Comparative Transcriptome Analysis Reveals the Molecular Mechanism of Bacillus velezensis GJ-7 Assisting Panax notoginseng against Meloidogyne hapla
Abstract
:1. Introduction
2. Results
2.1. Quality Evaluation of RNA-Seq Data
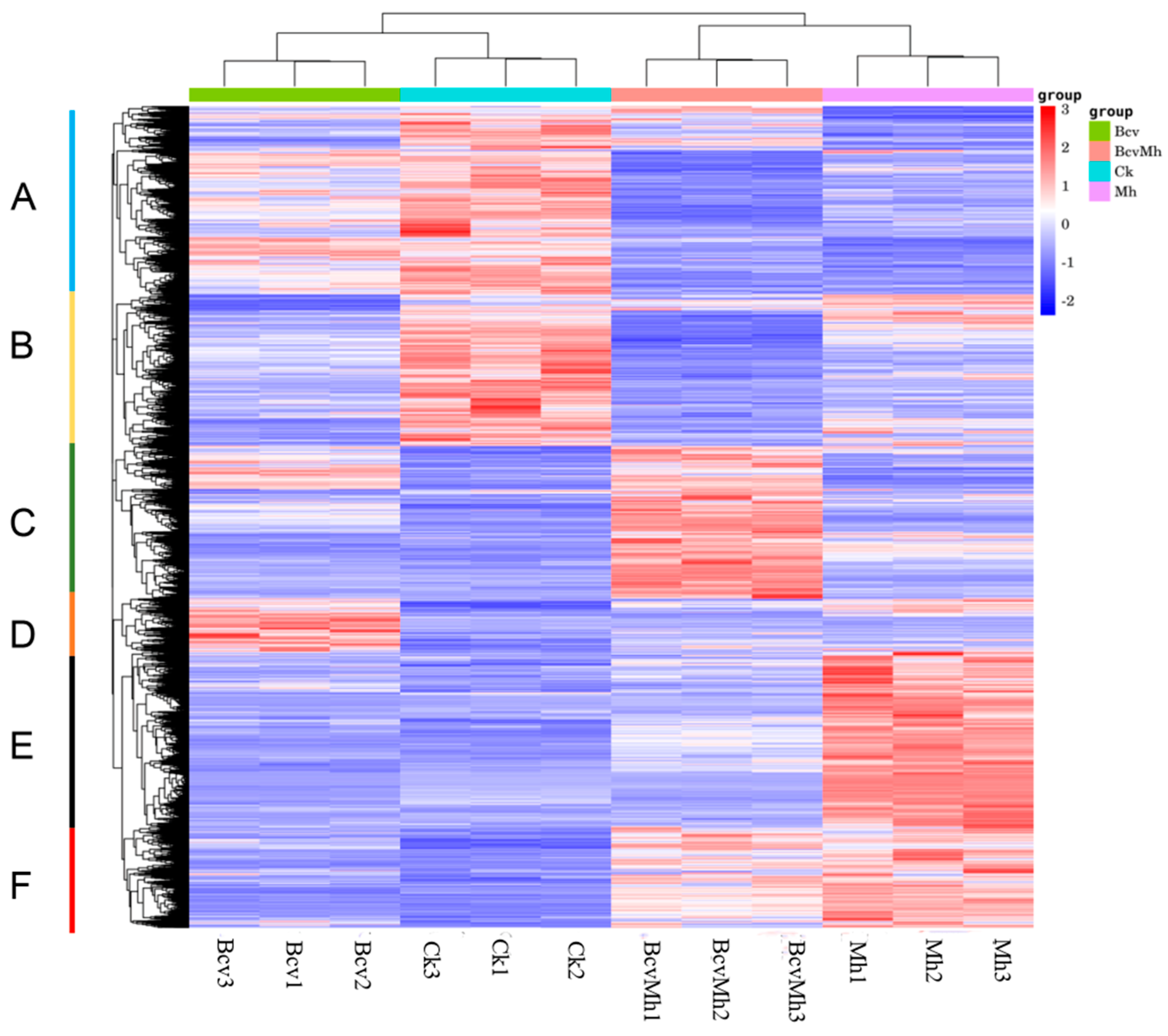
2.2. Identification and Hierarchical Cluster Analysis (HCA) of Differentially Expressed Genes (DEGs)
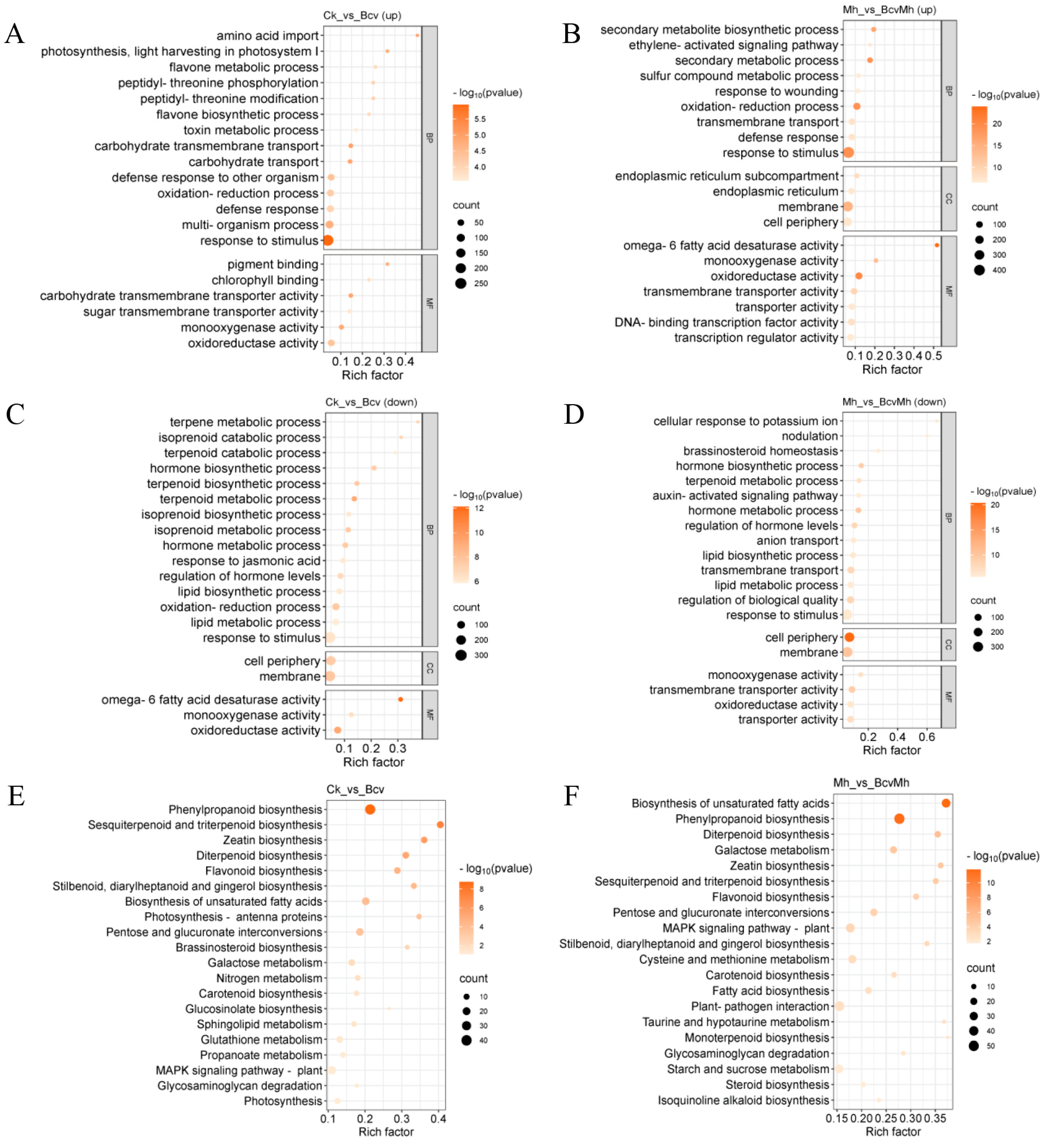
2.3. GO and KEGG Enrichment Analyses of DEGs
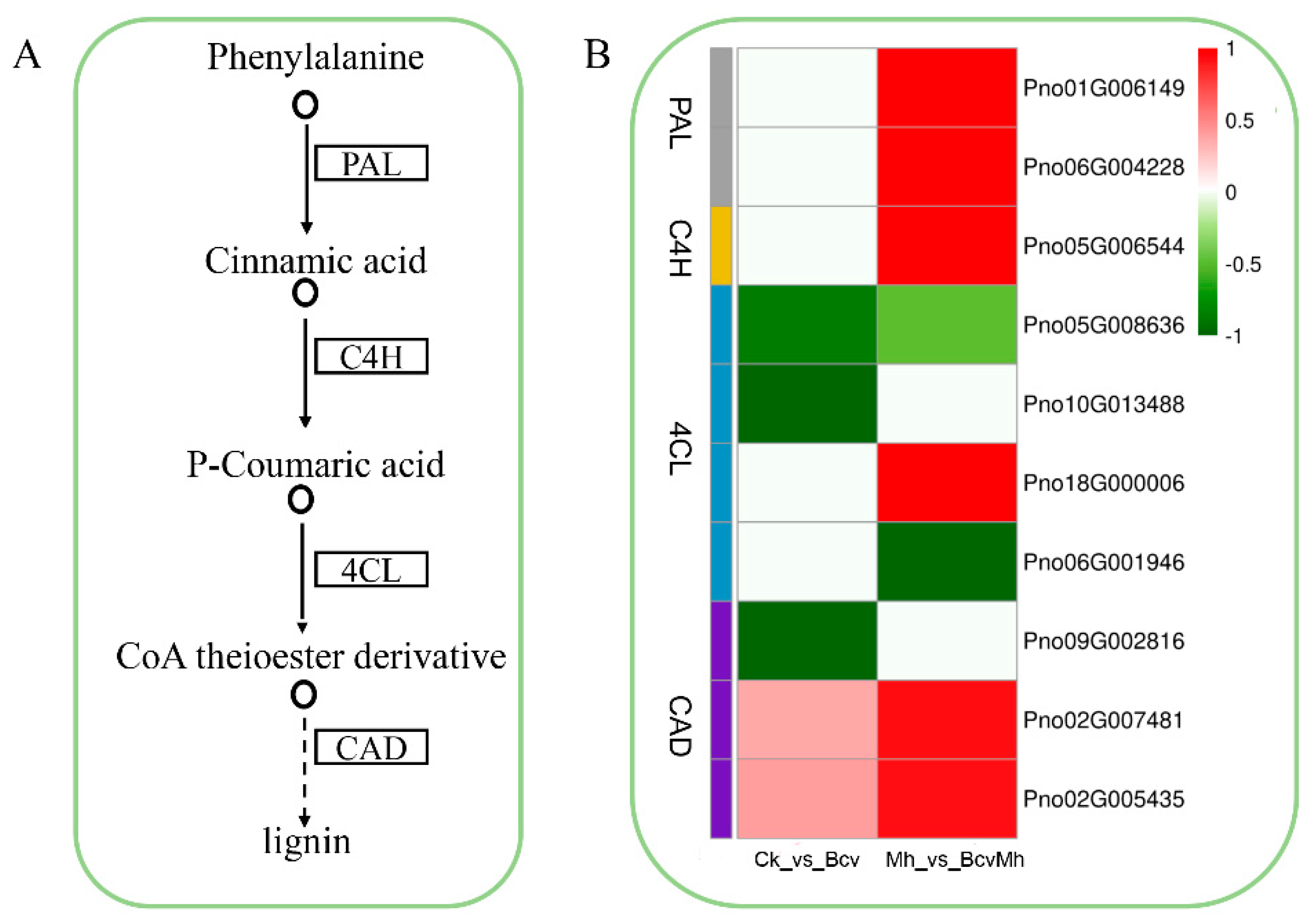
2.4. Differential Gene Expression Analysis of the Phenylpropane Biosynthesis Pathway
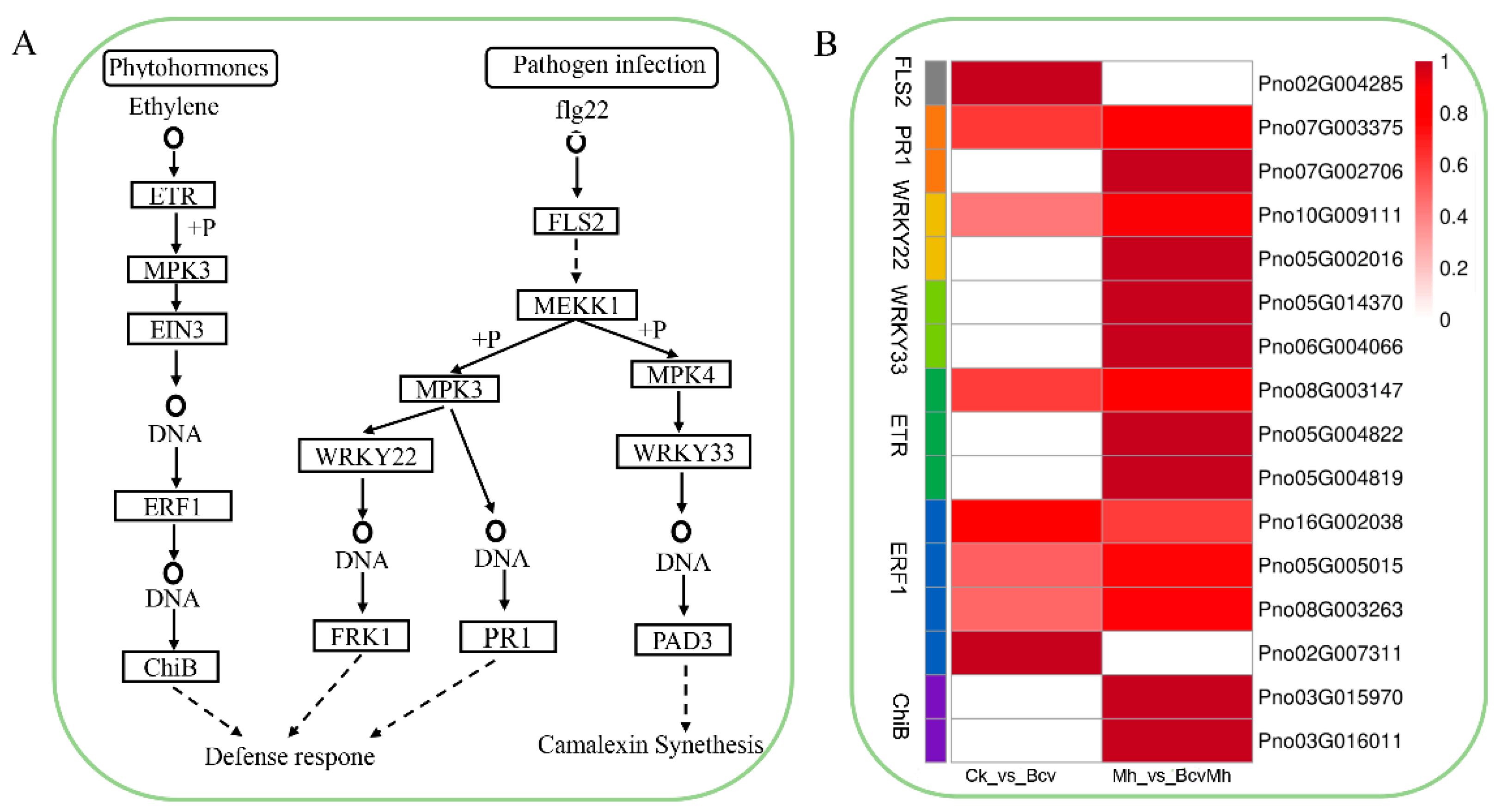
2.5. Differential Gene Expression Analysis of MAPK Signaling Pathway
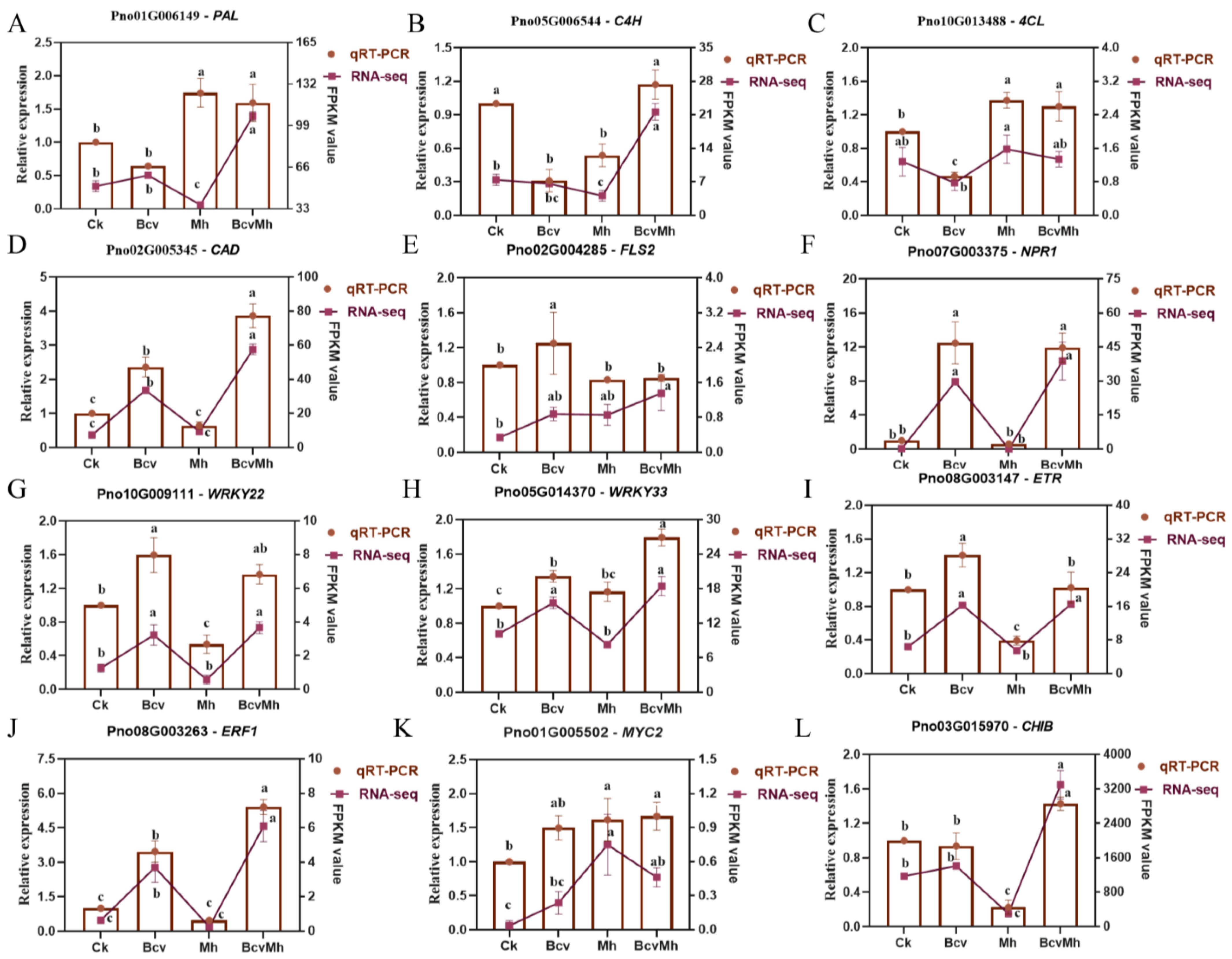
2.6. Verification of RNA-seq Data via qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Plant Material, Bacteria, and Nematode inoculum
4.2. Root Inoculation and Sampling
4.3. RNA-seq of Panax notoginseng Roots and Data Analysis
4.4. Verification of DEGs via Quantitative Real-Time PCR (qRT-PCR)
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, M.; Zhang, X.D.; Xu, Y.G.; Mei, X.Y.; Jiang, B.B.; Liao, J.J.; Yin, Z.B.; Zheng, J.F.; Zhao, Z.; Fan, L.M.; et al. Autotoxic ginsenosides in the rhizosphere contribute to the replant failure of Panax notoginseng. PLoS ONE 2015, 10, e0118555. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Wang, J.J.; Wang, Z.H.; Guo, L.W.; Zhu, S.S.; Wang, Y.; He, X.H. Rhizosphere bacteria from Panax notoginseng against Meloidogyne hapla by rapid colonization and mediated resistance. Front. Microbiol. 2022, 13, 877082. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.H.; Wang, W.P.; Liu, Y.B.; Jiang, C.H.; Yang, K.; Zhu, Y.Y.; Wang, Y.; He, X.H. Investigation and Infection Source Analysis of Root Knot Nematode Disease of Panax notoginseng in Lancang County, Yunnan Province. J. Yunnan Agric. Univ. Nat. Sci. 2021, 36, 60–68. [Google Scholar]
