Tissue Injury Protection: The Other Face of Anticoagulant Treatments in the Context of Ischemia and Reperfusion Injury with a Focus on Transplantation
Abstract
:1. Introduction
2. Thrombo-Inflammation in Ischemia–Reperfusion Injuries
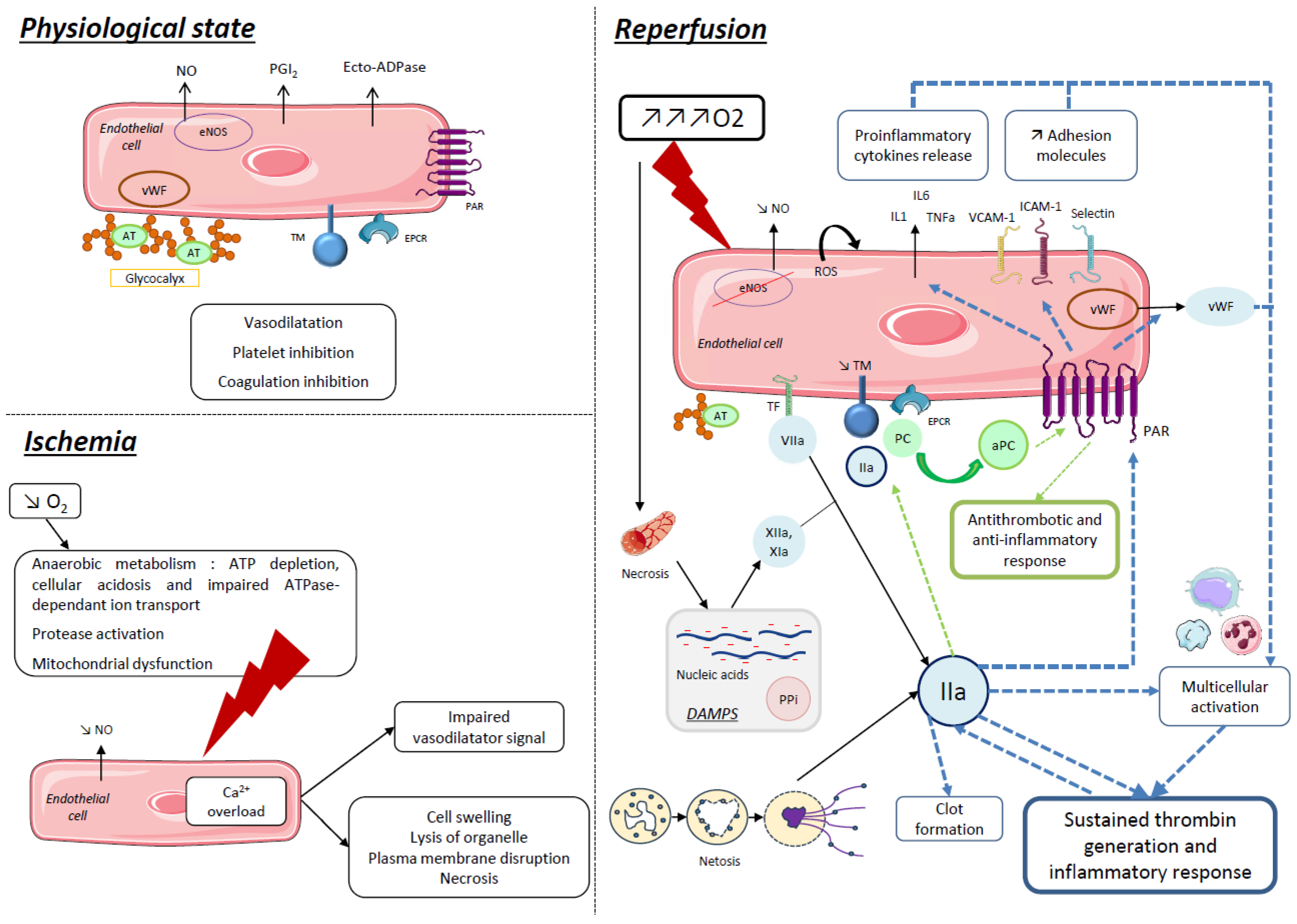
2.1. Ischemia Reperfusion Injury
2.2. Thrombo-Inflammation: Central Role of Endothelial Cells
2.3. Modulation of Thrombo-Inflammation by Coagulation Serine Proteases and Protease-Activated Receptors
2.3.1. Procoagulant Factors
2.3.2. Anticoagulant Factors
2.3.3. Protease-Activated Receptors
2.4. Ischemia–Reperfusion Injury and Thrombo-Inflammation in the Field of Transplantation
3. Anticoagulant Therapies and Ischemia–Reperfusion Injuries
3.1. Indirect Anticoagulant Therapies and Tissue Injury
3.1.1. Unfractionated Heparin
3.1.2. Low Molecular-Weight Heparin
3.1.3. Fondaparinux
3.2. Direct Anticoagulant Therapies and Tissue Injury
3.2.1. Xabans (Rivaroxaban, Apixaban)
3.2.2. Direct Thrombin Inhibitors
- Hirudin
- 2.
- Melagatran
- 3.
