. Chem. Soc. 2017, 139, 4152–4156. [Google Scholar] [CrossRef]
- Schwan, R.; Qu, C.; Mani, D.; Pal, N.; Schwaab, G.; Bowman, J.M.; Tschumper, G.S.; Havenith, M. Observation of the Low-Frequency Spectrum of the Water Trimer as a Sensitive Test of the Water-Trimer Potential and the Dipole-Moment Surface. Angew. Chem. 2020, 59, 11399–11407. [Google Scholar] [CrossRef]
- Anderson, D.T.; Davis, S.; Nesbitt, D.J. Sequential solvation of HCl in argon: High resolution infrared spectroscopy of ArnHCl (n = 1, 2, 3). J. Chem. Phys. 1997, 107, 1114–1127. [Google Scholar] [CrossRef]
- Leung, H.O.; Gangwani, D.; Grabow, J.U. Nuclear Quadrupole Hyperfine Structure in the Microwave Spectrum of Ar-N2O. J. Mol. Spectrosc. 1997, 184, 106–112. [Google Scholar] [CrossRef]
- Häber, T. FTIR-Spectroscopy of isolated and argon coated (HBr)n≤4 clusters in supersonic slit-jet expansions. Phys. Chem. Chem. Phys. 2003, 5, 1365–1369. [Google Scholar] [CrossRef]
- Gregoire, G.; Brinkmann, N.R.; van Heijnsbergen, D.; Schaefer, H.F.; Duncan, M.A. Infrared Photodissociation Spectroscopy of Mg+(CO2)n and Mg+(CO2)nAr Clusters. J. Phys. Chem. A 2003, 107, 218–227. [Google Scholar] [CrossRef]
- Bochenkova, A.V.; Suhm, M.A.; Granovsky, A.A.; Nemukhin, A.V. Hybrid diatomics-in-molecules-based quantum mechanical/molecular mechanical approach applied to the modeling of structures and spectra of mixed molecular clusters Arn(HCl)m and Arn(HF)m. J. Chem. Phys. 2004, 120, 3732–3743. [Google Scholar] [CrossRef]
- Walker, N.R.; Walters, R.S.; Tsai, M.K.; Jordan, K.D.; Duncan, M.A. Infrared Photodissociation Spectroscopy of Mg+(H2O)Arn Complexes: Isomers in Progressive Microsolvation. J. Phys. Chem. A 2005, 109, 7057–7067. [Google Scholar] [CrossRef] [PubMed]
- Douberly, G.E.; Walters, R.S.; Cui, J.; Jordan, K.D.; Duncan, M.A. Infrared Spectroscopy of Small Protonated Water Clusters, H+(H2O)n (n = 2–5): Isomers, Argon Tagging, and Deuteration. J. Phys. Chem. A 2010, 114, 4570–4579. [Google Scholar] [CrossRef]
- Carnegie, P.D.; Bandyopadhyay, B.; Duncan, M.A. Infrared Spectroscopy of Mn+(H2O) and Mn2+(H2O) via Argon Complex Predissociation. J. Phys. Chem. A 2011, 115, 7602–7609. [Google Scholar] [CrossRef]
- Marshall, M.D.; Leung, H.O.; Calvert, C.E. Molecular structure of the argon-(Z)-1-chloro-2-fluoroethylene complex from chirped-pusle and narrow-band Fourier transform microwave spectroscopy. J. Mol. Spectrosc. 2012, 280, 97–103. [Google Scholar] [CrossRef]
- Fujii, A.; Mizuse, K. Infrared spectroscopic studies on hydrogen-bonded water networks in gas phase clusters. Int. Rev. Phys. Chem. 2013, 32, 266–307. [Google Scholar] [CrossRef]
- Leung, H.O.; Marshall, M.D.; Mueller, J.L.; Amberger, B.K. The molecular structure of and interconversion tunneling in the argon-cis-1,2-difluoroethylene complex. J. Chem. Phys. 2013, 139, 134303. [Google Scholar] [CrossRef]
- Leung, H.O.; Marshall, M.D.; Messinger, J.P.; Knowlton, G.S.; Sundheim, K.M.; Cheung-Lau, J.C. The microwave spectra and molecular structures of 2-chloro-1,1-difluoroethylene and its complex with the argon atom. J. Mol. Spectrosc. 2014, 305, 25–33. [Google Scholar] [CrossRef]
- Kaledin, M.; Adedeji, D.T. Driven Molecular Dynamics Studies of the Shared Proton Motion in the H5O2+·Ar Cluster: The Effect of Argon Tagging and Deuteration on Vibrational Spectra. J. Phys. Chem. A 2015, 119, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Morita, M.; Takahashi, K.; Kuo, J.L. Features in Vibrational Spectra Induced by Ar-Tagging for H3O+Arm, m=0-3. J. Phys. Chem. A 2015, 119, 10887–10892. [Google Scholar] [CrossRef] [PubMed]
- Leung, H.O.; Marshall, M.D.; Wronkovich, M.A. The microwave spectrum and molecular structure of Ar-2,3,3,3-tetrafluoropropene. J. Mol. Spectrosc. 2017, 337, 80–85. [Google Scholar] [CrossRef]
- Marshall, M.D.; Leung, H.O. The microwave spectrum and molecular structure of 3,3-difluoro-1,2-epoxypropane and its complex with the argon atom. J. Mol. Spectrosc. 2018, 350, 18–26. [Google Scholar] [CrossRef]
