Effect of Low Protein Diets Supplemented with Sodium Butyrate, Medium-Chain Fatty Acids, or n-3 Polyunsaturated Fatty Acids on the Growth Performance, Immune Function, and Microbiome of Weaned Piglets
Abstract
:1. Introduction
2. Results
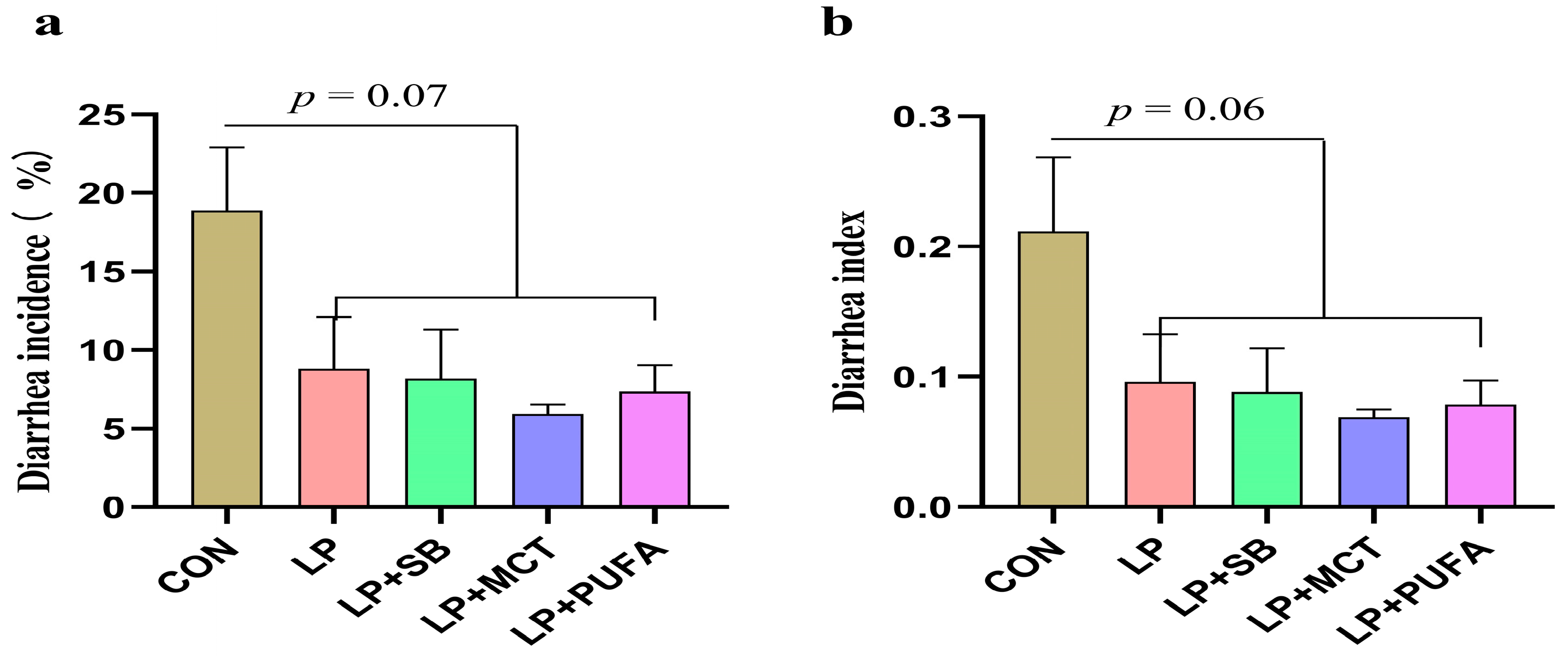
2.1. Growth Gerformance and Diarrhea Incidence
2.2. Plasma Immunity, Inflammatory Factors, and Jejunal Lymphocytes
2.3. Anti-Oxidation Capacity
2.4. The mRNA Expression of TLR4-IKKa and Keap1-Nrf2
2.5. Colonic Microbiome
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Management
4.2. Recording and Sample Collection
4.3. Chemical Analysis
4.4. The Gene Expression in Colonic Mucosa
4.5. The Microbiome in the Colon
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Wang, Y.; Zeng, X.; Zhang, T.; Li, P.; Yao, B.; Wang, L.; Qiao, S.; Zeng, X. Effect of antibiotic-free, low-protein diets with specific amino acid compositions on growth and intestinal flora in weaned pigs. Food Funct. 2019, 11, 493–507. [Google Scholar] [CrossRef]
- Sun, W.; Li, Y.; Tang, Z.; Chen, H.; Wan, K.; An, R.; Wu, L.; Sun, Z. Effects of adding sodium dichloroacetate to low-protein diets on nitrogen balance and amino acid metabolism in the portal-drained viscera and liver of pigs. J. Anim. Sci. Biotechnol. 2020, 11, 36. [Google Scholar] [CrossRef]
- Roudbar, M.A.; Mohammadabadi, M.; Mehrgardi, A.A.; Abdollahi-Arpanahi, R. Estimates of variance components due to parent-of-origin effects for body weight in Iran-Black sheep. Small Rumin. Res. 2017, 149, 1–5. [Google Scholar] [CrossRef]
- Shokri, S.; Khezri, A.; Mohammadabadi, M.; Kheyrodin, H. The expression of MYH7 gene in femur, humeral muscle and back muscle tissues of fattening lambs of the Kermani breed. Agric. Biotechnol. J. 2023, 15, 217–236. [Google Scholar] [CrossRef]
- Pluske, J.R.; Pethick, D.W.; Hopwood, D.E.; Hampson, D.J. Nutritional influences on some major enteric bacterial diseases of pig. Nutr. Res. Rev. 2002, 15, 333–371. [Google Scholar] [CrossRef] [PubMed]
- Nyachoti, C.M.; Omogbenigun, F.O.; Rademacher, M.; Blank, G. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets1. J. Anim. Sci. 2006, 84, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Guay, F.; Donovan, S.M.; Trottier, N.L. Biochemical and morphological developments are partially impaired in intestinal mucosa from growing pigs fed reduced-protein diets supplemented with crystalline amino acids. J. Anim. Sci. 2006, 84, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Gloaguen, M.; Le Floc’H, N.; Corrent, E.; Primot, Y.; van Milgen, J. The use of free amino acids allows formulating very low crude protein diets for piglets1. J. Anim. Sci. 2014, 92, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12, e0179586. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, E.; Leonel, A.; Teixeira, L.; Silva, A.; Silva, J.; Pelaez, J.; Capettini, L.; Lemos, V.; Santos, R.; Alvarez-Leite, J. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NFκB activation. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Song, P.; Fan, P.; Hou, C.; Thacker, P.; Ma, X. Dietary Sodium Butyrate Decreases Postweaning Diarrhea by Modulating Intestinal Permeability and Changing the Bacterial Communities in Weaned Piglets. J. Nutr. 2015, 145, 2774–2780. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, K.; Dierick, N.; Kanto, U.; Hongsapak, T.; Buyens, G.; Kuterna, L.; Vanderbeke, E. Medium-chain glycerides affect gut morphology, immune- and goblet cells in post-weaning piglets: In vitro fatty acid screening with Escherichia coli and in vivo consolidation with LPS challenge. J. Anim. Physiol. Anim. Nutr. 2018, 103, 221–230. [Google Scholar] [CrossRef]
- Jin, J.; Lu, Z.; Li, Y.; Cowart, L.A.; Lopes-Virella, M.F.; Huang, Y. Docosahexaenoic acid antagonizes the boosting effect of palmitic acid on LPS inflammatory signaling by inhibiting gene transcription and ceramide synthesis. PLoS ONE 2018, 13, e0193343. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Brendemuhl, J.H.; Jeong, K.C.; Badinga, L. Effects of dietary omega-3 polyunsaturated fatty acids on growth and immune response of weanling pigs. J. Anim. Sci. Technol. 2014, 56, 7. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef]
- Lauridsen, C. Effects of dietary fatty acids on gut health and function of pigs pre- and post-weaning. J. Anim. Sci. 2020, 98, skaa086. [Google Scholar] [CrossRef]
- Wu, G. Dietary requirements of synthesizable amino acids by animals: A paradigm shift in protein nutrition. J. Anim. Sci. Biotechnol. 2014, 5, 34. [Google Scholar] [CrossRef]
- Lynegaard, J.; Kjeldsen, N.; Bache, J.; Weber, N.; Hansen, C.; Nielsen, J.; Amdi, C. Low protein diets without medicinal zinc oxide for weaned pigs reduced diarrhea treatments and average daily gain. Animal 2020, 15, 100075. [Google Scholar] [CrossRef]
- Caprarulo, V.; Turin, L.; Hejna, M.; Reggi, S.; Dell’anno, M.; Riccaboni, P.; Trevisi, P.; Luise, D.; Baldi, A.; Rossi, L. Protective effect of phytogenic plus short and medium-chain fatty acids-based additives in enterotoxigenic Escherichia coli challenged piglets. Veter- Res. Commun. 2022, 47, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Wan, K.; Li, Y.; Sun, W.; An, R.; Tang, Z.; Wu, L.; Chen, H.; Sun, Z. Effects of dietary calcium pyruvate on gastrointestinal tract development, intestinal health and growth performance of newly weaned piglets fed low-protein diets. J. Appl. Microbiol. 2019, 128, 355–365. [Google Scholar] [CrossRef]
- Xu, J.; Chen, X.; Yu, S.; Su, Y.; Zhu, W. Effects of Early Intervention with Sodium Butyrate on Gut Microbiota and the Expression of Inflammatory Cytokines in Neonatal Piglets. PLoS ONE 2016, 11, e0162461. [Google Scholar] [CrossRef]
