Effects of Radioactive 56MnO2 Particle Inhalation on Mouse Lungs: A Comparison between C57BL and BALB/c
Abstract
:1. Introduction
2. Results
2.1. Estimated Absorbed Doses of Internal Irradiation in the Lungs
2.2. Body and Organ Weights
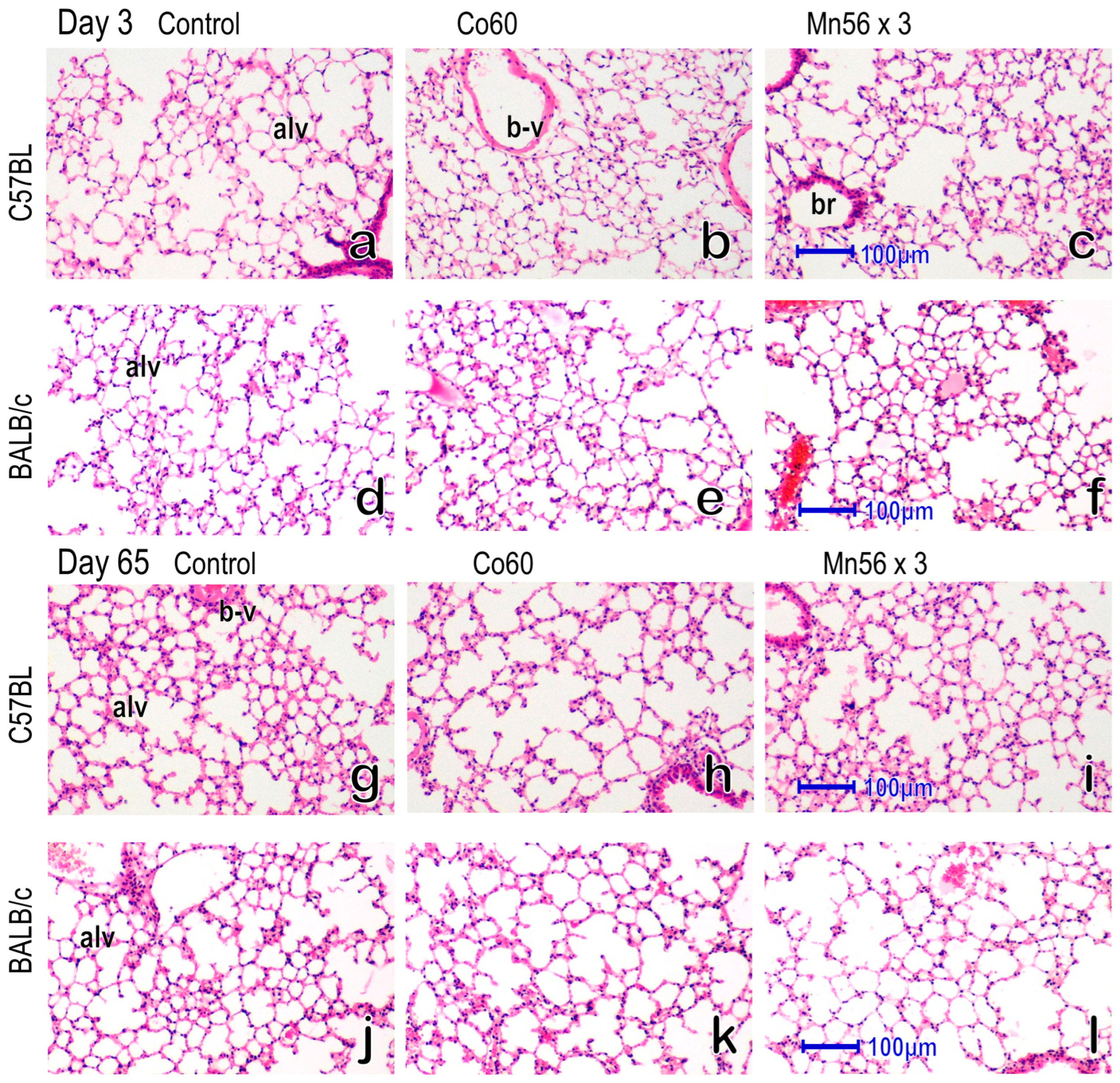
2.3. Histology of the Lung
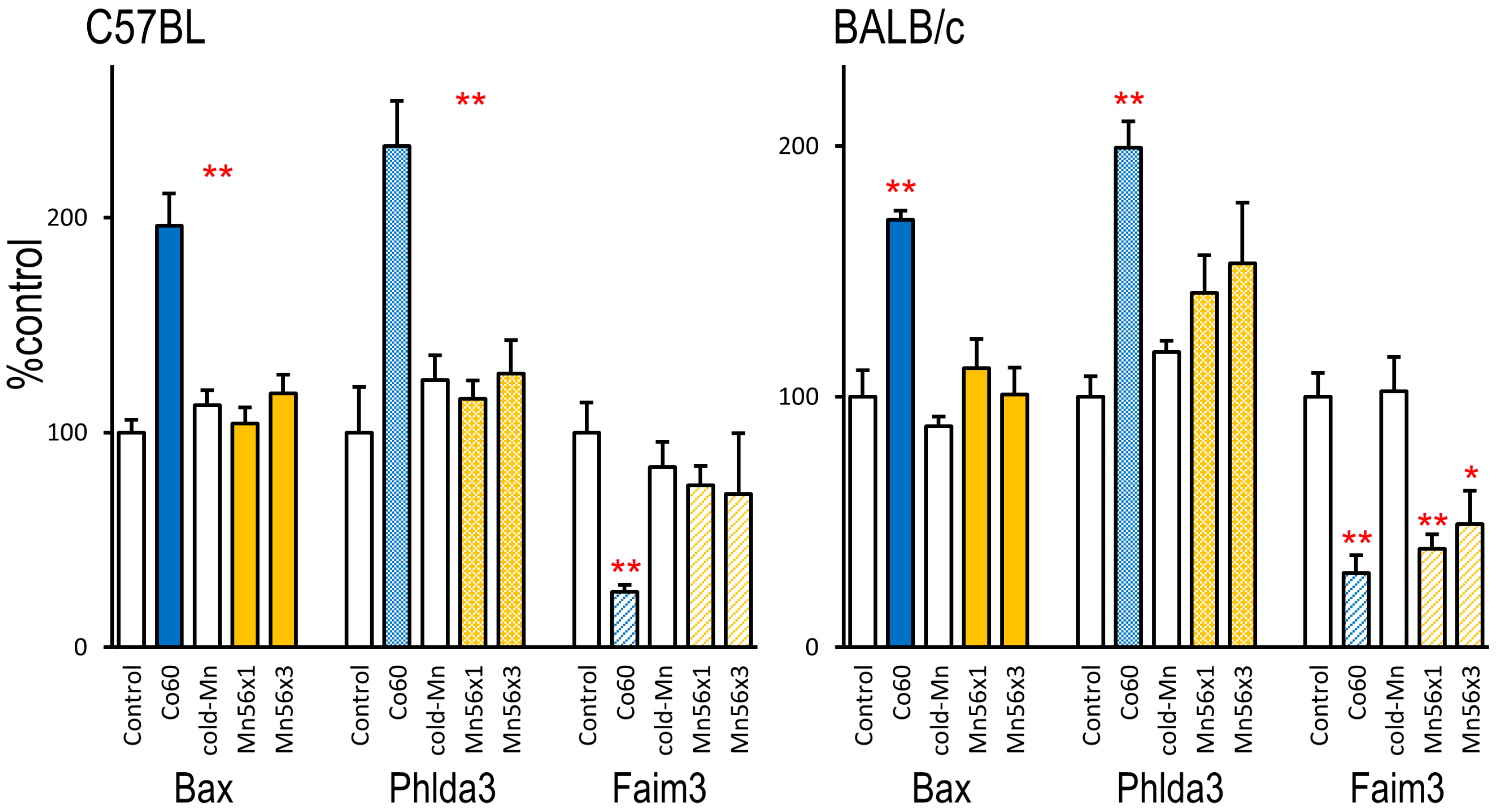
2.4. Expression of Biodosimetry Marker Genes
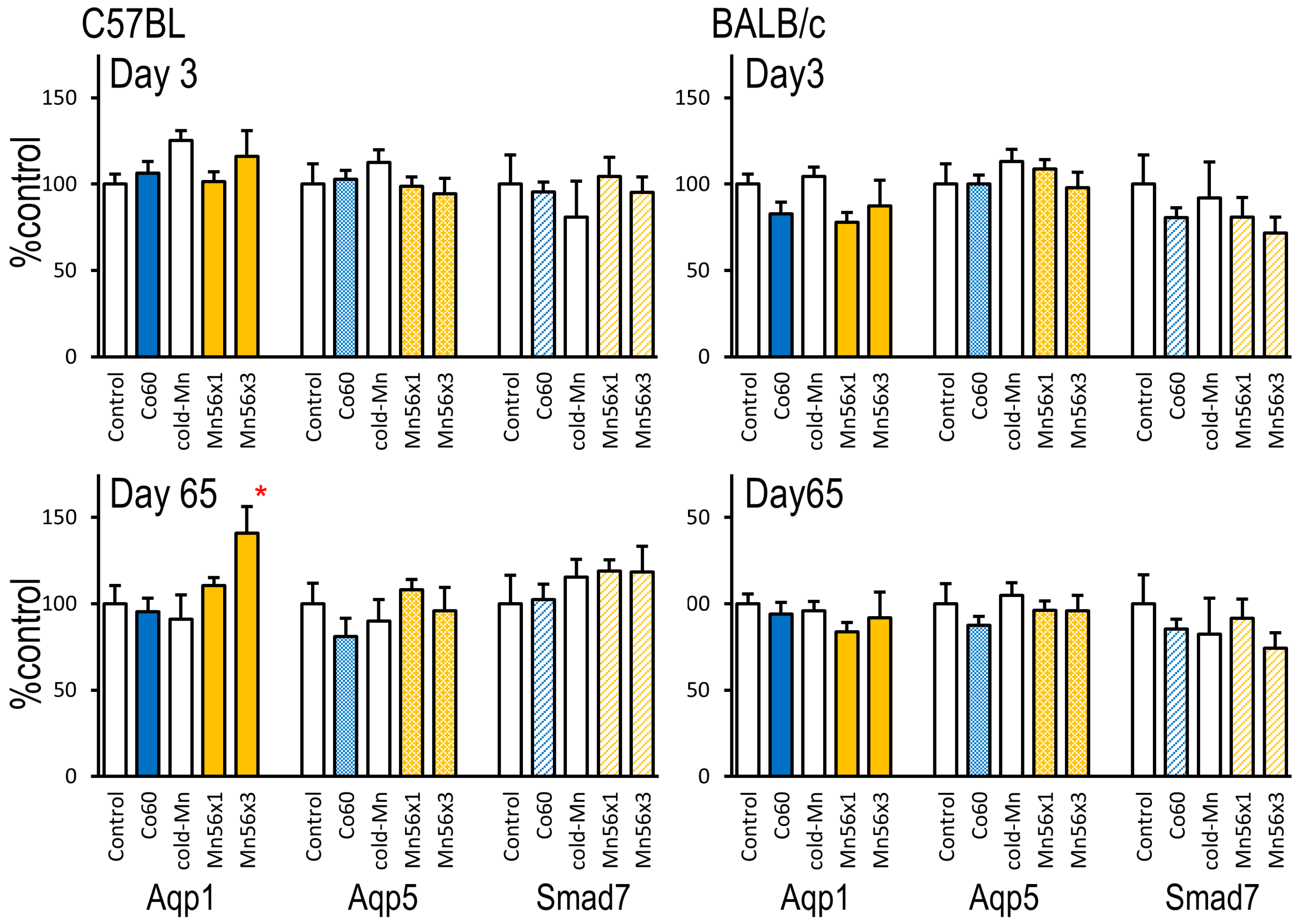
2.5. Gene Expression of Aqp1, Aqp5, and Smad7
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Irradiation and Dosimetry
4.3. Measurement of mRNA Levels Using Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kerr, G.D.; Egbert, S.D.; Al-Nabulsi, I.; Bailiff, I.K.; Beck, H.L.; Belukha, I.G.; Cockayne, J.E.; Cullings, H.M.; Eckerman, K.F.; Granovskaya, E.; et al. Workshop Report on Atomic Bomb Dosimetry—Review of Dose Related Factors for the Evaluation of Exposures to Residual Radiation at Hiroshima and Nagasaki. Health Phys. 2015, 109, 582–600. [Google Scholar] [CrossRef] [PubMed]
- Imanaka, T.; Endo, S.; Tanaka, K.; Shizuma, K. Gamma-ray exposure from neutron-induced radionuclides in soil in Hiroshima and Nagasaki based on DS02 calculations. Radiat. Environ. Biophys. 2008, 47, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Otani, K.; Ohtaki, M.; Yasuda, H. Solid cancer mortality risk among a cohort of Hiroshima early entrants after the atomic bombing, 1970–2010: Implications regarding health effects of residual radiation. J. Radiat. Res. 2022, 63 (Suppl.1), i45–i53. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Endo, S.; Imanaka, T.; Shizuma, K.; Hasai, H.; Hoshi, M. Skin dose from neutron-activated soil for early entrants following the A-bomb detonation in Hiroshima: Contribution from β and γ rays. Radiat. Environ. Biophys. 