- Dabigatran
3.2.3. Inhibitors of the TF/FVIIa Complex
4. Anticoagulant Therapies in the Field of Transplantation
4.1. Risk of Bleeding
4.1.1. Association with the Multi-Organ Procurement Procedure (MOPP)
4.1.2. Association with Anticoagulant Agents
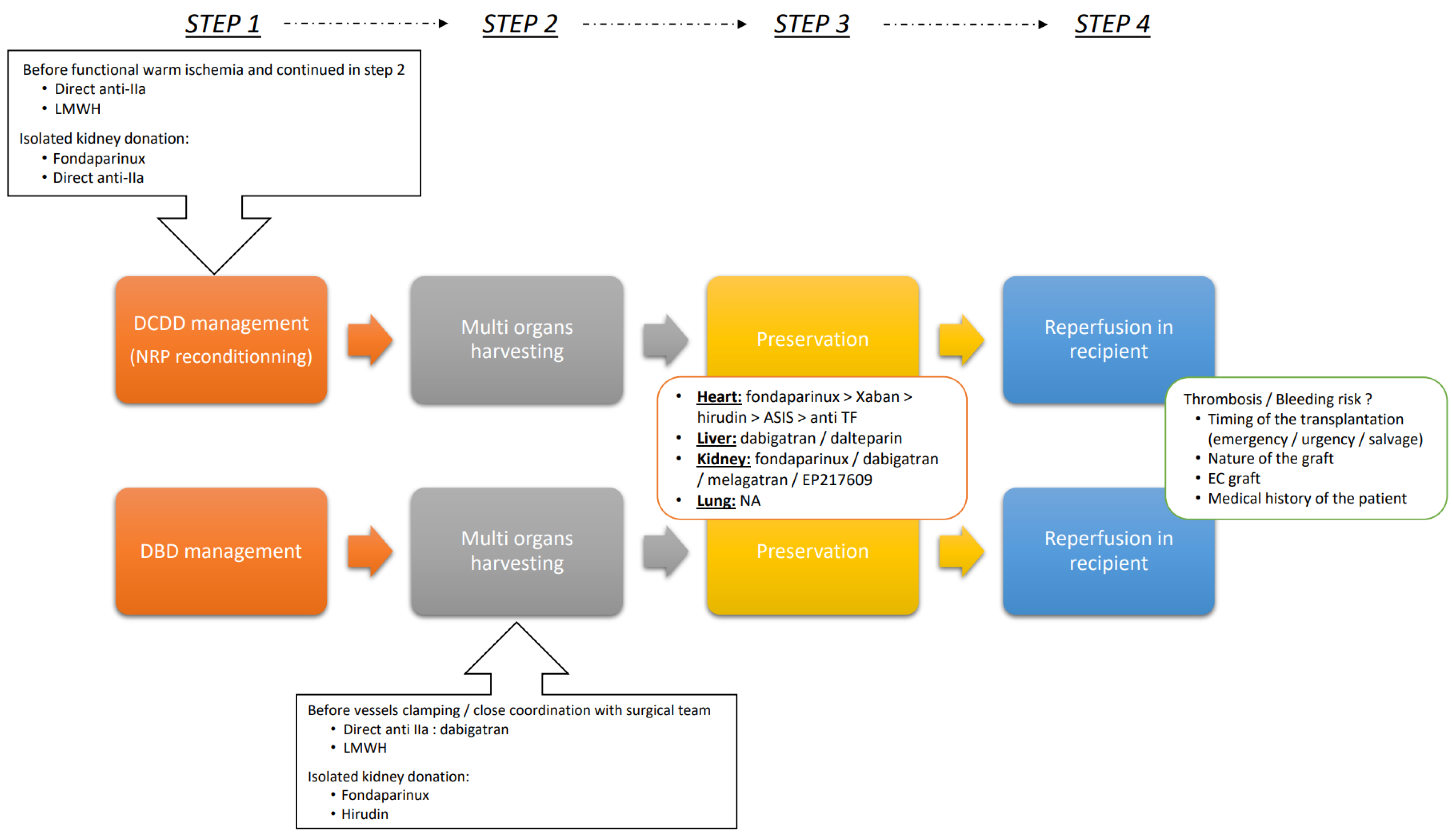
4.2. Normothermic In Situ and Ex Vivo Reconditioning
4.3. Optimizing Outcomes: A Strategic Guide to Implementing AC Therapies during Transplantation Procedures
- Deceased donor management
- Multi organs harvesting
- Preservation
- Reperfusion in recipient
5. Perspectives
5.1. Targeting Contact Phase
5.2. Targeting PAR Signaling
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wicks, P.; Sulham, K.A.; Gnanasakthy, A. Quality of Life in Organ Transplant Recipients Participating in an Online Transplant Community. Patient 2014, 7, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.S.; Shan, L.L.; Saxena, A.; Morris, D.L. Liver Transplantation: A Systematic Review of Long-term Quality of Life. Liver Int. 2014, 34, 1298–1313. [Google Scholar] [CrossRef] [PubMed]
- Bezinover, D.; Saner, F. Organ Transplantation in the Modern Era. BMC Anesth. 2019, 19, 32. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, A.C. Assessment of Quality of Life in Lung Transplantation Using a Simple Generic Tool. Thorax 2001, 56, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Merion, R.M.; Schaubel, D.E.; Dykstra, D.M.; Freeman, R.B.; Port, F.K.; Wolfe, R.A. The Survival Benefit of Liver Transplantation. Am. J. Transplant. 2005, 5, 307–313. [Google Scholar] [CrossRef] [PubMed]
- ABM. Rapport Médical et Scientifique. Available online: https://rams.agence-biomedecine.fr/ (accessed on 6 November 2023).
- Pessione, F.; Cohen, S.; Durand, D.; Hourmant, M.; Kessler, M.; Legendre, C.; Mourad, G.; Noël, C.; Peraldi, M.-N.; Pouteil-Noble, C.; et al. Multivariate Analysis of Donor Risk Factors for Graft Survival in Kidney Transplantation. Transplantation 2003, 75, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Bragg-Gresham, J.L.; Metzger, R.A.; Dykstra, D.M.; Gillespie, B.W.; Young, E.W.; Delmonico, F.L.; Wynn, J.J.; Merion, R.M.; Wolfe, R.A.; et al. Donor Characteristics Associated with Reduced Graft Survival: An Approach to Expanding the Pool of Kidney Donors. Transplantation 2002, 74, 1281–1286. [Google Scholar] [CrossRef]
- Merion, R.M. Expanded Criteria Donors for Kidney Transplantation. Transplant. Proc. 2005, 37, 3655–3657. [Google Scholar] [CrossRef]
- Smith, M.; Dominguez-Gil, B.; Greer, D.M.; Manara, A.R.; Souter, M.J. Organ Donation after Circulatory Death: Current Status and Future Potential. Intensive Care Med. 2019, 45, 310–321. [Google Scholar] [CrossRef]
- Lepoittevin, M.; Giraud, S.; Kerforne, T.; Allain, G.; Thuillier, R.; Hauet, T. How to Improve Results after DCD (Donation after Circulation Death). Presse Méd. 2022, 51, 104143. [Google Scholar] [CrossRef]
- Sandroni, C.; Cronberg, T.; Sekhon, M. Brain Injury after Cardiac Arrest: Pathophysiology, Treatment, and Prognosis. Intensive Care Med. 2021, 47, 1393–1414. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Hausenloy, D.J. Myocardial Reperfusion Injury. N. Engl. J. Med. 2007, 357, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.R.; Sánchez-Tarjuelo, R.; Cravedi, P.; Ochando, J.; López-Hoyos, M. Review: Ischemia Reperfusion Injury—A Translational Perspective in Organ Transplantation. Int. J. Mol. Sci. 2020, 21, 8549. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Castro, M.B.; Cornide-Petronio, M.E.; Gracia-Sancho, J.; Peralta, C. Inflammasome-Mediated Inflammation in Liver Ischemia-Reperfusion Injury. Cells 2019, 8, 1131. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Kim, M.; Brown, K.M.; D’Agati, V.D.; Lee, H.T. Paneth Cell-Derived Interleukin-17A Causes Multiorgan Dysfunction after Hepatic Ischemia and Reperfusion Injury. Hepatology 2011, 53, 1662–1675. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion—From Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kalogeris, T.; Korthuis, R.J. Reactive Species-Induced Microvascular Dysfunction in Ischemia/Reperfusion. Free Radic. Biol. Med. 2019, 135, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Darbousset, R.; Schoenwaelder, S.M. Thromboinflammation: Challenges of Therapeutically Targeting Coagulation and Other Host Defense Mechanisms. Blood 2019, 133, 906–918. [Google Scholar] [CrossRef]
- Neubauer, K.; Zieger, B. Endothelial Cells and Coagulation. Cell Tissue Res. 2022, 387, 391–398. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 113–170. ISBN 978-0-470-65071-4. [Google Scholar]
- Barba, J.; Zudaire, J.J.; Robles, J.E.; Tienza, A.; Rosell, D.; Berián, J.M.; Pascual, I. ¿Existe un intervalo de tiempo de isquemia fría seguro para el injerto renal? Actas Urol. Esp. 2011, 35, 475–480. [Google Scholar] [CrossRef]
- Sert, I.; Colak, H.; Tugmen, C.; Dogan, S.; Karaca, C. The Effect of Cold Ischemia Time on Delayed Graft Function and Acute Rejection in Kidney Transplantation. Saudi J. Kidney Dis. Transpl. 2014, 25, 960. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How It All Starts: Initiation of the Clotting Cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Gajsiewicz, J.M.; Smith, S.A.; Morrissey, J.H. Polyphosphate and RNA Differentially Modulate the Contact Pathway of Blood Clotting. J. Biol. Chem. 2017, 292, 1808–1814. [Google Scholar] [CrossRef]