- Liu, Y.; Azad, A.K.; Zhao, X.; Zhu, Q.; Kong, X. Dietary Crude Protein Levels Alter Diarrhea Incidence, Immunity, and Intestinal Barrier Function of Huanjiang Mini-Pigs During Different Growth Stages. Front. Immunol. 2022, 13, 908753. [Google Scholar] [CrossRef]
- Tanaka, S.; Saitoh, O.; Tabata, K.; Matsuse, R.; Kojima, K.; Sugi, K.; Nakagawa, K.; Kayazawa, M.; Teranishi, T.; Uchida, K.; et al. Medium-chain fatty acids stimulate interleukin-8 production in Caco-2 cells with different mechanisms from long-chain fatty acids. J. Gastroenterol. Hepatol. 2001, 16, 748–754. [Google Scholar] [CrossRef]
- Cao, W.; Wang, C.; Chin, Y.; Chen, X.; Gao, Y.; Yuan, S.; Xue, C.; Wang, Y.; Tang, Q. DHA-phospholipids (DHA-PL) and EPA-phospholipids (EPA-PL) prevent intestinal dysfunction induced by chronic stress. Food Funct. 2018, 10, 277–288. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Urso, M.; Clarkson, M. Oxidative stress, exercise, and antioxidant supplementation. Toxicology 2003, 189, 41–54. [Google Scholar] [CrossRef]
- Wu, X.; Guo, X.; Xie, C.; Zhang, T.; Gao, P.; Gao, T.; Yin, Y. Effects of a two-meal daily feeding pattern with varied crude protein levels on growth performance and antioxidant indexes in pigs. Anim. Nutr. 2016, 2, 267–270. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, Y.; Zhu, Q.; Gao, F.; Dai, S.; Chen, J.; Zhou, G. Sodium butyrate maintains growth performance by regulating the immune response in broiler chickens. Br. Poult. Sci. 2011, 52, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kang, K.S. Function of capric acid in cyclophosphamide-induced intestinal inflammation, oxidative stress, and barrier function in pigs. Sci. Rep. 2017, 7, 16530. [Google Scholar] [CrossRef]
- Qi, X.; Qin, Z.; Tang, J.; Han, P.; Xing, Q.; Wang, K.; Yu, J.; Zhou, G.; Tang, M.; Wang, W.; et al. Omega-3 polyunsaturated fatty acids ameliorates testicular ischemia-reperfusion injury through the induction of Nrf2 and inhibition of NF-κB in rats. Exp. Mol. Pathol. 2017, 103, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, M.; Yang, Y.; Mu, C.; Su, Y.; Zhu, W. Differential effect of early antibiotic intervention on bacterial fermentation patterns and mucosal gene expression in the colon of pigs under diets with different protein levels. Appl. Microbiol. Biotechnol. 2016, 101, 2493–2505. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, Q.; Li, Y.; Tang, Z.; Sun, W.; Zhang, X.; Sun, J.; Sun, Z. Comparative effects of dietary supplementations with sodium butyrate, medium-chain fatty acids, and n-3 polyunsaturated fatty acids in late pregnancy and lactation on the reproductive performance of sows and growth performance of suckling piglets. J. Anim. Sci. 2019, 97, 4256–4267. [Google Scholar] [CrossRef] [PubMed]