- Gravato-Nobre, M.J.; McClure, M.A.; Dolan, L.; Calder, G.; Davies, K.G.; Mulligan, B.; Evans, K.; Mende, N.V. Meloidogyne incognita surface antigen epitopes in infected Arabidopsis roots. J. Nematol. 1999, 31, 212–223. [Google Scholar] [PubMed]
- Vos, C.; Schouteden, N.; van Tuinen, D.; Chatagnier, O.; Elsen, A.; Waele, D.D.; Panis, B.; Gianinazzi-Pearson, V. Mycorrhiza induced resistance against the root-knot nematode Meloidogyne incognita involves priming of defense gene responses in tomato. Soil Biol. Biochem. 2013, 60, 45–54. [Google Scholar] [CrossRef]
- Anwar, S.A.; McKenry, M.V. Incidence and reproduction of Meloidogyne incognita on vegetable crop genotypes. Pak. J. Zool. 2010, 42, 135–141. [Google Scholar]
- Anwar, S.A.; McKenry, M.V. Incidence and population density of plant-parasitic nematodes infecting vegetable crops and associated yield losses. Pak. J. Zool. 2012, 44, 327–333. [Google Scholar]
- Kim, T.Y.; Jang, J.Y.; Jeon, S.J.; Lee, H.W.; Bae, C.H.; Yeo, J.H.; Lee, H.B.; Kim, I.S.; Park, H.W.; Kim, J.C. Nematicidal activity of kojic acid produced by Aspergillus oryzae against Meloidogyne incognita. J. Microbiol. Biotechnol. 2016, 26, 1383–1391. [Google Scholar] [CrossRef]
- Li, J.; Zou, C.G.; Xu, J.P.; Ji, X.L.; Niu, X.M.; Yang, G.K.; Huang, X.W.; Zhang, K.Q. Molecular mechanisms of nematode-nematophagous microbe interactions: Basis for biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2015, 53, 67–95. [Google Scholar] [CrossRef]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J., Gheysen, G., Fenoll, C., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Baimey, H.; Coyne, D.; Dagnenonbakin, G.; James, B. Plant-parasitic nematodes associated with vegetable crops in Benin: Relationship with soil physicochemical properties. Nematol. Mediterr. 2009, 37, 225–234. [Google Scholar]