2008, 47, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Abishev, Z.; Ruslanova, B.; Apbassova, S.; Chaizhunussova, N.; Shabdarbayeva, D.; Azimkhanov, A.; Zhumadilov, K.; Stepanenko, V.; Ivanov, S.; Shegay, P.; et al. Effects of Internal Exposure of Radioactive 56MnO2 Particles on the Lung in C57BL Mice. Curr. Issues Mol. Biol. 2023, 45, 3208–3218. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, N.; Ruslanova, B.; Abishev, Z.; Chaizhunussova, N.; Shabdarbayeva, D.; Amantayeva, G.; Farida, R.; Sandybayev, M.; Nagano, K.; Zhumadilov, K.; et al. Biological impacts on the lungs in rats internally exposed to radioactive 56MnO2 particle. Sci. Rep. 2021, 11, 11055. [Google Scholar] [CrossRef]
- Stepanenko, V.; Kaprin, A.; Ivanov, S.; Shegay, P.; Zhumadilov, K.; Petukhov, A.; Kolyzhenkov, T.; Bogacheva, V.; Zharova, E.; Iaskova, E.; et al. Internal doses in experimental mice and rats following exposure to neutron-activated 56MnO2 powder: Results of an international, multicenter study. Radiat. Environ. Biophys. 2020, 59, 683–692. [Google Scholar] [CrossRef]
- Yuhas, J.M.; Storer, J.B. On mouse strain differences in radiation resistance: Hematopoietic death and the endogenous colony-forming unit. Radiat. Res. 1969, 39, 608–622. [Google Scholar] [CrossRef]
- Hanson, W.R.; Fry, R.J.; Sallese, A.R.; Frischer, H.; Ahmad, T.; Ainsworth, E.J. Comparison of intestine and bone marrow radiosensitivity of the BALB/c and the C57BL/6 mouse strains and their B6CF1 offspring. Radiat. Res. 1987, 110, 340–352. [Google Scholar] [CrossRef]
- Paun, A.; Haston, C.K. Genomic and genome-wide association of susceptibility to radiation-induced fibrotic lung disease in mice. Radiother. Oncol. 2012, 105, 350–357. [Google Scholar] [CrossRef]
- Walkin, L.; Herrick, S.E.; Summers, A.; Brenchley, P.E.; Hoff, C.M.; Korstanje, R.; Margetts, P.J. The role of mouse strain differences in the susceptibility to fibrosis: A systematic review. Fibrogenes. Tissue Repair 2013, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S. Role of aquaporins in lung liquid physiology. Respir. Physiol. Neurobiol. 