- Marshall, M.D.; Leung, H.O.; Wang, K.; Acha, M.D. Microwave Spectrum and Molecular Structure of the Chiral Tagging Candidate, 3,3,3-Trifluoro-1,2-epoxypropane and Its Complex with the Argon Atom. J. Phys. Chem. A 2018, 122, 4670–4680. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, J.; Shirkov, L. Theoretical study of the complexes of dichlorobenzene isomers with argon. I. Global potential energy surface for all the isomers with application to intermolecular vibrations. J. Chem. Phys. 2019, 150, 074301. [Google Scholar] [CrossRef] [PubMed]
- Shirkov, L.; Makarewicz, J. Theoretical study of the complexes of dichlorobenzene isomers with argon. II. SAPT analysis of the intermolecular interaction. J. Chem. Phys. 2019, 150, 074302. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M.D.; Leung, H.O.; Febles, O.; Gomez, A. The microwave spectra and molecular structures of (E)-1,3,3,3-tetrafluoropropene and its complex with the argon atom. J. Mol. Spectrosc. 2020, 374, 111379. [Google Scholar] [CrossRef]
- Leung, H.O.; Marshall, M.D.; Stuart, D.J. Microwave Spectrum and Molecular Structure of 3-fluoro-1,2-epoxypropane and the Unexpected Structure of Its Complex with the Argon Atom. J. Phys. Chem. A 2020, 124, 1798–1810. [Google Scholar] [CrossRef]
- Leung, H.O.; Marshall, M.D.; Horowitz, J.R.; Stuart, D.J. The microwave spectrum and molecular structure of the gas-phase heterodimer formed between argon and glycidol. J. Mol. Spectrosc. 2021, 375, 111407. [Google Scholar] [CrossRef]
- Leung, H.O.; Marshall, M.D.; Bozzi, A.T.; Horowitz, J.R.; Nino, A.C.; Tandon, H.K.; Yoon, L. The microwave spectra and molecular structures of (E)-1-chloro-1,2-difluoroethylene and its complex with the argon atom. J. Mol. Spectrosc. 2021, 381, 111520. [Google Scholar] [CrossRef]
- Punyain, W.; Takahashi, K. Evaluation of Ar tagging toward the vibrational spectra and zero point energy of X-HOH, X-DOH, and X-HOD, for X = F, Cl, Br. Phys. Chem. Chem. Phys. 2021, 23, 9492–9499. [Google Scholar] [CrossRef] [PubMed]
- Marks, J.H.; Miliordos, E.; Duncan, M.A. Infrared spectroscopy of RG-Co+(H2O) complexes (RG = Ar, Ne, He): The role of rare gas “tag” atoms. J. Chem. Phys. 2021, 154, 064306. [Google Scholar] [CrossRef]
- Leung, H.O.; Marshall, M.D.; Ahmad, T.Z.; Borden, D.W.; Hoffman, C.A.; Kim, N.A. The microwave spectra and molecular structures of the chiral and achiral rotamers of 2,3,3-trifluoropropene and their gas-phase heterodimers with the argon atom. J. Mol. Spectrosc. 2022, 387, 111656. [Google Scholar] [CrossRef]
- Das, A.; Arunan, E. Measurement of Donor-Acceptor Interchange Tunnelling in Ar(H2O)2 using Rotational Spectroscopy and a Re-look at Its Structure and Bonding. J. Mol. Struct. 2022, 1252, 132094. [Google Scholar] [CrossRef]
- Ito, Y.; Kominato, M.; Nakashima, Y.; Ohshimo, K.; Misaizu, F. Fragment imaging in the infrared photodissociation of the Ar-tagged protonated water clusters H3O+-Ar and H+(H2O)2-Ar. Phys. Chem. Chem. Phys. 2023, 25, 9404–9412. [Google Scholar] [CrossRef]
- Leung, H.O.; Marshall, M.D.; Hong, S.; Hoque, L. The microwave spectra and molecular structures of (Z)-1-chloro-3,3,3-trifluoropropene and its gas-phase heterodimer with the argon atom. J. Mol. Spectrosc. 2023, 394, 111779. [Google Scholar] [CrossRef]
- Wassermann, T.N.; Luckhaus, D.; Coussan, S.; Suhm, M.A. Proton tunneling estimates for malonaldehyde vibrations from supersonic jet and matrix quenching experiments. Phys. Chem. Chem. Phys. 2006, 8, 2344–2348. [Google Scholar] [CrossRef] [PubMed]
- Wassermann, T.N.; Zielke, P.; Lee, J.J.; Cezard, C.; Suhm, M.A. Structural preferences, argon nanocoating, and dimerization of n-alkanols as revealed by OH stretching spectroscopy in supersonic jets. J. Phys. Chem. A 2007, 111, 7437–7448. [Google Scholar] [CrossRef]
- Wassermann, T.N.; Suhm, M.A. Ethanol monomers and dimers revisisted: A raman study of conformational preferences and argon nanocoating effects. J. Phys. Chem. A 2010, 114, 8223–8233. [Google Scholar] [CrossRef]