- James, B.; Godonou, I.; Atcha, C.; Baimey, H. Healthy Vegetables Through Participatory IPM in Peri-urban Areas of Benin. In Summary of Activities and Achievements, 2003–2006; IITA Benin: Abomey-Calavi, Benin, 2006; 134p. [Google Scholar]
- Nyczepir, A.P.; Thomas, S.H. Current and future management strategies in intensive crop production systems. In Root-Knot Nematodes; Perry, R.N., Moens, M., Starr, J.L., Eds.; CAB International: Wallingford, UK, 2009; pp. 412–443. [Google Scholar]
- Schneider, S.M.; Rosskopf, E.N.; Leesch, J.G.; Chellemi, D.O.; Bull, C.T.; Mazzola, M. United States Department of Agriculture-Agricultural Research Service research on alternatives to methyl bromide: Pre-plant and post-harvest. Pest Manag. Sci. 2003, 59, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Riga, E. The effects of Brassica green manures on plant parasitic and freeliving nematodes used in combination with reduced rates of synthetic nematicides. J. Nematol. 2011, 43, 119–121. [Google Scholar] [PubMed]
- Heydari, A.; Pessarakli, M. A review on biological control of fungal plant pathogens using microbial antagonists. J. Biol. Sci. 2010, 10, 273–290. [Google Scholar] [CrossRef]
- Abd-Elgawad, M.M.M. Biological control agents of plant-parasitic nematodes. Egypt. J. Biol. Pest Control 2016, 26, 423–429. [Google Scholar]