2007, 159, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Yadav, E.; Yadav, N.; Hus, A.; Yadav, J.S. Aquaporins in lung health and disease: Emerging roles, regulation, and clinical implications. Respir. Med. 2020, 174, 106193. [Google Scholar] [CrossRef] [PubMed]
- Kabacik, S.; MacKay, A.; Tamber, N.; Manning, G.; Finnon, P.; Paillier, F.; Ashworth, A.; Bouffler, S.; Badie, C. Gene expression following ionising radiation: Identification of biomarkers for dose estimation and prediction of individual response. Int. J. Radiat. Biol. 2011, 87, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Wang, W.W.; Chen, S.W.; Shen, Q.; Min, R. Radiation dose effect of DNA repair-related gene expression in mouse white blood cells. Med. Sci. Monit. 2011, 17, 290–297. [Google Scholar] [CrossRef]
- Paul, S.; Smilenov, L.B.; Elliston, C.D.; Amundson, S.A. Radiation Dose-Rate Effects on Gene Expression in a Mouse Biodosimetry Model. Rediat. Res. 2015, 184, 24–32. [Google Scholar] [CrossRef]
- Hamasaki, K.; Imai, K.; Hayashi, T.; Nakachi, K.; Kusunoki, Y. Radiation sensitivity and genomic instability in the hematopoietic system: Frequencies of micronucleated reticulocytes in whole-body X-irradiated BALB/c and C57BL/6 mice. Cancer Sci. 2007, 98, 1840–1844. [Google Scholar] [CrossRef]
- Kallman, R.F.; Kohn, H.I. The reaction of the mouse thymus to x-rays measured by changes in organ weight. Radiat. Res. 1955, 2, 280–293. [Google Scholar] [CrossRef]
- Takada, A.; Takada, Y.; Huang, C.C.; Ambrus, J.L. Biphasic pattern of thymus regeneration after whole-body irradiation. J. Exp. Med. 1969, 129, 445–457. [Google Scholar] [CrossRef]
- Kong, X.; Yu, D.; Wang, Z.; Li, S. Relationship between p53 status and the bioeffect of ionizing radiation (Review). Oncol. Lett. 2021, 22, 661. [Google Scholar] [CrossRef]
- Okazaki, R. Role of p53 in Regulating Radiation Responses. Life 2022, 12, 1099. [Google Scholar] [CrossRef] [PubMed]