- Okada, M.; Suzuki, K.; Takada, K.; Nakashima, M.; Nakanishi, T.; Shinohara, T. Detection of Up-Regulated Genes in Thrombin-Stimulated Human Umbilical Vein Endothelial Cells. Thromb. Res. 2006, 118, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Ziętek, Z. Endothelial Markers: Thrombomodulin and Von Willebrand Factor and Risk of Kidney Thrombosis After Transplantation. Transplant. Proc. 2021, 53, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Ammollo, C.T.; Morrissey, J.H.; Dale, G.L.; Friese, P.; Esmon, N.L.; Esmon, C.T. Extracellular Histones Promote Thrombin Generation through Platelet-Dependent Mechanisms: Involvement of Platelet TLR2 and TLR4. Blood 2011, 118, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Folco, E.J.; Mawson, T.L.; Vromman, A.; Bernardes-Souza, B.; Franck, G.; Persson, O.; Nakamura, M.; Newton, G.; Luscinskas, F.W.; Libby, P. Neutrophil Extracellular Traps Induce Endothelial Cell Activation and Tissue Factor Production Through Interleukin-1α and Cathepsin G. Arter. Thromb. Vasc. Biol. 2018, 38, 1901–1912. [Google Scholar] [CrossRef]
- Di Minno, M.N.D.; Ambrosino, P.; Ageno, W.; Rosendaal, F.; Di Minno, G.; Dentali, F. Natural Anticoagulants Deficiency and the Risk of Venous Thromboembolism: A Meta-Analysis of Observational Studies. Thromb. Res. 2015, 135, 923–932. [Google Scholar] [CrossRef]
- Reitsma, S.; Slaaf, D.W.; Vink, H.; Van Zandvoort, M.A.M.J.; Oude Egbrink, M.G.A. The Endothelial Glycocalyx: Composition, Functions, and Visualization. Pflug. Arch. Eur. J. Physiol. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Schiefer, J.; Lebherz-Eichinger, D.; Erdoes, G.; Berlakovich, G.; Bacher, A.; Krenn, C.G.; Faybik, P. Alterations of Endothelial Glycocalyx During Orthotopic Liver Transplantation in Patients with End-Stage Liver Disease. Transplantation 2015, 99, 2118–2123. [Google Scholar] [CrossRef]
- Schiefer, J.; Faybik, P.; Koch, S.; Tudor, B.; Kollmann, D.; Kuessel, L.; Krenn, C.G.; Berlakovich, G.; Baron, D.M.; Baron-Stefaniak, J. Glycocalyx Damage within Human Liver Grafts Correlates with Graft Injury and Postoperative Graft Function After Orthotopic Liver Transplantation. Transplantation 2020, 104, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sladden, T.M.; Yerkovich, S.; Grant, M.; Zhang, F.; Liu, X.; Trotter, M.; Hopkins, P.; Linhardt, R.J.; Chambers, D.C. Endothelial Glycocalyx Shedding Predicts Donor Organ Acceptability and Is Associated with Primary Graft Dysfunction in Lung Transplant Recipients. Transplantation 2019, 103, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Castellino, F.J.; Ploplis, V.A. The Protein C Pathway and Pathologic Processes. J. Thromb. Haemost. 2009, 7, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Sohn, R.H.; Deming, C.B.; Johns, D.C.; Champion, H.C.; Bian, C.; Gardner, K.; Rade, J.J. Regulation of Endothelial Thrombomodulin Expression by Inflammatory Cytokines Is Mediated by Activation of Nuclear Factor-Kappa B. Blood 2005, 105, 3910–3917. [Google Scholar] [CrossRef] [PubMed]
- Dufourcq, P.; Seigneur, M.; Pruvost, A.; Dumain, P.; Belloc, F.; Amiral, J.; Boisseau, M.R. Membrane Thrombomodulin Levels Are Decreased during Hypoxia and Restored by cAMP and IBMX. Thromb. Res. 1995, 77, 305–310. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Tashiro, H.; Kuroda, S.; Okimoto, S.; Kobayashi, T.; Hinoi, T.; Ohdan, H. Downregulation of Thrombomodulin Contributes to Ischemia-reperfusion Injury in Mice with Steatotic Liver. Hepatol. Res. 2022, 52, 762–772. [Google Scholar] [CrossRef]
- Ossovskaya, V.S.; Bunnett, N.W. Protease-Activated Receptors: Contribution to Physiology and Disease. Physiol. Rev. 2004, 84, 579–621. [Google Scholar] [CrossRef]
- Künze, G.; Isermann, B. Targeting Biased Signaling by PAR1: Function and Molecular Mechanism of Parmodulins. Blood 2023, 141, 2675–2684. [Google Scholar] [CrossRef]
- Tena, B.; Vendrell, M. Perioperative Considerations for Kidney and Pancreas-Kidney Transplantation. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 3–14. [Google Scholar] [CrossRef]
- Sakai, T.; Ko, J.S.; Crouch, C.E.; Kumar, S.; Little, M.B.; Chae, M.S.; Ganoza, A.; Gómez-Salinas, L.; Humar, A.; Kim, S.H.; et al. Perioperative Management of Adult Living Donor Liver Transplantation: Part 1—Recipients. Clin. Transplant. 2022, 36, e14667. [Google Scholar] [CrossRef]
- Iske, J.; Schroeter, A.; Knoedler, S.; Nazari-Shafti, T.Z.; Wert, L.; Roesel, M.J.; Hennig, F.; Niehaus, A.; Kuehn, C.; Ius, F.; et al. Pushing the Boundaries of Innovation: The Potential of Ex Vivo Organ Perfusion from an Interdisciplinary Point of View. Front. Cardiovasc. Med. 2023, 10, 1272945. [Google Scholar] [CrossRef]