- Tayyeb, J.Z.; Popeijus, H.E.; Mensink, R.P.; Konings, M.C.J.M.; Mokhtar, F.B.A.; Plat, J. Short-Chain Fatty Acids (Except Hexanoic Acid) Lower NF-kB Transactivation, Which Rescues Inflammation-Induced Decreased Apolipoprotein A-I Transcription in HepG2 Cells. Int. J. Mol. Sci. 2020, 21, 5088. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Xu, W.; Zhang, J.; Yao, J.; Xu, J. The Maternal Diet with Fish Oil Might Decrease the Oxidative Stress and Inflammatory Response in Sows, but Increase the Susceptibility to Inflammatory Stimulation in their Offspring. Animals 2020, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Sczesnak, A.; Segata, N.; Qin, X.; Gevers, D.; Petrosino, J.F.; Huttenhower, C.; Littman, D.R.; Ivanov, I.I. The Genome of Th17 Cell-Inducing Segmented Filamentous Bacteria Reveals Extensive Auxotrophy and Adaptations to the Intestinal Environment. Cell Host Microbe 2011, 10, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, H.; Zhang, M.; Wu, T.; Liu, R. Altered short chain fatty acid profiles induced by dietary fiber intervention regulate AMPK levels and intestinal homeostasis. Food Funct. 2019, 10, 7174–7187. [Google Scholar] [CrossRef]
- Zhou, L.; Fang, L.; Sun, Y.; Su, Y.; Zhu, W. Effects of the dietary protein level on the microbial composition and metabolomic profile in the hindgut of the pig. Anaerobe 2016, 38, 61–69. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F.; Ling, Z.; Tong, X.; Xiang, C. Human Intestinal Lumen and Mucosa-Associated Microbiota in Patients with Colorectal Cancer. PLoS ONE 2012, 7, e39743. [Google Scholar] [CrossRef]
- Dey, N.; Soergel, D.A.; Repo, S.; Brenner, S.E. Association of gut microbiota with post-operative clinical course in Crohn’s disease. BMC Gastroenterol. 2013, 13, 131. [Google Scholar] [CrossRef]
- Gebeyew, K.; Yang, C.; He, Z.; Tan, Z. Low-protein diets supplemented with methionine and lysine alter the gut microbiota composition and improve the immune status of growing lambs. Appl. Microbiol. Biotechnol. 2021, 105, 8393–8410. [Google Scholar] [CrossRef]
- Wu, J.; Liu, M.; Zhou, M.; Wu, L.; Yang, H.; Huang, L.; Chen, C. Isolation and genomic characterization of five novel strains of Erysipelotrichaceae from commercial pigs. BMC Microbiol. 2021, 21, 125. [Google Scholar] [CrossRef]
- Sun, W.; Sun, J.; Li, M.; Xu, Q.; Zhang, X.; Tang, Z.; Chen, J.; Zhen, J.; Sun, Z. The effects of dietary sodium butyrate supplementation on the growth performance, carcass traits and intestinal microbiota of growing-finishing pigs. J. Appl. Microbiol. 2020, 128, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Vojinovic, D.; Radjabzadeh, D.; Kurilshikov, A.; Amin, N.; Wijmenga, C.; Franke, L.; Ikram, M.A.; Uitterlinden, A.G.; Zhernakova, A.; Fu, J.; et al. Relationship between Gut Microbiota and Circulating Metabolites in Population-Based Cohorts. Nat. Commun. 2019, 10, 5813. [Google Scholar] [CrossRef] [PubMed]