- Lee, J.J.; Hofener, S.; Klopper, W.; Wassermann, T.N.; Suhm, M.A. Origin of the argon nanocoating shift in the OH stretching fundamental of n-propanol: A combined experimental and quantum chemical study. J. Phys. Chem. C 2009, 113, 10929–10938. [Google Scholar] [CrossRef]
- Vasylieva, A.; Doroshenko, I.; Doroshenko, O.; Pogorelov, V. Effect of argon environment on small water clusters in matrix isolation. Low Temp. Phys. 2019, 45, 627–633. [Google Scholar] [CrossRef]
- Vasylieva, A.; Doroshenko, I.; Stepanian, S.; Adamowicz, L. The influence of low-temperature argon matrix on embedded water clusters. A DFT theoretical study. Low Temp. Phys. 2021, 47, 242–249. [Google Scholar] [CrossRef]
- Kisiel, Z.; Fowler, P.W.; Legon, A.C. Rotational spectra and structures of van der Waals dimers of Ar with a series of fluorocarbons. J. Chem. Phys. 1991, 95, 2283–2291. [Google Scholar] [CrossRef]
- Cohen, R.C.; Busarow, K.L.; Laughlin, K.B.; Blake, G.A.; Havenith, M.; Lee, Y.T.; Saykally, R.J. Tunable far infrared laser spectroscopy of van der Waals bonds: Vibration-rotation-tunneling spectra of Ar-H2O. J. Chem. Phys. 1988, 89, 4494–4504. [Google Scholar] [CrossRef][Green Version]
- Cohen, R.C.; Busarow, K.L.; Lee, Y.Y.; Saykally, R.J. Tunable far infrared laser spectroscopy of van der Waals bonds: The intermolecular stretching vibration and effective radial potentials for Ar-H2O. J. Chem. Phys. 1990, 92, 169–177. [Google Scholar] [CrossRef]
- Fraser, G.T.; Lovas, F.J.; Suenram, R.D.; Matsumura, K. Microwave spectrum of Ar-H2O: Dipole moment, isotopic studies, and 17O quadrupole coupling constants. J. Mol. Spectrosc. 1990, 144, 97–112. [Google Scholar] [CrossRef]
- Chałasiński, G.; Szczȩs̀niak, M.M.; Scheiner, S. Ab initio study of the intermolecular potential of Ar-H2O. J. Chem. Phys. 1991, 94, 2807–2816. [Google Scholar] [CrossRef]
- Cohen, R.C.; Saykally, R.J. Multidimensional intermolecular dynamics from tunable far-infrared laser spectroscopy: Angular-radial coupling in the intermolecular potential of argon-H2O. J. Chem. Phys. 1991, 95, 7891–7906. [Google Scholar] [CrossRef]
- Suzukí, S.; Bumgarner, R.E.; Stockman, P.A.; Green, P.G.; Blake, G.A. Tunable far-infrared laser spectroscopy of deuterated isotopomers of Ar-H2O. J. Chem. Phys. 1991, 94, 824–825. [Google Scholar] [CrossRef]
- Germann, T.C.; Gutowsky, H.S. Nuclear hyperfine interactions and dynamic state of H2O in Ar-H2O. J. Chem. Phys. 1993, 98, 5235–5238. [Google Scholar] [CrossRef]
- Tao, F.M.; Klemperer, W. Accurate ab initio potential energy surfaces of Ar-HF, Ar-H2O, and Ar-NH3. J. Chem. Phys. 1994, 101, 1129–1145. [Google Scholar] [CrossRef]
- Borges, E.; Ferreira, G.G.; Braga, J.P. Structures and energies of ArnH2O (n = 1–26) clusters using a nonrigid potential surface: A molecular dynamics simulation. Int. J. Quantum Chem. 2008, 108, 2523–2529. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y. New rovibrational bands of the Ar-H2O complex at the ν2 bend region of H2O. J. Mol. Spectrosc. 2014, 301, 1–8. [Google Scholar] [CrossRef]
- Zou, L.; Weaver, S.L.W. Direct measurement of additional Ar-H2O vibration-rotation-tunneling bands in the millimeter-submillimeter range. J. Mol. Spectrosc. 2016, 324, 12–19. [Google Scholar] [CrossRef]
- Hou, D.; Ma, Y.T.; Zhang, X.L.; Li, H. The origins of intra- and inter-molecular vibrational couplings: A case study of H2O-Ar on full and reduced-dimensional potential energy surface. J. Chem. Phys. 2016, 144, 014301. [Google Scholar] [CrossRef]
- Vanfleteren, T.; Földes, T.; Herman, M.; Liévin, J.; Loreau, J.; Coudert, L.H. Experimental and theoretical investigations of H2O-Ar. J. Chem. Phys. 2017, 147, 014302. [Google Scholar] [CrossRef]
- Yang, D.; Liu, L.; Xie, D.; Guo, H. Full-dimensional quantum studies of vibrational energy transfer dynamics between H2O and Ar: Theory assessing experiment. Phys. Chem. Chem. Phys. 2022, 24, 13542–13549. [Google Scholar] [CrossRef]