- Sharfuddin, A.A.; Sandoval, R.M.; Berg, D.T.; McDougal, G.E.; Campos, S.B.; Phillips, C.L.; Jones, B.E.; Gupta, A.; Grinnell, B.W.; Molitoris, B.A. Soluble Thrombomodulin Protects Ischemic Kidneys. J. Am. Soc. Nephrol. 2009, 20, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Legrand, M.; Mik, E.G.; Johannes, T.; Payen, D.; Ince, C. Renal Hypoxia and Dysoxia After Reperfusion of the Ischemic Kidney. Mol. Med. 2008, 14, 502–516. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral Anticoagulants. Chest 2012, 141, e24S–e43S. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.P.; Byrne, R.A.; Patel, R.K.; Arya, R. Progress in the Monitoring of Direct Oral Anticoagulant Therapy. Br. J. Haematol. 2019, 184, 912–924. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.; Samama, C.-M.; Greinacher, A.; Hunt, B.J. The Utility of Viscoelastic Methods in the Prevention and Treatment of Bleeding and Hospital-associated Venous Thromboembolism in Perioperative Care: Guidance from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- Friedrichs, G.S.; Kilgore, K.S.; Manley, P.J.; Gralinski, M.R.; Lucchesi, B.R. Effects of Heparin and N-Acetyl Heparin on Ischemia/Reperfusion-Induced Alterations in Myocardial Function in the Rabbit Isolated Heart. Circ. Res. 1994, 75, 701–710. [Google Scholar] [CrossRef]
- Black, S.C.; Gralinski, M.R.; Friedrichs, G.S.; Kilgore, K.S.; Driscoll, E.M.; Lucchesi, B.R. Cardioprotective Effects of Heparin or N-Acetylheparin in an in Vivo Model of Myocardial Ischaemic and Reperfusion Injury. Cardiovasc. Res. 1995, 29, 629–636. [Google Scholar] [CrossRef]
- Libersan, D.; Khalil, A.; Dagenais, P.; Quan, E.; Delorme, F.; Uzan, A.; Latour, J.G. The Low Molecular Weight Heparin, Enoxaparin, Limits Infarct Size at Reperfusion in the Dog. Cardiovasc. Res. 1998, 37, 656–666. [Google Scholar] [CrossRef]
- Kouretas, P.C.; Kim, Y.D.; Cahill, P.A.; Myers, A.K.; To, L.N.; Wang, Y.N.; Wallace, R.B.; Kron, I.L.; Hannan, R.L. Heparin Preserves Nitric Oxide Activity in Coronary Endothelium during Ischemia-Reperfusion Injury. Ann. Thorac. Surg. 1998, 66, 1210–1215. [Google Scholar] [CrossRef]
- Liu, W.; Pitcher, D.E.; Morris, S.L.; Pugmire, J.E.; Shores, J.T.; Ingram, C.E.; Glew, R.H.; Morris, D.M.; Fry, D.E. Differential Effects of Heparin on the Early and Late Phases of Hepatic Ischemia and Reperfusion Injury in the Pig. Shock 1999, 12, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Yamaguchi, M.; Kikuchi, H.; Nakano, H.; Midorikawa, T.; Kumada, K.; Takeda, M. Heparin Reduces Serum Levels of Endothelin-1 and Hepatic Ischemia Reperfusion Injury in Rabbits. Surg. Today 2000, 30, 523–525. [Google Scholar] [CrossRef]
- Schoots, I.G.; Levi, M.; van Vliet, A.K.; Maas, A.M.; Roossink, E.H.P.; van Gulik, T.M. Inhibition of Coagulation and Inflammation by Activated Protein C or Antithrombin Reduces Intestinal Ischemia/Reperfusion Injury in Rats. Crit. Care Med. 2004, 32, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, K.; Spellman, S.R.; McCarthy, J.B.; Low, W.C.; Camarata, P.J. Reduction of Brain Injury Using Heparin to Inhibit Leukocyte Accumulation in a Rat Model of Transient Focal Cerebral Ischemia. II. Dose-Response Effect and the Therapeutic Window. J. Neurosurg. 1996, 85, 1108–1112. [Google Scholar] [CrossRef]
- Gralinski, M.R.; Driscoll, E.M.; Friedrichs, G.S.; DeNardis, M.R.; Lucchesi, B.R. Reduction of Myocardial Necrosis After Glycosaminoglycan Administration: Effects of a Single Intravenous Administration of Heparin or N-Acetylheparin 2 Hours Before Regional Ischemia and Reperfusion. J. Cardiovasc. Pharmacol. Ther. 1996, 1, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Thourani, V.H.; Brar, S.S.; Kennedy, T.P.; Thornton, L.R.; Watts, J.A.; Ronson, R.S.; Zhao, Z.-Q.; Sturrock, A.L.; Hoidal, J.R.; Vinten-Johansen, J. Nonanticoagulant Heparin Inhibits NF-κB Activation and Attenuates Myocardial Reperfusion Injury. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H2084–H2093. [Google Scholar] [CrossRef] [PubMed]
- Walensi, M.; de Groot, H.; Schulz, R.; Hartmann, M.; Petrat, F. Mesenteric Ischemia-Reperfusion Injury: Clearly Improved Hemodynamics but Only Minor Protection of the Rat Small Intestine by (Sub)Therapeutic Heparin Sodium and Enoxaparin Doses. J. Surg. Res. 2013, 179, e57–e69. [Google Scholar] [CrossRef]
- Yazici, S.; Karahan, O.; Oral, M.K.; Bayramoğlu, Z.; Unal, M.; Caynak, B.; Sagbas, E. Comparison of Renoprotective Effect of Dabigatran with Low-Molecular-Weight Heparin. Clin. Appl. Thromb. Hemost. 2016, 22, 361–365. [Google Scholar] [CrossRef]
- Harada, N.; Okajima, K.; Uchiba, M. Dalteparin, a Low Molecular Weight Heparin, Attenuates Inflammatory Responses and Reduces Ischemia-Reperfusion-Induced Liver Injury in Rats. Crit. Care Med. 2006, 34, 1883–1891. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Cheng, Y.; Ji, S.; Du, G. Antagonistic Effects of Ultra-Low-Molecular-Weight Heparin against Cerebral Ischemia/Reperfusion Injury in Rats. Pharmacol. Res. 2007, 56, 350–355. [Google Scholar] [CrossRef]