- Koppel, N.; Rekdal, V.M.; Balskus, E.P. Chemical transformation of xenobiotics by the human gut microbiota. Science 2017, 356, 6344. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ding, X.L.; Li, N.; Zhang, X.Y.; Zeng, X.F.; Wang, S.; Liu, H.B.; Wang, Y.M.; Jia, H.M.; Qiao, S.Y. Dietary supplemented antimicrobial peptide microcin J25 improves the growth performance, apparent total tract digestibility, fecal microbiota, and intestinal barrier function of weaned pigs. J. Anim. Sci. 2017, 95, 5064–5076. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Items | Treatments 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + SB | LP + MCT | LP + PUFA | |||
| Initial BW (kg) | 7.93 | 7.93 | 7.93 | 7.93 | 7.93 | 0.05 | 1.00 |
| Final BW (kg) | 16.9 a | 14.1 b | 13.7 b | 13.3 b | 13.5 b | 0.27 | <0.01 |
| ADG, g/d | 332 a | 229 b | 215 b | 199 b | 205 b | 10.31 | <0.01 |
| ADFI, g/d | 523 a | 496 c | 517 ab | 497 c | 499 bc | 6.72 | 0.02 |
| F/G, g/g | 1.58 a | 2.18 b | 2.43 b | 2.54 b | 2.48 b | 0.11 | <0.01 |
| Items | Treatments 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + SB | LP + MCT | LP + PUFA | |||
| Ig A (pg/mL) | 68.0 | 51.8 | 40.7 | 46.0 | 56.6 | 17.6 | 0.85 |
| Ig G (pg/mL) | 4.17 | 1.79 | 1.45 | 2.32 | 3.60 | 1.18 | 0.43 |
| Ig M (pg/mL) | 0.98 | 0.72 | 0.69 | 0.70 | 0.77 | 0.27 | 0.94 |
| IL-6 (pg/mL) | 26.2 | 12.4 | 12.8 | 12.8 | 10.6 | 3.85 | 0.07 |
| IL-8 (pg/mL) | 2.23 ab | 2.45 a | 1.14 bc | 1.25 bc | 0.85 c | 0.38 | 0.03 |
| IL-10 (pg/mL) | 1.13 | 2.41 | 2.29 | 2.36 | 5.65 | 1.17 | 0.13 |
| IL-17 (pg/mL) | 0.28 | 0.39 | 0.32 | 0.20 | 0.74 | 0.15 | 0.15 |
| TGF-β (pg/mL) | 22.2 | 20.7 | 21.4 | 20.8 | 20.4 | 1.04 | 0.74 |
| IFN-γ (pg/mL) | 2.13 | 1.19 | 1.23 | 0.92 | 0.73 | 0.35 | 0.09 |
| Lymphocytes goblet cell amount | 60.8 ab | 50.6 b | 40.2 b | 86.6 a | 50.6 b | 9.35 | 0.02 |
| Items | Treatments 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + SB | LP + MCT | LP + PUFA | |||
| T-AOC (mM) | 1.59 | 1.56 | 1.56 | 1.53 | 1.54 | 0.03 | 0.42 |
| CAT (U/mL) | 4.27 | 2.29 | 3.50 | 4.97 | 4.18 | 0.67 | 0.10 |
| T-SOD (U/mL) | 37.2 | 37.9 | 38.6 | 26.6 | 28.0 | 4.67 | 0.21 |
| GSH-Px (U/mL) | 522 c | 645 c | 1014 b | 1351 a | 1281 ab | 102 | <0.001 |
| TNOS (U/mL) | 16.2 | 17.0 | 16.0 | 16.5 | 16.7 | 1.04 | 0.96 |
| iNOS (U/mL) | 4.30 | 5.40 | 4.59 | 5.01 | 4.59 | 0.65 | 0.78 |
| Items | Treatments 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + SB | LP + MCT | LP + PUFA | |||
| ACE | 560 | 577 | 581 | 542 | 527 | 21.0 | 0.36 |
| Chao1 | 563 | 576 | 585 | 556 | 532 | 21.6 | 0.50 |
| Simpson | 0.97 | 0.98 | 0.98 | 0.97 | 0.96 | 0.004 | 0.14 |
| Shannon | 6.61 | 6.81 | 6.85 | 6.54 | 6.35 | 0.14 | 0.13 |
| Items (%) | Treatments 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + SB | LP + MCT | LP + PUFA | |||
| Ruminococcaceae | 0.193 | 0.268 | 0.194 | 0.238 | 0.207 | 0.027 | 0.27 |
| Muribaculaceae | 0.162 | 0.103 | 0.122 | 0.15 | 0.095 | 0.027 | 0.37 |
| Lachnospiraceae | 0.099 | 0.118 | 0.088 | 0.077 | 0.149 | 0.024 | 0.28 |