- Liu, L.; Yang, D.; Guo, H.; Xie, D. Full-Dimensional Quantum Dynamics Studies of Ro-vibrationally Inelastic Scattering of H2O with Ar: A Benchmark Test of the Rigid-Rotor Approximation. J. Phys. Chem. A 2023, 127, 195–202. [Google Scholar] [CrossRef]
- Arunan, E.; Emilsson, T.; Gutowsky, H.S. Rotational Spectra, Structure, and Dynamics of Arm-(H2O)n Clusters: The Ar-(H2O)2 trimer. J. Chem. Phys. 2002, 116, 4886–4895. [Google Scholar] [CrossRef]
- Arunan, E.; Emilsson, T.; Gutowsky, H.S. Rotational Spectra, Structure, and Dynamics of Arm-(H2O)n Clusters: Ar2-H2O, Ar3-H2O, Ar-(H2O)2 and Ar-(H2O)3. J. Am. Chem. Soc. 1994, 116, 8418–8419. [Google Scholar] [CrossRef]
- Moudens, A.; Georges, R.; Goubet, M.; Makarewicz, J.; Lokshtanov, S.E.; Vigasin, A.A. Direct absorption spectroscopy of water clusters formed in a continuous slit nozzle expansion. J. Chem. Phys. 2009, 131, 204312. [Google Scholar] [CrossRef] [PubMed]
- Mó, O.; Yáñez, M.; Elguero, J. Cooperative (nonpairwise) effects in water trimers: An ab initio molecular orbital study. J. Chem. Phys. 1992, 97, 6628–6638. [Google Scholar] [CrossRef]
- Pugliano, N.; Saykally, R.J. Measurement of Quantum Tunneling Between Chiral Isomers of the Cyclic Water Trimer. Science 1992, 257, 1937–1940. [Google Scholar] [CrossRef]
- Schütz, M.; Bürgi, T.; Leutwyler, S.; Bürgi, H.B. Fluxionality and low-lying transition structures of the water trimer. J. Chem. Phys. 1993, 99, 5228–5238. [Google Scholar] [CrossRef]
- Wales, D.J. Theoretical study of water trimer. J. Am. Chem. Soc. 1993, 115, 11180–11190. [Google Scholar] [CrossRef]
- Xantheas, S.S.; Dunning, T.H. The structure of the water trimer from abinitio calculations. J. Chem. Phys. 1993, 98, 8037–8040. [Google Scholar] [CrossRef]
- Xantheas, S.S.; Dunning, T.H. Ab initio studies of cyclic water clusters (H2O)n, n = 1–6. I. Optimal structures and vibrational spectra. J. Chem. Phys. 1993, 99, 8774–8792. [Google Scholar] [CrossRef]
- Xantheas, S.S. Ab initio studies of cyclic water clusters (H2O)n, n = 1–6. II. Analysis of many-body interactions. J. Chem. Phys. 1994, 100, 7523–7534. [Google Scholar] [CrossRef]
- Pribble, R.N.; Zwier, T.S. Size-Specific Infrared Spectra of Benzene-(H2O)n Clusters (n = 1 through 7): Evidence for Noncyclic (H2O)n Structures. Science 1994, 265, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Loeser, J.G.; Elrod, M.J.; Host, B.C.; Rzepiela, J.A.; Pugliano, N.; Saykally, R.J. Dynamics of Structural Rearrangements in the Water Trimer. J. Am. Chem. Soc. 1994, 116, 3507–3512. [Google Scholar] [CrossRef]
- Xantheas, S.S. Ab initio studies of cyclic water clusters (H2O)n, n = 1–6. III. Comparison of density functional with MP2 results. J. Chem. Phys. 1995, 102, 4505–4517. [Google Scholar] [CrossRef]
- Schütz, M.; Klopper, W.; Lüthi, H.P.; Leutwyler, S. Low-lying stationary points and torsional interconversions of cyclic (H2O)4: An abinitio study. J. Chem. Phys. 1995, 103, 6114–6126. [Google Scholar] [CrossRef]
- Fowler, J.E.; Schaefer, H.F.I. Detailed Study of the Water Trimer Potential Energy Surface. J. Am. Chem. Soc. 1995, 117, 446–452. [Google Scholar] [CrossRef]
- Wales, D.J.; Walsh, T.R. Theoretical study of the water pentamer. J. Chem. Phys. 1996, 105, 6957–6971. [Google Scholar] [CrossRef]
- Huisken, F.; Kaloudis, M.; Kulcke, A. Infrared spectroscopy of small size-selected water clusters. J. Chem. Phys. 1996, 104, 17–25. [Google Scholar] [CrossRef]
- Cruzan, J.D.; Braly, L.B.; Liu, K.; Brown, M.G.; Loeser, J.G.; Saykally, R.J. Quantifying Hydrogen Bond Cooperativity in Water: VRT Spectroscopy of the Water Tetramer. Science 1996, 271, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Brown, M.G.; Cruzan, J.D.; Saykally, R.J. Vibration-Rotation Tunneling Spectra of the Water Pentamer: Structure and Dynamics. Science 1996, 271, 62–64. [Google Scholar] [CrossRef]
- Paul, J.B.; Collier, C.P.; Saykally, R.J.; Scherer, J.J.; O’Keefe, A. Direct Measurement of Water Cluster Concentrations by Infrared Cavity Ringdown Laser Absorption Spectroscopy. J. Phys. Chem. A 1997, 101, 5211–5214. [Google Scholar] [CrossRef]