- Coggle, J.E.; Lambert, B.E.; Moores, S.R. Radiation effects in the lung. Environ. Health Perspect. 1986, 70, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Down, J.D. The nature and relevance of late lung pathology following localised irradiation of the thorax in mice and rats. Br. J. Cancer Suppl. 1986, 7, 330–332. [Google Scholar] [PubMed]
- Travis, E.L.; Harley, R.A.; Fenn, J.O.; Klobukowski, C.J.; Hargrove, H.B. Pathologic changes in the lung following single and multi-fraction irradiation. Int. J. Radiat. Oncol. Biol. Phys. 1977, 2, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Ding, N.-H.; Li, J.J.; Sun, L.-Q. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr. Drug Targets 2013, 14, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Rübe, C.E.; Uthe, D.; Schmid, K.W.; Richter, K.D.; Wessel, J.; Schuck, A.; Willich, N.; Rübe, C. Dose-dependent induction of transforming growth factor β (TGF-β) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1033–1042. [Google Scholar] [CrossRef]
- Straub, J.M.; New, J.; Hamilton, C.D.; Lominska, C.; Shnayder, Y.; Thomas, S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J. Cancer Res. Clin. Oncol. 2015, 141, 1985–1994. [Google Scholar] [CrossRef]
- Xie, L.; Zhou, J.; Zhang, S.; Chen, Q.; Lai, R.; Ding, W.; Song, C.; Meng, X.; Wu, J. Integrating microRNA and mRNA expression profiles in response to radiation-induced injury in rat lung. Radiat. Oncol. 2014, 9, 111. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Hata, H.; Ozawa, H.; Takata, K. Immunohistochemical localization of the aquaporins AQP1, AQP3, AQP4, and AQP5 in the mouse respiratory system. Acta Histochem. Cytochem. 2009, 42, 159–169. [Google Scholar] [CrossRef]