| Prevotellaceae | 0.132 | 0.079 | 0.104 | 0.085 | 0.091 | 0.018 | 0.29 |
| Lactobacillaceae | 0.113 | 0.098 | 0.077 | 0.091 | 0.088 | 0.029 | 0.93 |
| Rikenellaceae | 0.061 | 0.044 | 0.069 | 0.041 | 0.055 | 0.016 | 0.73 |
| Spirochaetaceae | 0.042 | 0.037 | 0.06 | 0.032 | 0.064 | 0.021 | 0.74 |
| Erysipelotrichaceae | 0.025 b | 0.064 a | 0.036 b | 0.038 b | 0.043 b | 0.007 | 0.01 |
| Clostridiaceae-1 | 0.009 | 0.016 | 0.04 | 0.023 | 0.03 | 0.01 | 0.30 |
| Christensenellaceae | 0.013 | 0.023 | 0.027 | 0.013 | 0.037 | 0.008 | 0.21 |
| Acidaminococcaceae | 0.019 | 0.021 | 0.006 | 0.034 | 0.025 | 0.014 | 0.76 |
| Veillonellaceae | 0.018 | 0.015 | 0.012 | 0.046 | 0.011 | 0.015 | 0.48 |
| Streptococcaceae | 0.03 | 0.004 | 0.044 | 0.001 | 0.002 | 0.021 | 0.49 |
| Uncultured bacterium-o- Mollicutes RF39 | 0.009 | 0.016 | 0.02 | 0.01 | 0.017 | 0.004 | 0.29 |
| Family XIII | 0.01 | 0.011 | 0.013 | 0.014 | 0.013 | 0.003 | 0.90 |
| Peptostreptococcaceae | 0.007 | 0.006 | 0.018 | 0.008 | 0.011 | 0.004 | 0.34 |
| Tannerellaceae | 0.012 | 0.011 | 0.008 | 0.014 | 0.006 | 0.005 | 0.86 |
| Items (%) | Treatments 2 | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| CON | LP | LP + SB | LP + MCT | LP + PUFA | |||
| Uncultured bacterium-f-Muribaculaceae | 0.162 | 0.103 | 0.122 | 0.150 | 0.095 | 0.027 | 0.37 |
| Lactobacillus | 0.113 | 0.098 | 0.077 | 0.091 | 0.088 | 0.029 | 0.93 |
| Rikenellaceae-RC9-gut-group | 0.059 | 0.043 | 0.068 | 0.039 | 0.053 | 0.015 | 0.69 |
| Treponema-2 | 0.038 | 0.033 | 0.055 | 0.030 | 0.060 | 0.020 | 0.75 |
| Prevotellaceae-NK3B31-group | 0.045 | 0.026 | 0.040 | 0.028 | 0.036 | 0.009 | 0.58 |
| Uncultured bacterium-f-Lachnospiraceae | 0.025 | 0.026 | 0.029 | 0.025 | 0.032 | 0.007 | 0.93 |
| Ruminococcaceae-UCG-014 | 0.021 | 0.048 | 0.026 | 0.017 | 0.022 | 0.008 | 0.09 |
| Faecalibacterium | 0.035 | 0.047 | 0.008 | 0.022 | 0.018 | 0.016 | 0.47 |
| [Eubacterium]-coprostanoligenes_group | 0.021 | 0.029 | 0.019 | 0.036 | 0.021 | 0.004 | 0.08 |
| Ruminococcaceae-UCG-005 | 0.013 | 0.033 | 0.027 | 0.023 | 0.029 | 0.007 | 0.39 |
| Items | Treatments 1 | ||||
|---|---|---|---|---|---|
| CON | LP | LP + SB | LP + MCT | LP + PUFA | |
| Ingredients, % | |||||
| Corn | 61.45 | 69.37 | 69.23 | 69.23 | 69.23 |
| Soybean meal | 12.90 | 8.30 | 8.28 | 8.28 | 8.28 |
| Puffed soybean | 12.14 | 8.20 | 8.18 | 8.18 | 8.18 |
| Fish meal | 4.80 | 4.70 | 4.69 | 4.69 | 4.69 |
| Soybean oil | 1.80 | 1.90 | 1.90 | 1.90 | 1.90 |
| Whey powder | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| CaHPO4 | 1.10 | 1.24 | 1.24 | 1.24 | 1.24 |
| Limestone powder | 0.80 | 0.80 | 0.80 | 0.80 | 0.80 |
| Salt | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| L-lysine HCl | 0.66 | 0.91 | 0.91 | 0.91 | 0.91 |
| Methionine | 0.25 | 0.32 | 0.32 | 0.32 | 0.32 |
| Tryptophan | 0.05 | 0.09 | 0.09 | 0.09 | 0.09 |
| Threonine | 0.25 | 0.37 | 0.37 | 0.37 | 0.37 |