- Hu, H.J.; Chen, Y.L.; Wang, Y.F.; Tang, Y.Y.; Chen, S.L.; Yan, S.Z. Endophytic Bacillus cereus effectively controls Meloidogyne incognita on tomato plants through rapid rhizosphere occupation and repellent action. Plant Dis. 2017, 101, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhao, H.; Zhao, D.; Zhu, X.F.; Wang, Y.Y.; Chen, L.J. Isolation and Identification of Bacteria from Rhizosphere Soil and Their Effect on Plant Growth Promotion and Root-knot Nematode Disease. Biol. Control 2018, 119, 12–19. [Google Scholar] [CrossRef]
- Yin, N.; Zhao, J.L.; Liu, R.; Li, Y.; Ling, J.; Yang, Y.H.; Xie, B.Y.; Mao, Z.C. Biocontrol efficacy of Bacillus cereus strain Bc-cm103 against Meloidogyne incognita. Plant Dis. 2021, 105, 2061–2070. [Google Scholar] [CrossRef]
- Ye, L.; Wang, J.Y.; Liu, X.F.; Guan, Q.; Dou, N.X.; Li, J.; Zhang, Q.; Gao, Y.M.; Wang, M.; Li, J.S.; et al. Nematicidal activity of volatile organic compounds produced by Bacillus altitudinis AMCC1040 against Meloidogyne incognita. Arch. Microbiol. 2022, 204, 521. [Google Scholar] [CrossRef]
- Zhai, Y.L.; Shao, Z.Z.; Cai, M.M.; Zheng, L.Y.; Li, G.Y.; Huang, D.; Chen, W.L.; Zhang, J.B. Multiple modes of nematode control by volatiles of Pseudomonas putida 1A00316 from Antarctic soil against Meloidogyne incognita. Front. Microbiol. 2018, 9, 253. [Google Scholar] [CrossRef]
- Kerry, B.R. Rhizosphere interactions and the exploitation of microbial agents for the biological control of plant-parasitic nematodes. Annu. Rev. Phytopathol. 2000, 38, 423–441. [Google Scholar] [CrossRef]
- Hashem, M.; Abo-Elyousr, K.A.M. Management of the root-knot nematode Meloidogyne incognita on tomato with combinations of different biocontrol organisms. Crop Prot. 2011, 30, 285–292. [Google Scholar] [CrossRef]