- Gottmann, U.; Mueller-Falcke, A.; Schnuelle, P.; Birck, R.; Nickeleit, V.; van der Woude, F.J.; Yard, B.A.; Braun, C. Influence of Hypersulfated and Low Molecular Weight Heparins on Ischemia/Reperfusion: Injury and Allograft Rejection in Rat Kidneys. Transpl. Int. 2007, 20, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.D.; Schabbauer, G.; Holscher, T.; Sato, Y.; Tencati, M.; Pawlinski, R.; Mackman, N. The Synthetic Pentasaccharide Fondaparinux Reduces Coagulation, Inflammation and Neutrophil Accumulation in Kidney Ischemia-Reperfusion Injury. J. Thromb. Haemost. 2005, 3, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Olanders, K.; Borjesson, A.; Zhao, X.; Andersson, R. Effects of Anticoagulant Treatment on Intestinal Ischaemia and Reperfusion Injury in Rats. Acta Anaesthesiol. Scand. 2005, 49, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Marechal, X.; Lancel, S.; Decoster, B.; Asseman, P.; Neviere, R. The Synthetic Pentasaccharide Fondaparinux Prevents Coronary Microvascular Injury and Myocardial Dysfunction in the Ischemic Heart. Thromb. Haemost. 2008, 100, 912–919. [Google Scholar] [PubMed]
- Macchi, L.; Moussa, W.B.; Guillou, S.; Tamareille, S.; Lamon, D.; Prunier, D.; Prunier, F. The Synthetic Pentasaccharide Fondaparinux Attenuates Myocardial Ischemia-Reperfusion Injury in Rats via STAT-3. Shock 2014, 41, 166–171. [Google Scholar] [CrossRef]
- Tillet, S.; Giraud, S.; Delpech, P.O.; Thuillier, R.; Ameteau, V.; Goujon, J.M.; Renelier, B.; Macchi, L.; Hauet, T.; Mauco, G. Kidney Graft Outcome Using an Anti-Xa Therapeutic Strategy in an Experimental Model of Severe Ischaemia-Reperfusion Injury. Br. J. Surg. 2015, 102, 132–142; discussion 142. [Google Scholar] [CrossRef]
- Iba, T.; Aihara, K.; Yamada, A.; Nagayama, M.; Tabe, Y.; Ohsaka, A. Rivaroxaban Attenuates Leukocyte Adhesion in the Microvasculature and Thrombus Formation in an Experimental Mouse Model of Type 2 Diabetes Mellitus. Thromb. Res. 2014, 133, 276–280. [Google Scholar] [CrossRef]
- Kono, S.; Yamashita, T.; Deguchi, K.; Omote, Y.; Yunoki, T.; Sato, K.; Kurata, T.; Hishikawa, N.; Abe, K. Rivaroxaban and Apixaban Reduce Hemorrhagic Transformation After Thrombolysis by Protection of Neurovascular Unit in Rat. Stroke 2014, 45, 2404–2410. [Google Scholar] [CrossRef]
- Dittmeier, M.; Kraft, P.; Schuhmann, M.K.; Fluri, F.; Kleinschnitz, C. Pretreatment with Rivaroxaban Attenuates Stroke Severity in Rats by a Dual Antithrombotic and Anti-Inflammatory Mechanism. Thromb. Haemost. 2016, 115, 835–843. [Google Scholar] [CrossRef]
- Morihara, R.; Yamashita, T.; Kono, S.; Shang, J.; Nakano, Y.; Sato, K.; Hishikawa, N.; Ohta, Y.; Heitmeier, S.; Perzborn, E.; et al. Reduction of Intracerebral Hemorrhage by Rivaroxaban after tPA Thrombolysis Is Associated with Downregulation of PAR-1 and PAR-2: Downregulation of PAR-1 and PAR-2 by Rivaroxaban. J. Neurosci. Res. 2017, 95, 1818–1828. [Google Scholar] [CrossRef]
- Guillou, S.; Beaumont, J.; Tamareille, S.; Giraud, S.; Mirebeau-Prunier, D.; Prunier, F.; Macchi, L. Direct Rivaroxaban-Induced Factor XA Inhibition Proves to Be Cardioprotective in Rats. Shock. 2020, 53, 730–736. [Google Scholar] [CrossRef]
- Erlich, J.H.; Boyle, E.M.; Labriola, J.; Kovacich, J.C.; Santucci, R.A.; Fearns, C.; Morgan, E.N.; Yun, W.; Luther, T.; Kojikawa, O.; et al. Inhibition of the Tissue Factor-Thrombin Pathway Limits Infarct Size after Myocardial Ischemia-Reperfusion Injury by Reducing Inflammation. Am. J. Pathol. 2000, 157, 1849–1862. [Google Scholar] [CrossRef] [PubMed]
- Karabiyikoglu, M.; Hua, Y.; Keep, R.F.; Ennis, S.R.; Xi, G. Intracerebral Hirudin Injection Attenuates Ischemic Damage and Neurologic Deficits without Altering Local Cerebral Blood Flow. J. Cereb. Blood Flow. Metab. 2004, 24, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Sevastos, J.; Kennedy, S.E.; Davis, D.R.; Sam, M.; Peake, P.W.; Charlesworth, J.A.; Mackman, N.; Erlich, J.H. Tissue Factor Deficiency and PAR-1 Deficiency Are Protective against Renal Ischemia Reperfusion Injury. Blood 2007, 109, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Nitescu, N.; Grimberg, E.; Ricksten, S.-E.; Marcussen, N.; Guron, G. Thrombin Inhibition with Melagatran Does Not Attenuate Renal Ischaemia-Reperfusion Injury in Rats. Nephrol. Dial. Transpl. 2007, 22, 2149–2155. [Google Scholar] [CrossRef] [PubMed]
- Giraud, S.; Thuillier, R.; Belliard, A.; Hebrard, W.; Nadeau, C.; Milin, S.; Goujon, J.-M.; Manguy, E.; Mauco, G.; Hauet, T.; et al. Direct Thrombin Inhibitor Prevents Delayed Graft Function in a Porcine Model of Renal Transplantation. Transplantation 2009, 87, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhou, W.; Ploen, R.; Zorn, M.; Veltkamp, R. Anticoagulation with Dabigatran Does Not Increase Secondary Intracerebral Haemorrhage after Thrombolysis in Experimental Cerebral Ischaemia. Thromb. Haemost. 2013, 110, 153–161. [Google Scholar] [CrossRef]
- Kono, S.; Deguchi, K.; Omote, Y.; Yunoki, T.; Yamashita, T.; Kurata, T.; Ikeda, Y.; Abe, K. Reducing Hemorrhagic Complication by Dabigatran via Neurovascular Protection after Recanalization with Tissue Plasminogen Activator in Ischemic Stroke of Rat. J. Neurosci. Res. 2014, 92, 46–53. [Google Scholar] [CrossRef]
- Hale, S.L.; Kloner, R.A. Dabigatran Treatment: Effects on Infarct Size and the No-Reflow Phenomenon in a Model of Acute Myocardial Ischemia/Reperfusion. J. Thromb. Thrombolysis 2015, 39, 50–54. [Google Scholar] [CrossRef]
- Noguchi, D.; Kuriyama, N.; Hibi, T.; Maeda, K.; Shinkai, T.; Gyoten, K.; Hayasaki, A.; Fujii, T.; Iizawa, Y.; Tanemura, A.; et al. The Impact of Dabigatran Treatment on Sinusoidal Protection Against Hepatic Ischemia/Reperfusion Injury in Mice. Liver Transpl. 2021, 27, 363–384. [Google Scholar] [CrossRef]