- Bai, C.X.; Fukuda, N.; Song, Y.L.; Ma, T.H.; Matthay, M.A.; Verkman, A.S. Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice. J. Clin. Investig. 1999, 103, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, G.; Zhang, W.; Peng, Q.; Xue, M.; Jinhong, H. Expression of pulmonary aquaporin 1 is dramatically upregulated in mice with pulmonary fibrosis induced by bleomycin. Arch. Med. Sci. 2013, 9, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, K.; Wang, Y.; Zhang, R.; Shang, J.; Jiang, W.; Wang, A. The effects of aquaporin-1 in pulmonary edema induced by fat embolism syndrome. Int. J. Mol. Sci. 2016, 17, 1183. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Dong, L.; Li, M.; Cai, W. Qidonghuoxue Decoction Ameliorates Pulmonary Edema in Acute Lung Injury Mice through the Upregulation of Epithelial Sodium Channel and Aquaporin-1. J. Evid. Based Complement. Altern. Med. 2020, 2020, 2492304. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Y.; Wu, C.; Wang, T.; Wu, M. Aquaporin-1 inhibition exacerbates ischemia-reperfusion-induced lung injury in mouse. Am. J. Med. Sci. 2023, 365, 84–92. [Google Scholar] [CrossRef]
- Su, X.; Song, Y.; Jiang, J.; Bai, C. The role of aquaporin-1 (AQP1) expression in a murine model of lipopolysaccharide-induced acute lung injury. Respir. Physiol. Neurobiol. 2004, 142, 1–11. [Google Scholar] [CrossRef]
- Hasan, B.; Li, F.S.; Siyit, A.; Tuyghun, E.; Luo, J.H.; Upur, H.; Ablimit, A. Expression of aquaporins in the lungs of mice with acute injury caused by LPS treatment. Respir. Physiol. Neurobiol. 2014, 200, 40–45. [Google Scholar] [CrossRef]
- Moritake, T.; Fujita, H.; Yanagisawa, M.; Nakawatari, M.; Imadome, K.; Nakamura, E.; Iwakawa, M.; Imai, T. Strain-dependent damage in mouse lung after carbon ion irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e95–e102. [Google Scholar] [CrossRef]
- Siamwala, J.H.; Mossman, J.A.; Schorl, C.; Borgas, D.; Sakhatskyy, P.; Rand, D.M.; Lu, Q.; Rounds, S. Strain-dependent lung transcriptomic differences in cigarette smoke and LPS models of lung injury in mice. Physiol. Genom 2023, 55, 259–274. [Google Scholar] [CrossRef]