- Tohge, T.; Nishiyama, Y.; Hirai, M.Y.; Yano, M.; Nakajima, J.I.; Awazuhara, M.; Inoue, E.; Takahashi, H.; Goodenowe, D.B.; Kitayama, M.; et al. Functional genomics by integrated analysis of metabolome and transcriptome of Arabidopsis plants over-expressing an MYB transcription factor. Plant J. 2005, 42, 218–235. [Google Scholar] [CrossRef] [PubMed]
- Mauch-Mani, B.; Baccelli, L.; Luna, E.; Victor, F. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The chemistry of plant-microbe interactions in the rhizosphere and the potential for metabolomics to reveal signaling related to defense priming and induced systemic resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; David, M.W.; Wees, S.C.M.V.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Gond, S.K.; Bergen, M.S.; Torres, M.S.; White, J.F.; Kharwar, R.N. Endophytic Bacillus spp. produce antifungal lipopeptides and induce host defence gene expression in maize. Microbiol. Res. 2015, 66, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Choudhary, D.K.; Sharma, K.P.; Aggarwal, R. Bacterial mediated plant protection: Induced systemic resistance in soybean. In Microbial Biotechnology; Patra, J., Das, G., Shin, H.S., Eds.; Springer: Singapore, 2018; pp. 193–206. [Google Scholar]
- Hamamouch, N.; Li, C.; Seo, P.J.; Park, C.M.; Davis, E.L. Expression of Arabidopsis pathogenesis-related genes during nematode infection. Mol. Plant Pathol. 2011, 12, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Y.; Wu, J.J.; Fan, S.J.; Li, W.B.; Dong, L.D.; Cheng, Q.; Xu, P.F.; Zhang, S.Z. Isolation and characterization of a novel pathogenesis-related protein gene (GmPRP) with induced expression in soybean (Glycine max) during infection with Phytophthora sojae. PLoS ONE 2015, 41, e0129932. [Google Scholar] [CrossRef]
- Spoel, S.H.; Dong, X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012, 12, 89–100. [Google Scholar] [CrossRef]