- Wales, D.J.; Walsh, T.R. Theoretical study of the water tetramer. J. Chem. Phys. 1997, 106, 7193–7207. [Google Scholar] [CrossRef]
- Nielsen, I.M.B.; Seidl, E.T.; Janssen, C.L. Accurate structures and binding energies for small water clusters: The water trimer. J. Chem. Phys. 1999, 110, 9435. [Google Scholar] [CrossRef]
- Xantheas, S.S.; Burnham, C.J.; Harrison, R.J. Development of transferable interaction models for water. II. Accurate energetics of the first few water clusters from first principles. J. Chem. Phys. 2002, 116, 1493–1499. [Google Scholar] [CrossRef]
- Taketsugu, T.; Wales, D.J. Theoretical study of rearrangements in water dimer and trimer. Mol. Phys. 2002, 100, 2793–2806. [Google Scholar] [CrossRef]
- Anderson, J.A.; Crager, K.; Fedoroff, L.; Tschumper, G.S. Anchoring the potential energy surface of the cyclic water trimer. J. Chem. Phys. 2004, 121, 11023–11029. [Google Scholar] [CrossRef]
- Day, M.B.; Kirschner, K.N.; Shields, G.C. Global Search for Minimum Energy (H2O)n Clusters, n = 3–5. J. Phys. Chem. A 2005, 109, 6773–6778. [Google Scholar] [CrossRef]
- Dunn, M.E.; Evans, T.M.; Kirschner, K.N.; Shields, G.C. Prediction of Accurate Anharmonic Experimental Vibrational Frequencies for Water Clusters, (H2O)n, n = 2–5. J. Phys. Chem. A 2006, 110, 303–309. [Google Scholar] [CrossRef]
- Slipchenko, M.N.; Kuyanov, K.E.; Sartakov, B.G.; Vilesov, A.F. Infrared intensity in small ammonia and water clusters. J. Chem. Phys. 2006, 124, 241101. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, S.; Yamada, K.M.T. Infrared spectra and structure of water clusters trapped in argon and krypton matrices. J. Mol. Struct. 2006, 795, 78–83. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Yamada, K.M.T. The monocyclic water hexamer detected in neon matrices by infrared spectroscopy. Chem. Phys. Lett. 2007, 435, 74–78. [Google Scholar] [CrossRef]
- Pérez, J.F.; Hadad, C.Z.; Restrepo, A. Structural Studies of the Water Tetramer. Int. J. Quantum Chem. 2008, 108, 1653–1659. [Google Scholar] [CrossRef]
- Watanabe, Y.; Maeda, S.; Ohno, K. Intramolecular vibrational frequencies of water clusters (H2O)n (n = 2–5): Anharmonic analyses using potential functions based on the scaled hypersphere search method. J. Chem. Phys. 2008, 129, 074315. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Dai, J.; Hou, Y.; Yuan, J. Structure and vibrational spectra of small water clusters from first principles simulations. J. Chem. Phys. 2010, 133, 014302. [Google Scholar] [CrossRef] [PubMed]
- Bégué, D.; Baraille, I.; Garrain, P.A.; Dargelos, A.; Tassaing, T. Calculation of IR frequencies and intensities in electrical and mechanical anharmonicity approximations: Applications to small water clusters. J. Chem. Phys. 2010, 133, 034102. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.M.; Temelso, B.; Archer, K.A.; Morrell, T.E.; Shields, G.C. Accurate Predictions of Water Cluster Formation, (H2O)n=2--10. J. Phys. Chem. A 2010, 114, 11725–11737. [Google Scholar] [CrossRef]
- Ramirez, F.; Hadad, C.Z.; Guerra, D.; David, J.; Restrepo, A. Structural Studies of the Water Pentamer. Chem. Phys. Lett. 2011, 507, 229–233. [Google Scholar] [CrossRef]
- Temelso, B.; Archer, K.A.; Shields, G.C. Benchmark Structures and Binding Energies of Small Water Clusters with Anharmonicity Corrections. J. Phys. Chem. A 2011, 115, 12034–12046. [Google Scholar] [CrossRef]
- Temelso, B.; Shields, G.C. The Role of Anharmonicity in Hydrogen-Bonded Systems: The Case of Water Clusters. J. Chem. Theory Comput. 2011, 7, 2804–2817. [Google Scholar] [CrossRef]
- Yoo, S.; Xantheas, S.S. Structures, Energetics and Spectroscopic Fingerprints of Water Clusters n = 2–24. In Handbook of Computational Chemistry; Leszczynski, J., Ed.; Springer: New York, NY, USA, 2012; Volume 2, pp. 761–792. [Google Scholar]