- Golino, P.; Ragni, M.; Cirillo, P.; Scognamiglio, A.; Ravera, A.; Buono, C.; Guarino, A.; Piro, O.; Lambiase, C.; Botticella, F.; et al. Recombinant Human, Active Site-Blocked Factor VIIa Reduces Infarct Size and No-Reflow Phenomenon in Rabbits. Am. J. Physiol. Heart Circ. Physiol. 2000, 278, H1507–H1516. [Google Scholar] [CrossRef] [PubMed]
- Loubele, S.T.B.G.; Spek, C.A.; Leenders, P.; Van Oerle, R.; Aberson, H.L.; Van Der Voort, D.; Hamulyák, K.; Petersen, L.C.; Spronk, H.M.H.; Ten Cate, H. Active Site Inhibited Factor VIIa Attenuates Myocardial Ischemia/Reperfusion Injury in Mice. J. Thromb. Haemost. 2009, 7, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Tillet, S.; Giraud, S.; Kerforne, T.; Saint-Yves, T.; Joffrion, S.; Goujon, J.-M.; Cau, J.; Mauco, G.; Petitou, M.; Hauet, T. Inhibition of Coagulation Proteases Xa and IIa Decreases Ischemia–Reperfusion Injuries in a Preclinical Renal Transplantation Model. Transl. Res. 2016, 178, 95–106.e1. [Google Scholar] [CrossRef] [PubMed]
- Frank, R.D.; Holscher, T.; Schabbauer, G.; Tencati, M.; Pawlinski, R.; Weitz, J.I.; Mackman, N. A Non-Anticoagulant Synthetic Pentasaccharide Reduces Inflammation in a Murine Model of Kidney Ischemia-Reperfusion Injury. Thromb. Haemost. 2006, 96, 802–806. [Google Scholar] [CrossRef]
- Guillou, S.; Tamareille, S.; Giraud, S.; Poitevin, G.; Prunier-Mirebeau, D.; Nguyen, P.; Prunier, F.; Macchi, L. Fondaparinux Upregulates Thrombomodulin and the Endothelial Protein C Receptor during Early-Stage Reperfusion in a Rat Model of Myocardial Infarction. Thromb. Res. 2016, 141, 98–103. [Google Scholar] [CrossRef]
- Shang, J.; Yamashita, T.; Kono, S.; Morihara, R.; Nakano, Y.; Fukui, Y.; Li, X.; Hishikawa, N.; Ohta, Y.; Abe, K. Effects of Pretreatment with Warfarin or Rivaroxaban on Neurovascular Unit Dissociation after Tissue Plasminogen Activator Thrombolysis in Ischemic Rat Brain. J. Stroke Cerebrovasc. Dis. 2016, 25, 1997–2003. [Google Scholar] [CrossRef]
- McCourtie, A.S.; Merry, H.E.; Wolf, P.S.; FitzSullivan, E.; Keech, J.C.; Farivar, A.S.; Mulligan, M.S. Synergistic Protection in Lung Ischemia-Reperfusion Injury with Calcineurin and Thrombin Inhibition. Ann. Thorac. Surg. 2010, 89, 1766–1771. [Google Scholar] [CrossRef]
- Habazettl, H.; Lindert, J.; Baeter, S.; Neumann, K.; Kuppe, H.; Kuebler, W.M.; Pries, A.R.; Koster, A. Effects of Unfractionated Heparin, Low Molecular Weight Heparin and r-Hirudin on Leukocyte Adhesion in Ischemia/Reperfusion. Blood Coagul. Fibrinolysis 2004, 15, 375. [Google Scholar] [CrossRef]
- Thuillier, R.; Favreau, F.; Celhay, O.; Macchi, L.; Milin, S.; Hauet, T. Thrombin Inhibition during Kidney Ischemia-Reperfusion Reduces Chronic Graft Inflammation and Tubular Atrophy. Transplantation 2010, 90, 612–621. [Google Scholar] [CrossRef]
- Favreau, F.; Thuillier, R.; Cau, J.; Milin, S.; Manguy, E.; Mauco, G.; Zhu, X.; Lerman, L.O.; Hauet, T. Anti-Thrombin Therapy during Warm Ischemia and Cold Preservation Prevents Chronic Kidney Graft Fibrosis in a DCD Model. Am. J. Transpl. 2010, 10, 30–39. [Google Scholar] [CrossRef]
- Bonhomme, F.; Ajzenberg, N.; Schved, J.-F.; Molliex, S.; Samama, C.-M. Pre-Interventional Haemostatic Assessment: Guidelines from the French Society of Anaesthesia and Intensive Care. Eur. J. Anaesthesiol. 2013, 30, 142–162. [Google Scholar] [CrossRef]
- Cosmi, B.; Alatri, A.; Cattaneo, M.; Gresele, P.; Marietta, M.; Rodeghiero, F.; Tripodi, A.; Ansaloni, L.; Fusari, M.; Taddei, S. Assessment of the Risk of Bleeding in Patients Undergoing Surgery or Invasive Procedures: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET). Thromb. Res. 2009, 124, e6–e12. [Google Scholar] [CrossRef] [PubMed]
- Chee, Y.L.; Crawford, J.C.; Watson, H.G.; Greaves, M. Guidelines on the Assessment of Bleeding Risk Prior to Surgery or Invasive Procedures: British Committee for Standards in Haematology. Br. J. Haematol. 2008, 140, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Apfelbaum, J.L.; Connis, R.T.; Nickinovich, D.G.; Pasternak, L.R.; Arens, J.F.; Caplan, R.A.; Fleisher, L.A.; Flowerdew, R.; Gold, B.S.; Mayhew, J.F.; et al. Practice Advisory for Preanesthesia Evaluation: An Updated Report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012, 116, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Fletcher-Sandersjöö, A.; Thelin, E.P.; Maegele, M.; Svensson, M.; Bellander, B.-M. Time Course of Hemostatic Disruptions After Traumatic Brain Injury: A Systematic Review of the Literature. Neurocrit Care 2021, 34, 635–656. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Kozar, R.; Zhang, J.; Dong, J. Diverse Activities of von Willebrand Factor in Traumatic Brain Injury and Associated Coagulopathy. J. Thromb. Haemost. 2020, 18, 3154–3162. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, S.; Yamazaki, M.; Hara, Y.; Iwata, M. Alterations of Platelet, Coagulation, and Fibrinolysis Markers in Patients with Acute Ischemic Stroke. Semin. Thromb. Hemost. 1997, 23, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.F.J.; Lai, K.W.; Tsang, S.F. Value of Preoperative Coagulation Tests: Reappraisal of Major Noncardiac Surgery. World J. Surg. 2002, 26, 515–520. [Google Scholar] [CrossRef]