- Vlot, A.C.; Sales, J.H.; Lenk, M.; Bauer, K.; Barmbilla, A.; Sommer, A.; Chen, Y.Y.; Wenig, M.; Nayem, S. Systemic propagation of immunity in plants. New Phytol. 2020, 229, 1234–1250. [Google Scholar] [CrossRef]
- Chinchilla, D.; Shan, L.; He, P.; de Vries, S.; Kemmerling, B. One for all: The receptor-associated kinase BAK1. Trends Plant Sci. 2009, 14, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Claus, L.A.N.; Leslie, M.E.; Tao, K.; Wu, Z.P.; Liu, J.; Yu, X.; Li, B.; Zhou, J.G.; Shan, L.B. Ligand-induced monoubiquitination of BIK1 regulates plant immunity. Nature 2020, 581, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Tena, G.; Plotnikova, J.; Willmann, M.R.; Chiu, W.L.; Gomez, L.; Boller, T.; Ausubel, F.M.; Sheen, J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 2002, 415, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr. Opin. Plant Biol. 2010, 13, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, G.K.; Rakwal, R.; Jwa, N.S.; Agrawal, V.P. Signalling molecules and blast pathogen attack activates rice OsPRla and OsPRlb genes: A model illustrating components participating during defence/stress response. Plant Physiol. Biol. 2001, 39, 1095–1103. [Google Scholar]
- Bellincampi, D.; Cervone, F.; Lionetti, V. Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Front. Plant Sci. 2014, 5, 228. [Google Scholar] [CrossRef]
- Sattler, S.E.; Funnell-Harris, D.L. Modifying lignin to improve bioenergy feedstocks: Strengthening the barrier against pathogens? Front. Plant Sci. 2013, 4, 70. [Google Scholar] [CrossRef]
- Thomma, B.; Nelissen, I.; Eggermont, K.; Broekaert, W.F. Deficiency in phytoalexin production causes enhanced susceptibilty of Arabidopsis thaliana to the fungus Alternaria brassicola. Plant J. 1999, 19, 163–171. [Google Scholar] [CrossRef]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef]