- Howard, J.C.; Tschumper, G.S. Wavefunction methods for the accurate characterization of water clusters. WIREs Comput. Mol. Sci. 2014, 4, 199–224. [Google Scholar] [CrossRef]
- Howard, J.C.; Tschumper, G.S. Benchmark Structures and Harmonic Vibrational Frequencies Near the CCSD(T) Complete Basis Set Limit for Small Water Clusters: (H2O)n=2,3,4,5,6. J. Chem. Theory Comput. 2015, 11, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Miliordos, E.; Xantheas, S.S. An accurate and efficient computational protocol for obtaining the complete basis set limits of the binding energies of water clusters at the MP2 and CCSD(T) levels of theory: Application to (H2O)m, m = 2–6, 8, 11, 16, and 17. J. Chem. Phys. 2015, 142, 234303. [Google Scholar] [CrossRef]
- Malloum, A.; Fifen, J.J.; Dhaouadi, Z.; Engo, S.G.N.; Conradie, J. Structures, relative stability and binding energies of neutral water clusters, (H2O)2-30. New J. Chem. 2019, 43, 13020–13037. [Google Scholar] [CrossRef]
- Møller, C.; Plesset, M.S. Note on an Approximation Treatment for Many-electron Systems. Phys. Rev. 1934, 46, 618–622. [Google Scholar] [CrossRef]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358. [Google Scholar] [CrossRef]
- Bates, D.M.; Smith, J.R.; Janowski, T.; Tschumper, G.S. Development of a 3-body:many-body integrated fragmentation method for weakly bound clusters and application to water clusters (H2O)n=3–10,16,17. J. Chem. Phys. 2011, 135, 044123. [Google Scholar] [CrossRef]
- Bates, D.M.; Smith, J.R.; Tschumper, G.S. Efficient and Accurate Methods for the Geometry Optimization of Water Clusters: Application of Analytic Gradients for the Two-Body:Many-Body QM:QM Fragmentation Method to (H2O)n, n = 3–10. J. Chem. Theory Comput. 2011, 7, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.C.; Tschumper, G.S. N-body:Many-body QM:QM vibrational frequencies: Application to small hydrogen-bonded clusters. J. Chem. Phys. 2013, 139, 184113. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, B.W.; Tschumper, G.S. A multicentered approach to integrated QM/QM calculations. Applications to multiply hydrogen-bonded systems. J. Comput. Chem. 2003, 24, 1563–1568. [Google Scholar] [CrossRef]
- Tschumper, G.S. Multicentered integrated QM:QM methods for weakly bound clusters: An efficient and accurate 2-body:many-body treatment of hydrogen bonding and van der Waals interactions. Chem. Phys. Lett. 2006, 427, 185–191. [Google Scholar] [CrossRef]
- Elsohly, A.M.; Shaw, C.L.; Guice, M.E.; Smith, B.D.; Tschumper, G.S. Analytic gradients for the multicentered integrated QM:QM method for weakly bound clusters: Efficient and accurate 2-body:many-body geometry optimizations. Mol. Phys. 2007, 105, 2777–2782. [Google Scholar] [CrossRef]
- Tschumper, G.S.; Ellington, T.L.; Johnson, S.N. Chapter Two—Dissociation in Binary Acid/Base Clusters: An Examination of Inconsistencies Introduced Into the Many-Body Expansion by Naíve Fragmentation Schemes. Annu. Rep. Comput. Chem. 2017, 13, 93–115. [Google Scholar] [CrossRef]
- Purvis, G.D., III; Stanton, R.J.. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Stanton, J.F.; Gauss, J.; Cheng, L.; Harding, M.E.; Matthews, D.A.; Szalay, P.G. CFOUR, Coupled-Cluster Techniques for Computational Chemistry, a Quantum-Chemical Program Package. With Contributions from A.A. Auer, R.J. Bartlett, U. Benedikt, C. Berger, D.E. Bernholdt, Y.J. Bomble, O. Christiansen, F. Engel, R. Faber, M. Heckert, O. Heun, M. Hilgenberg, C. Huber, T.-C. Jagau, D. Jonsson, J. Jusélius, T. Kirsch, K. Klein, W.J. Lauderdale, F. Lipparini, T. Metzroth, L.A. Mück, D.P. O’Neill, D.R. Price, E. Prochnow, C. Puzzarini, K. Ruud, F. Schiffmann, W. Schwalbach, C. Simmons, S. Stopkowicz, A. Tajti, J. Vázquez, F. Wang, J.D. Watts and the Integral Packages MOLECULE (J. Almlöf and P.R. Taylor), PROPS (P.R. Taylor), ABACUS (T. Helgaker, H.J. Aa. Jensen, P. Jørgensen, and J. Olsen), and ECP routines by A. V. Mitin and C. van Wüllen. Current Version. Available online: http://www.cfour.de (accessed on 1 December 2023).