- Boutin, C.; Vachiéry-Lahaye, F.; Alonso, S.; Louart, G.; Bouju, A.; Lazarovici, S.; Perrigault, P.-F.; Capdevila, X.; Jaber, S.; Colson, P.; et al. Pratiques anesthésiques pour prélèvement d’organes chez le sujet en mort encéphalique et pronostic du greffon rénal. Ann. Fr. Anesth. Réanim. 2012, 31, 427–436. [Google Scholar] [CrossRef]
- Chhaya, V.Y.; Kibbe, M.R. What Surgeons Need to Know About the Recent Updates to the CHEST Guidelines on Antithrombotic Therapy for Venous Thromboembolic Disease and Perioperative Management of Antithrombotic Therapy. Ann. Surg. 2023, 278, e695–e697. [Google Scholar] [CrossRef]
- Douketis, J.D.; Spyropoulos, A.C.; Murad, M.H.; Arcelus, J.I.; Dager, W.E.; Dunn, A.S.; Fargo, R.A.; Levy, J.H.; Samama, C.M.; Shah, S.H.; et al. Perioperative Management of Antithrombotic Therapy. CHEST 2022, 162, e207–e243. [Google Scholar] [CrossRef]
- Shore-Lesserson, L.; Baker, R.A.; Ferraris, V.; Greilich, P.E.; Fitzgerald, D.; Roman, P.; Hammon, J. STS/SCA/AmSECT Clinical Practice Guidelines: Anticoagulation during Cardiopulmonary Bypass. J. Extra Corpor. Technol. 2018, 50, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Matar, C.F.; Kahale, L.A.; Hakoum, M.B.; Tsolakian, I.G.; Etxeandia-Ikobaltzeta, I.; Yosuico, V.E.; Terrenato, I.; Sperati, F.; Barba, M.; Schünemann, H.; et al. Anticoagulation for Perioperative Thromboprophylaxis in People with Cancer. Cochrane Database Syst. Rev. 2018, 2019, CD009447. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.H.; ElShafei, M.N.; Ahmed, M.B.; Abdalla, L.O.; Ahmed, I.; Elzouki, A.-N.; Danjuma, M.I.-M. The Net Clinical Benefit of Rivaroxaban Compared to Low-Molecular-Weight Heparin in the Treatment of Cancer-Associated Thrombosis: Systematic Review and Meta-Analysis. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029620940046. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Hessheimer, A.J.; Neyrinck, A.P.; Paredes, D.; Bellini, M.I.; Dark, J.H.; Kimenai, H.J.A.N.; Pengel, L.H.M.; Watson, C.J.E.; ESOT Workstream 04 of the TLJ (Transplant Learning Journey) Project. Consensus Statement on Normothermic Regional Perfusion in Donation after Circulatory Death: Report from the European Society for Organ Transplantation’s Transplant Learning Journey. Transpl. Int. 2021, 34, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, S.; Simón, C.; Santamaria, X. Normothermic Machine Perfusion Systems: Where Do We Go From Here? Transplantation 2023, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, I.H.; Fredenburgh, J.C.; Hirsh, J.; Weitz, J.I. Medical Device-induced Thrombosis: What Causes It and How Can We Prevent It? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef]
- Loike, J.D.; Sodeik, B.; Cao, L.; Leucona, S.; Weitz, J.I.; Detmers, P.A.; Wright, S.D.; Silverstein, S.C. CD11c/CD18 on Neutrophils Recognizes a Domain at the N Terminus of the A Alpha Chain of Fibrinogen. Proc. Natl. Acad. Sci. USA 1991, 88, 1044–1048. [Google Scholar] [CrossRef]
- Amara, U.; Rittirsch, D.; Flierl, M.; Bruckner, U.; Klos, A.; Gebhard, F.; Lambris, J.D.; Huber-Lang, M. Interaction between the Coagulation and Complement System. Adv. Exp. Med. Biol. 2008, 632, 71–79. [Google Scholar] [CrossRef]
- Tsai, W.B.; Grunkemeier, J.M.; Horbett, T.A. Human Plasma Fibrinogen Adsorption and Platelet Adhesion to Polystyrene. J. Biomed. Mater. Res. 1999, 44, 130–139. [Google Scholar] [CrossRef]
- Srivastava, P.; Gailani, D. The Rebirth of the Contact Pathway: A New Therapeutic Target. Curr. Opin. Hematol. 2020, 27, 311–319. [Google Scholar] [CrossRef]
- Larsson, M.; Rayzman, V.; Nolte, M.W.; Nickel, K.F.; Björkqvist, J.; Jämsä, A.; Hardy, M.P.; Fries, M.; Schmidbauer, S.; Hedenqvist, P.; et al. A Factor XIIa Inhibitory Antibody Provides Thromboprotection in Extracorporeal Circulation without Increasing Bleeding Risk. Sci. Transl. Med. 2014, 6, 222ra17. [Google Scholar] [CrossRef] [PubMed]
- Mailer, R.K.; Rangaswamy, C.; Konrath, S.; Emsley, J.; Renné, T. An Update on Factor XII-Driven Vascular Inflammation. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119166. [Google Scholar] [CrossRef] [PubMed]
- Tricoci, P.; Huang, Z.; Held, C.; Moliterno, D.J.; Armstrong, P.W.; Van De Werf, F.; White, H.D.; Aylward, P.E.; Wallentin, L.; Chen, E.; et al. Thrombin-Receptor Antagonist Vorapaxar in Acute Coronary Syndromes. N. Engl. J. Med. 2012, 366, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Dowal, L.; Sim, D.S.; Dilks, J.R.; Blair, P.; Beaudry, S.; Denker, B.M.; Koukos, G.; Kuliopulos, A.; Flaumenhaft, R. Identification of an Antithrombotic Allosteric Modulator That Acts through Helix 8 of PAR1. Proc. Natl. Acad. Sci. USA 2011, 108, 2951–2956. [Google Scholar] [CrossRef]
- Gandhi, D.M.; Rosas, R.; Greve, E.; Kentala, K.; Diby, N.D.-R.; Snyder, V.A.; Stephans, A.; Yeung, T.H.W.; Subramaniam, S.; DiMilo, E.; et al. The Parmodulin NRD-21 Is an Allosteric Inhibitor of PAR1 Gq Signaling with Improved Anti-Inflammatory Activity and Stability. Bioorg. Med. Chem. 2019, 27, 3788–3796. [Google Scholar] [CrossRef]