- Kang, X.X.; Guo, Y.; Leng, S.; Xiao, L.; Wang, L.H.; Xue, Y.R.; Liu, C.H. Comparative Transcriptome Profiling of Gaeumannomyces graminis var. tritici in Wheat Roots in the Absence and Presence of Biocontrol Bacillus velezensis CC09. Front. Microbiol. 2019, 10, 1474. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. Comparison of methods of collecting inocula of Meloidogyne spp., including a new technique. Plant Dis. Rep. 1973, 57, 1025–1028. [Google Scholar]
- Huang, Y.H.; Mao, Z.C.; Xie, B.Y. Chinese leek (Allium tuberosum Rottler ex Sprengel) reduced disease symptom caused by root-knot nematode. J. Integr. Agric. 2016, 15, 364–372. [Google Scholar] [CrossRef]
- Yang, L.R.; Xie, L.H.; Xue, B.G.; Goodwin, P.H.; Quan, X.; Zheng, C.L.; Liu, T.G.; Lei, Z.S.; Yang, X.J.; Chao, Y.N.; et al. Comparative transcriptome profiling of the early infection of wheat roots by Gaeumannomyces graminis var. tritici. PLoS ONE 2015, 10, e0120691. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Liu, G.Z.; Zhang, G.H.; Jing, Y.; Dong, Y.; Lu, Y.C.; Yang, S.C. The chromosome-scale high-quality genome assembly of Panax notoginseng provides insight into dencichine biosynthesis. Plant Biotechnol. J. 2021, 19, 869–871. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Livak, K.; Schmittgen, T. Analysis of relative gene expression data using realtime quantitative PCR and the 2-DDCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Gene ID | Gene Description | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|---|
| Pno01G006149 | PAL | TTGGATGAGGTGAAGCGGAT | CGACAGCTCCACCTTAATGC |
| Pno05G006544 | C4H | CCATATCTACAGGCCACGGT | AGGCGTTAACCACCACCTTA |