- Tovar, A.; Crouse, W.L.; Smith, G.J.; Thomas, J.M.; Keith, B.P.; McFadden, K.M.; Moran, T.P.; Furey, T.S.; Kelada, S.N.P. Integrative analysis reveals mouse strain-dependent responses to acute ozone exposure associated with airway macrophage transcriptional activity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022, 322, L33–L49. [Google Scholar] [CrossRef]
- O’Neal, S.L.; Zheng, W. Manganese Toxicity Upon Overexposure: A Decade in Review. Curr. Environ. Health Rep. 2015, 2, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Adkins, B.; Luginbuhl, G.H.; Gardner, D.E. Acute exposure of laboratory mice to manganese oxide. Am. Ind. Hyg. Assoc. J. 1980, 41, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Camner, P.; Curstedt, T.; Jarstrand, C.; Johannsson, A.; Robertson, B.; Wiernik, A. Rabbit lung after inhalation of manganese chloride: A comparison with the effects of chlorides of nickel, cadmium, cobalt, and copper. Environ. Res. 1985, 38, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, V.; Kaprin, A.; Ivanov, S.; Shegay, P.; Bogacheva, V.; Sato, H.; Shichijo, K.; Toyoda, S.; Kawano, N.; Ohtaki, M.; et al. Microdistribution of internal radiation dose in biological tissues exposed to 56Mn dioxide microparticles. J. Radiat. Res. 2022, 63 (Suppl. 1), i21. [Google Scholar] [CrossRef]
| Body Weights (g) | Thymus (g/kg bw) | Spleen (g/kg bw) | Lung (g/kg bw) | Heart (g/kg bw) | Liver (g/kg bw) | Kidney (g/kg bw) | Testis (g/kg bw) | |
|---|---|---|---|---|---|---|---|---|
| Day 3/C57BL | ||||||||
| Control | 28.7 ± 1.0 | 1.7 ± 0.27 | 2.9 ± 0.30 | 10.2 ± 1.1 | 5.7 ± 0.12 | 52.5 ± 3.7 | 15.4 ± 0.58 | 6.8 ± 0.28 |
| Co60 | 28.8 ± 1.2 | 0.8 ± 0.09 * | 2.8 ± 0.32 | 8.5 ± 0.73 | 5.6 ± 0.37 | 60.5 ± 3.5 | 15.6 ± 0.60 | 7.1 ± 0.64 |
| Cold-Mn | 29.0 ± 0.9 | 1.4 ± 0.18 | 3.5 ± 0.30 | 9.4 ± 0.36 | 5.8 ± 0.23 | 51.9 ± 1.0 | 15.2 ± 0.33 | 6.5 ± 0.33 |