- Nazir, S.; Gadi, I.; Al-Dabet, M.M.; Elwakiel, A.; Kohli, S.; Ghosh, S.; Manoharan, J.; Ranjan, S.; Bock, F.; Braun-Dullaeus, R.C.; et al. Cytoprotective Activated Protein C Averts Nlrp3 Inflammasome-Induced Ischemia-Reperfusion Injury via mTORC1 Inhibition. Blood 2017, 130, 2664–2677. [Google Scholar] [CrossRef]
| Species | Models | In Vivo Effect | Reference | ||
|---|---|---|---|---|---|
| UFH | Rabbit | Global MI | Decreases creatine kinase, improves left ventricular end-diastolic pressure | Friedrich, 1994 [49] | |
| Dog | MI | Reduces the extent of myocardial injury | Black, 1995 [50] | ||
| Dog | MI | Decreases infarct size | Libersan, 1998 [51] | ||
| Rat | Cerebral IR | Reduces infarct size | Yanaka, 1996 [56] | ||
| Pig | Liver IR | Decreases hepatic enzymes compared to control | Liu, 1999 [53] | ||
| Rabbit | Hepatic IR | Improves hepatic IR injury caused by sinusoidal microcirculatory disturbance | Matsumoto, 2000 [54] | ||
| Rat | Intestinal IR | No effect on inflammatory response | Schoots, 2004 [55] | ||
| LMWH | Enoxaparin | Dog | MI | Decreases infarct size | Gralinski, 1996 [57] |
| Dog | MI | Decreases infarct size | Libersan, 1998 [51] | ||
| Dog | MI | Decreases infarct size | Thourani, 2000 [58] | ||
| Rat | Mesenteric IR | Improves hemodynamics parameters during reperfusion | Walensi, 2013 [59] | ||
| Rat | Kidney IR | Decreases prolidase and malondialdehyde levels | Yazici, 2016 [60] | ||
| Dalteparin | Rat | Liver IR | Prevents increase of serum transaminases and increases hepatic tissue blood flow | Harada, 2006 [61] | |
| ULMWH | Rat | Cerebral IR | Decreases infarct size and increase neurological deficit scores | Zhang, 2007 [62] | |
| Reviparin | Rat | Kidney transplantation | Decreases albuminemia and cellular infiltration | Gottman, 2007 [63] | |
| Fondaparinux | Rat | Kidney IR | Increases survival and decrease creatinine levels | Frank, 2005 [64] | |
| Rat | Intestinal IR | No effect | Olenders, 2005 [65] | ||
| Rat | Heart IR | Improves post ischemic myocardial contractile performance and tissue damage | Montaigne, 2008 [66] | ||
| Rat | MI | Decreases infarct size | Macchi, 2014 [67] | ||
| Pig | Kidney transplantation | Improves graft outcome in both the acute and chronic phases | Tillet, 2015 [68] | ||
| Direct anti-Xa | Rivaroxaban | Mouse | Mesenteric IR | Attenuates the leukocyte–platelet–endothelial interaction | Iba, 2014 [69] |
| Rat | Cerebral IR | No effect on infarct size but improves paraparesis score | Kono, 2014 [70] | ||
| Rat | Cerebral IR | Develops significantly smaller strokes and less severe functional deficits | Dittmeier, 2016 [71] | ||
| Rat | Cerebral IR | No effect on infarct size | Morihara, 2017 [72] | ||
| Rat | MI | Decreases infarct size | Guillou, 2020 [73] | ||
| Apixaban | Rat | Cerebral IR | No effect on infarct size | Kono, 2014 [70] | |
| Direct anti-IIa | Hirudin | Rabbit | MI | Decreases infarct size | Erlich, 2000 [74] |
| Rat | Cerebral IR | Decreases infarct size | Karabiyikoglu, 2004 [75] | ||
| Mouse | Bilateral kidney IR | Protects from renal failure | Sevastos, 2007 [76] | ||
| Melagatran | Rat | Kidney IR | No effect | Nitescu, 2007 [77] | |
| Pig | Kidney transplantation | Improves graft outcome and reduces renal injury | Giraud, 2009 [78] | ||
| Dabigatran | Mouse | Cerebral IR | Does not increase hemorrhagic risk after thrombolysis | Sun, 2013 [79] | |
| Rat | Cerebral IR | Reduces hemorrhagic complication after thrombolysis | Kono, 2014 [80] | ||
| Rat | Cerebral IR | Reduces infarct size without an increase in intracerebral hemorrhage | Dittmeier, 2016 [71] | ||
| Mouse | MI | No effect on infarct size | Hale, 2015 [81] | ||
| Rat | Kidney IR | Decreases prolidase and malondialdehyde levels | Yazici, 2016 [60] | ||
| Mouse | Liver IR | Prevents increase of serum transaminases and improves vascular integrity | Noguchi, 2021 [82] | ||
| Other ACs | ASIS | Rabbit | MI | Decreases infarct size | Golino, 2000 [83] |
| Rat | Intestinal IR | Improves endothelial permeability in the ileum | Olanders, 2005 [65] | ||
| Mouse | MI | Decreases infarct size | Loubele, 2009 [84] | ||
| Anti-TF (MoAbs) | Rabbit | MI | Decreases infarct size | Erlich, 2000 [74] | |
| EP217609 | Pig | Kidney transplantation | Improves graft outcome and reduces renal injury | Tillet, 2016 [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carré, J.; Kerforne, T.; Hauet, T.; Macchi, L. Tissue Injury Protection: The Other Face of Anticoagulant Treatments in the Context of Ischemia and Reperfusion Injury with a Focus on Transplantation. Int. J. Mol. Sci. 2023, 24, 17491. https://doi.org/10.3390/ijms242417491
Carré J, Kerforne T, Hauet T, Macchi L. Tissue Injury Protection: The Other Face of Anticoagulant Treatments in the Context of Ischemia and Reperfusion Injury with a Focus on Transplantation. International Journal of Molecular Sciences. 2023; 24(24):17491. https://doi.org/10.3390/ijms242417491
Chicago/Turabian StyleCarré, Julie, Thomas Kerforne, Thierry Hauet, and Laurent Macchi. 2023. "Tissue Injury Protection: The Other Face of Anticoagulant Treatments in the Context of Ischemia and Reperfusion Injury with a Focus on Transplantation" International Journal of Molecular Sciences 24, no. 24: 17491. https://doi.org/10.3390/ijms242417491