- Matthews, D.A.; Cheng, L.; Harding, M.E.; Lipparini, F.; Stopkowicz, S.; Jagau, T.C.; Szalay, P.G.; Gauss, J.; Stanton, J.F. Coupled-cluster techniques for computational chemistry: The CFOUR program package. J. Chem. Phys. 2020, 152, 214108. [Google Scholar] [CrossRef]
- Kestner, N.R. He-He Interaction in the SCF-MO Approximation. J. Chem. Phys. 1968, 48, 252–257. [Google Scholar] [CrossRef]
- Liu, B.; McLean, A.D. Accurate calculation of the attractive interaction of two ground state helium atoms. J. Chem. Phys. 1973, 59, 4557–4558. [Google Scholar] [CrossRef]
- Jansen, H.B.; Ros, P. Nonempirical molecular orbital calculations on the protonation of carbon monoxide. Chem. Phys. Lett. 1969, 3, 140–143. [Google Scholar] [CrossRef]
- Boys, S.F.; Bernardi, F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol. Phys. 1970, 19, 553–566. [Google Scholar] [CrossRef]
- Tschumper, G.S. Reliable Electronic Structure Computations for Weak Non-Covalent Interactions in Clusters. In Reviews in Computational Chemistry; Lipkowitz, K.B., Cundari, T.R., Eds.; Wiley-VCH, Inc.: Hoboken, NJ, USA, 2008; Volume 26, Chapter 2; pp. 39–90. [Google Scholar] [CrossRef]
- Jeziorski, B.; Moszynski, R.; Szalewicz, K. Perturbation Theory Approach to Intermolecular Potential Energy Surfaces of van der Waals Complexes. Chem. Rev. 1994, 94, 1887–1930. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Sherrill, C.D. Wavefunction methods for noncovalent interactions. WIREs Comput. Mol. Sci. 2012, 2, 304–326. [Google Scholar] [CrossRef]
- Szalewicz, K. Symmetry-adapted perturbation theory of intermolecular forces. WIREs Comput. Mol. Sci. 2012, 2, 254–272. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Sherrill, C.D. Density fitting of intramonomer correlation effects in symmetry-adapted perturbation theory. J. Chem. Phys. 2010, 133, 014101. [Google Scholar] [CrossRef]
- Hohenstein, E.G.; Jaeger, H.M.; Carrell, E.J.; Tschumper, G.S.; Sherrill, C.D. Accurate Interaction Energies for Problematic Dispersion-Bound Complexes: Homogeneous Dimers of NCCN, P2 and PCCP. J. Chem. Theory. Comput. 2011, 7, 2842–2851. [Google Scholar] [CrossRef] [PubMed]
- Turney, J.M.; Simmonett, A.C.; Parrish, R.M.; Hohenstein, E.G.; Evangelista, F.A.; Fermann, J.T.; Mintz, B.J.; Burns, L.A.; Wilke, J.J.; Abrams, M.L.; et al. PSI4: An open-source ab initio electronic structure program. WIREs Comput. Mol. Sci. 2012, 2, 556–565. [Google Scholar] [CrossRef]
- Smith, D.G.A.; Burns, L.A.; Simmonett, A.C.; Parrish, R.M.; Schieber, M.C.; Galvelis, R.; Kraus, P.; Kruse, H.; Di Remigio, R.; Alenaizan, A.; et al. PSI4 1.4: Open-source software for high-throughput quantum chemistry. J. Chem. Phys. 2020, 152, 184108. [Google Scholar] [CrossRef]
- Parrish, R.M.; Sherrill, C.D. Tractability gains in symmetry-adapted perturbation theory including coupled double excitations: CCD+ST(CCD) dispersion with natural orbital truncations. J. Chem. Phys. 2013, 139, 174102. [Google Scholar] [CrossRef]
| MP2 | 2b:Mb | 3b:Mb | |||||
|---|---|---|---|---|---|---|---|
| Complex | Label | ||||||
| (HO) | C | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| (HO) | C | 3.24 | 1.72 | 3.45 | 1.97 | 3.43 | 1.95 |
| (HO) | S | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| (HO) | C | 3.88 | 2.96 | 3.92 | 3.00 | 3.91 | 2.98 |
| (HO) | C | 9.11 | … | 9.34 | … | 9.31 | … |
| Ar(HO) | C Face | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ar(HO) | C Face | 0.36 | 0.23 | 0.27 | 0.17 | 0.27 | 0.17 |
| Ar(HO) | C Edge | 1.34 | 0.90 | 1.39 | 0.98 | 1.26 | 0.87 |
| Ar(HO) | C Face | 3.10 | 1.75 | 3.40 | 2.00 | 3.37 | 1.98 |
| Ar(HO) | C Face | 4.17 | 2.28 | 4.18 | 2.41 | 4.16 | 2.39 |
| Ar(HO) | C Face | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ar(HO) | C Face | 3.50 | 2.70 | 3.55 | 2.73 | 3.56 | 2.74 |