| Mn56x1 | 29.5 ± 1.4 | 1.3 ± 0.08 | 4.7 ± 0.21 * | 9.0 ± 0.38 | 5.9 ± 0.18 | 55.0 ± 2.3 | 14.8 ± 0.50 | 5.9 ± 0.73 |
| Mn56x3 | 29.4 ± 1.2 | 1.4 ± 0.10 | 3.3 ± 0.38 | 9.6 ± 0.55 | 4.8 ± 0.13 * | 55.2 ± 1.5 | 14.8 ± 0.82 | 6.5 ± 0.82 |
| Day 3/Balb/c | ||||||||
| Control | 26.3 ± 1.6 | 1.4 ± 0.14 | 3.8 ± 0.38 | 9.2 ± 0.70 | 5.6 ± 0.20 | 49.3 ± 2.5 | 17.0 ± 0.70 | 8.8 ± 0.61 |
| Co60 | 26.2 ± 1.9 | 0.9 ± 0.09 * | 3.1 ± 0.40 | 8.8 ± 0.51 | 5.6 ± 0.24 | 56.1 ± 1.7 | 18.2 ± 0.52 | 9.2 ± 0.63 |
| Cold-Mn | 27.5 ± 1.8 | 1.2 ± 0.11 | 4.8 ± 0.32 | 8.7 ± 0.54 | 5.5 ± 0.24 | 46.9 ± 2.7 | 17.8 ± 0.58 | 9.1 ± 0.58 |
| Mn56x1 | 27.0 ± 2.1 | 1.4 ± 0.14 | 4.1 ± 0.23 | 8.8 ± 0.50 | 5.9 ± 0.21 | 52.2 ± 1.6 | 16.2 ± 0.67 | 8.5 ± 0.87 |
| Mn56x3 | 27.3 ± 1.9 | 1.0 ± 0.06 * | 4.5 ± 0.53 | 9.0 ± 0.50 | 5.4 ± 0.12 | 56.9 ± 2.1 | 17.4 ± 0.50 | 7.5 ± 0.87 |
| Day 65/C57BL | ||||||||
| Control | 36.0 ± 1.3 | 1.6 ± 0.09 | 2.9 ± 0.28 | 7.4 ± 0.67 | 5.2 ± 0.25 | 51.1 ± 2.53 | 15.2 ± 0.59 | 5.3 ± 0.23 |
| Co60 | 32.6 ± 0.6 | 1.3 ± 0.10 | 3.4 ± 0.33 | 8.3 ± 0.45 | 6.0 ± 0.36 | 48.2 ± 2.44 | 16.0 ± 0.63 | 6.6 ± 0.22 * |
| Cold-Mn | 33.6 ± 1.0 | 1.4 ± 0.11 | 3.0 ± 0.29 | 7.2 ± 0.52 | 5.8 ± 0.25 | 49.2 ± 2.42 | 16.4 ± 1.06 | 6.2 ± 0.59 |
| Mn56x1 | 35.4 ± 2.1 | 1.3 ± 0.14 | 3.2 ± 0.40 | 9.4 ± 0.77 | 5.5 ± 0.21 | 47.6 ± 2.5 | 14.7 ± 0.80 | 5.9 ± 0.95 |
| Mn56x3 | 33.9 ± 1.5 | 1.6 ± 0.14 | 2.8 ± 0.31 | 8.3 ± 0.38 | 5.7 ± 0.33 | 43.7 ± 1.74 | 15.5 ± 0.46 | 6.9 ± 0.61 * |
| Day 65/Balb/c | ||||||||
| Control | 29.8 ± 2.5 | 1.2 ± 0.17 | 3.6 ± 0.22 | 8.9 ± 0.85 | 6.8 ± 1.02 | 49 ± 2.5 | 18.0 ± 0.57 | 8.7 ± 0.68 |
| Co60 | 32.1 ± 2.3 | 1.2 ± 0.11 | 3.1 ± 0.28 | 7.3 ± 0.42 | 5.6 ± 0.20 | 45.9 ± 3.6 | 17.0 ± 0.94 | 7.0 ± 0.68 |
| Cold-Mn | 28.6 ± 1.7 | 1.1 ± 0.09 | 3.2 ± 0.33 | 8.4 ± 0.47 | 5.6 ± 0.24 | 44.9 ± 2.1 | 17.8 ± 0.49 | 9.1 ± 0.36 |
| Mn56x1 | 28.4 ± 1.6 | 1.2 ± 0.09 | 3.9 ± 0.54 | 9.7 ± 0.59 | 5.9 ± 0.31 | 49.2 ± 3.3 | 18.4 ± 0.88 | 7.1 ± 1.07 |