| Premix 2 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| SB | 0.00 | 0.00 | 0.20 | 0.00 | 0.00 |
| MCT | 0.00 | 0.00 | 0.00 | 0.20 | 0.00 |
| n-3 PUFA | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Chemical composition 3 | |||||
| Digestive energy DE (MJ/kg) | 14.67 | 14.58 | 14.59 | 14.59 | 14.60 |
| Crude protein | 20.45 | 16.75 | 16.54 | 16.68 | 16.15 |
| Ca | 0.80 | 0.89 | 0.88 | 0.87 | 0.82 |
| Total P | 0.69 | 0.74 | 0.74. | 0.77 | 0.73 |
| Lysine | 1.56 | 1.55 | 1.55 | 1.55 | 1.55 |
| Methionine | 0.58 | 0.61 | 0.61 | 0.61 | 0.61 |
| Cystine | 0.31 | 0.27 | 0.26 | 0.26 | 0.26 |
| Threonine | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| Tryptophan | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| Arginine | 1.15 | 0.92 | 0.92 | 0.92 | 0.92 |
| Histidine | 0.45 | 0.39 | 0.39 | 0.39 | 0.39 |
| Isoleucine | 0.72 | 0.60 | 0.60 | 0.60 | 0.60 |
| Leucine | 1.60 | 1.41 | 1.40 | 1.40 | 1.40 |
| Phenylalanine | 0.81 | 0.68 | 0.67 | 0.67 | 0.67 |
| Tyrosine | 0.56 | 0.49 | 0.49 | 0.49 | 0.49 |
| Valine | 0.83 | 0.70 | 0.70 | 0.70 | 0.70 |
| Genes 1 | Primer Sequences (5′→3′) Primer Sequence (5′→3′) | Serial Number | Tm Value (°C) | Length of PCR (bp) |
|---|---|---|---|---|
| TLR4 | F: CTGCCTGTGCTGAGTTTCAGGAACG R: CCTCACCCAGTCTTCGTC | NM-001293316.1 | 66 57 | 219 |
| IKKα | F: AATCTGCTTCGGAACAACA R: GTCAATCTGGATGCTGGTT | XM-021077172.1 | 55 55 | 111 |
| Keap1 | F: AGCAGCGGCGTTTCTACGT R: TGGGCTTGTGCAGAGTGAGC | XM_021076667.1 | 63 63 | 168 |
| Nrf2 | F: TCAGACCCACCACTAGCCTT R: GTGATGCCAGCAGACCTCTT | XM_021075133.1 | 50 50 | 138 |
| β-actin | F: TGCGGCATCCACCAAACTA R: CGTAGAGGTCCTTGCGGATGT | XM_021086047.1 | 57 62 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Lan, T.; Ding, Q.; Ren, Z.; Tang, Z.; Tang, Q.; Peng, X.; Xu, Y.; Sun, Z. Effect of Low Protein Diets Supplemented with Sodium Butyrate, Medium-Chain Fatty Acids, or n-3 Polyunsaturated Fatty Acids on the Growth Performance, Immune Function, and Microbiome of Weaned Piglets. Int. J. Mol. Sci. 2023, 24, 17592. https://doi.org/10.3390/ijms242417592
Li W, Lan T, Ding Q, Ren Z, Tang Z, Tang Q, Peng X, Xu Y, Sun Z. Effect of Low Protein Diets Supplemented with Sodium Butyrate, Medium-Chain Fatty Acids, or n-3 Polyunsaturated Fatty Acids on the Growth Performance, Immune Function, and Microbiome of Weaned Piglets. International Journal of Molecular Sciences. 2023; 24(24):17592. https://doi.org/10.3390/ijms242417592
Chicago/Turabian StyleLi, Wenxue, Tianyi Lan, Qi Ding, Zhongxiang Ren, Zhiru Tang, Qingsong Tang, Xie Peng, Yetong Xu, and Zhihong Sun. 2023. "Effect of Low Protein Diets Supplemented with Sodium Butyrate, Medium-Chain Fatty Acids, or n-3 Polyunsaturated Fatty Acids on the Growth Performance, Immune Function, and Microbiome of Weaned Piglets" International Journal of Molecular Sciences 24, no. 24: 17592. https://doi.org/10.3390/ijms242417592