| Ar(HO) | C Edge | 5.70 | 4.50 | 5.88 | 4.65 | 5.63 | 4.43 |
| Ar(HO) | C Face | 8.30 | 5.50 | 8.75 | 5.82 | 8.73 | 5.77 |
| Ar(HO) | C Face | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ar(HO) | C Face | 0.49 | 0.30 | 0.36 | 0.22 | 0.36 | 0.22 |
| Ar(HO) | C Edge | 2.90 | 2.44 | 3.03 | 2.58 | 2.71 | 2.31 |
| MP2 | 2b:Mb | 3b:Mb | ||||
|---|---|---|---|---|---|---|
| Label | ||||||
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||
| C Face | −3.88 | −3.10 | −3.99 | −3.21 | −3.84 | −3.09 |
| C Face | −3.52 | −2.87 | −3.73 | −3.04 | −3.57 | −2.92 |
| C Edge | −2.54 | −2.20 | −2.60 | −2.23 | −2.58 | −2.23 |
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||
| C Face | −4.02 | −3.07 | −4.04 | −3.18 | −3.90 | −3.06 |
| C Face | −2.95 | −2.53 | −3.26 | −2.77 | −3.11 | −2.65 |
| Binding Process: S (HO) + Ar → C Ar(HO) | ||||||
| C Face | −4.65 | −3.92 | −4.84 | −4.08 | −4.62 | −3.90 |
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||
| C Face | −5.03 | −4.18 | −5.21 | −4.34 | −4.98 | −4.16 |
| C Edge | −2.82 | −2.39 | −2.88 | −2.42 | −2.91 | −2.46 |
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||
| C Face | −5.46 | … | −5.43 | … | −5.20 | … |
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||
| C Face | −5.85 | −4.92 | −6.03 | −5.09 | −5.75 | −4.86 |
| C Face | −5.36 | −4.61 | −5.67 | −4.87 | −5.39 | −4.64 |
| C Edge | −2.95 | −2.47 | −3.01 | −2.51 | −3.04 | −2.55 |
| SAPT Components | ||||||||
|---|---|---|---|---|---|---|---|---|
| Label | MP2 | 2b:Mb | 3b:Mb | SAPT | Exch | Elect | Ind | Disp |
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||||
| C Face | −3.90 | −4.00 | −3.85 | −3.13 | +5.62 | −1.73 | −0.66 | −6.37 |
| C Face | −3.53 | −3.74 | −3.58 | −2.92 | +5.42 | −1.71 | −0.55 | −6.08 |
| C Edge | −2.54 | −2.60 | −2.58 | −2.04 | +3.66 | −1.09 | −0.40 | −4.22 |
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||||
| C Face | −4.03 | −4.04 | −3.90 | −3.08 | +5.41 | −1.63 | −0.53 | −6.33 |
| C Face | −2.95 | −3.26 | −3.11 | −2.43 | +4.96 | −1.60 | −0.20 | −5.59 |
| Binding Process: S (HO) + Ar → C Ar(HO) | ||||||||
| C Face | −4.71 | −4.90 | −4.66 | −3.71 | +6.94 | −2.17 | −0.39 | −8.08 |
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||||
| C Face | −5.05 | −5.23 | −4.99 | −4.08 | +7.20 | −2.23 | −0.77 | −8.28 |
| C Edge | −2.83 | −2.89 | −2.91 | −2.28 | +4.05 | −1.23 | −0.44 | −4.65 |
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||||
| C Face | −5.48 | −5.44 | −5.20 | −4.09 | +7.30 | −2.16 | −0.53 | −8.69 |
| Binding Process: C (HO) + Ar → C Ar(HO) | ||||||||
| C Face | −5.97 | −6.15 | −5.84 | −4.79 | +8.51 | −2.64 | −0.57 | −10.01 |
| C Face | −5.50 | −5.81 | −5.50 | −4.42 | +8.29 | −2.63 | −0.45 | −9.62 |
| C Edge | −2.96 | −3.01 | −3.05 | −2.38 | +4.28 | −1.31 | −0.49 | −4.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rock, C.A.; Tschumper, G.S. Insight into the Binding of Argon to Cyclic Water Clusters from Symmetry-Adapted Perturbation Theory. Int. J. Mol. Sci. 2023, 24, 17480. https://doi.org/10.3390/ijms242417480
Rock CA, Tschumper GS. Insight into the Binding of Argon to Cyclic Water Clusters from Symmetry-Adapted Perturbation Theory. International Journal of Molecular Sciences. 2023; 24(24):17480. https://doi.org/10.3390/ijms242417480
Chicago/Turabian StyleRock, Carly A., and Gregory S. Tschumper. 2023. "Insight into the Binding of Argon to Cyclic Water Clusters from Symmetry-Adapted Perturbation Theory" International Journal of Molecular Sciences 24, no. 24: 17480. https://doi.org/10.3390/ijms242417480
APA StyleRock, C. A., & Tschumper, G. S. (2023). Insight into the Binding of Argon to Cyclic Water Clusters from Symmetry-Adapted Perturbation Theory. International Journal of Molecular Sciences, 24(24), 17480. https://doi.org/10.3390/ijms242417480