| Mn56x3 | 27.5 ± 1.6 | 1.2 ± 0.1 | 4.0 ± 0.33 | 9.1 ± 0.8 | 6.6 ± 0.74 | 48 ± 1.4 | 19.1 ± 0.74 | 9.1 ± 0.99 |
| Gene | GenBank Accession # | Q-PCR Primer Sequences (5′ -> 3′) | |
|---|---|---|---|
| Forward | Reverse | ||
| Bax | NM_007527 | CGTGGACACGGACTCCCCCC | TGATCAGCTCGGGCACTTTA |
| Phlda3 | NM_013750 | AAGCCGTGGAGTGCGTAGAG | GTCTGGATGGCCTGTTGATTC |
| Faim3 | NM_026976 | TTCATGAGCAAAGGACACGC | CACCAGTCCCAAAAGAACCAG |
| Aqp1 | NM_007472 | ACCTGCTGGCGATTGACTACA | CATAGATGAGCACTGCCAGGG |
| Aqp5 | NM_009701 | CTCCCCAGCCTTATCCATTG | CACGATCGGTCCTACCCAGA |
| Smad7 | AF015260 | TTGCTGTGAATCTTACGGGAAG | GGTTTGAGAAAATCCATTGGGT |
| Actb | NM_007393.5 | CTGTCCCTGTATGCCTCTGGTC | TGAGGTAGTCCGTCAGGTCCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abishev, Z.; Ruslanova, B.; Apbassova, S.; Shabdarbayeva, D.; Chaizhunussova, N.; Dyusupov, A.; Azhimkhanov, A.; Zhumadilov, K.; Stepanenko, V.; Ivanov, S.; et al. Effects of Radioactive 56MnO2 Particle Inhalation on Mouse Lungs: A Comparison between C57BL and BALB/c. Int. J. Mol. Sci. 2023, 24, 17605. https://doi.org/10.3390/ijms242417605
Abishev Z, Ruslanova B, Apbassova S, Shabdarbayeva D, Chaizhunussova N, Dyusupov A, Azhimkhanov A, Zhumadilov K, Stepanenko V, Ivanov S, et al. Effects of Radioactive 56MnO2 Particle Inhalation on Mouse Lungs: A Comparison between C57BL and BALB/c. International Journal of Molecular Sciences. 2023; 24(24):17605. https://doi.org/10.3390/ijms242417605
Chicago/Turabian StyleAbishev, Zhaslan, Bakhyt Ruslanova, Saulesh Apbassova, Dariya Shabdarbayeva, Nailya Chaizhunussova, Altai Dyusupov, Almas Azhimkhanov, Kassym Zhumadilov, Valeriy Stepanenko, Sergey Ivanov, and et al. 2023. "Effects of Radioactive 56MnO2 Particle Inhalation on Mouse Lungs: A Comparison between C57BL and BALB/c" International Journal of Molecular Sciences 24, no. 24: 17605. https://doi.org/10.3390/ijms242417605