| Pno10G013488 | 4CL | CGAGTCAACTGCTGTAGGGA | CACGTAACCAAAGCTCACCC |
| Pno02G005345 | CAD | GGGGTTAGGAGGAGTTGGTC | TAGGAATGAATCAGCGCCCA |
| Pno02G004285 | FLS2 | TGCTCAGCCACAACTACTCA | AACCCAACTGCAGCAAGATG |
| Pno07G003375 | PR1 | CATGCCCAAAACTCACCACA | GCAGTCTCCAATCCTCGAGT |
| Pno10G009111 | WRKY22 | CCACAACCATCCAACTCGAC | CTGGAGTCTTTGGGTGTTGC |
| Pno05G014370 | WRKY33 | ACCACATACGAAGGGAAGCA | GCTGGTGCCCTTGTATTCTG |
| Pno08G003147 | ETR | TTGGTCATTGCCTTGTCTGC | ACCACCTTGTTTGCTTGCAA |
| Pno08G003263 | ERF1 | TTCCACTCCCCAAATTCCGA | TGTGACGAAGCGCCAAATAG |
| Pno01G005502 | MYC2 | ATGCTGATTACCCGGGTGAA | ACATCCCATCTCCAACTGCT |
| Pno03G015970 | CHIB | GAAAACAACCGGGCTGCTTA | GCATTTCCAGCTTGCCCATA |
| PnACT2 | Actin | TCCAAGGGTGAATATGATGAATCG | AACCTCTCCAAAGAGAATTTCTGAGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, W.; Wang, J.; Wang, Z.; Yan, X.; Wang, Y.; He, X. Comparative Transcriptome Analysis Reveals the Molecular Mechanism of Bacillus velezensis GJ-7 Assisting Panax notoginseng against Meloidogyne hapla. Int. J. Mol. Sci. 2023, 24, 17581. https://doi.org/10.3390/ijms242417581
Wu W, Wang J, Wang Z, Yan X, Wang Y, He X. Comparative Transcriptome Analysis Reveals the Molecular Mechanism of Bacillus velezensis GJ-7 Assisting Panax notoginseng against Meloidogyne hapla. International Journal of Molecular Sciences. 2023; 24(24):17581. https://doi.org/10.3390/ijms242417581
Chicago/Turabian StyleWu, Wentao, Jingjing Wang, Zhuhua Wang, Xirui Yan, Yang Wang, and Xiahong He. 2023. "Comparative Transcriptome Analysis Reveals the Molecular Mechanism of Bacillus velezensis GJ-7 Assisting Panax notoginseng against Meloidogyne hapla" International Journal of Molecular Sciences 24, no. 24: 17581. https://doi.org/10.3390/ijms242417581




