Single-Nucleotide Polymorphisms of the PAR2 and IL-17A Genes Are Significantly Associated with Chronic Pain
Abstract
1. Introduction
2. Results
2.1. rs2243057 SNP of PAR2 Gene Is Associated with Chronic Pain
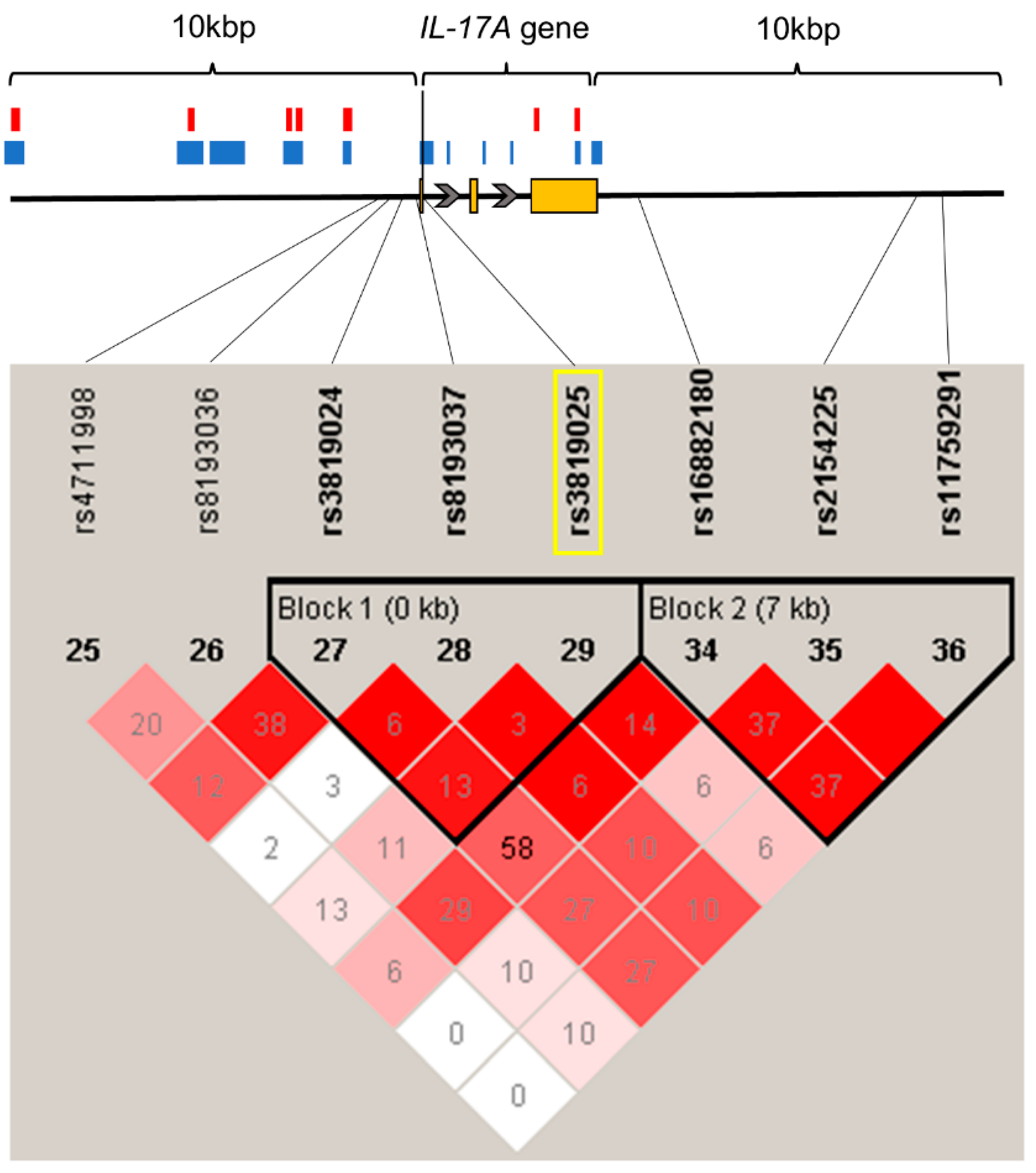
2.2. rs3819025 SNP of the IL-17A Gene Is Associated with Chronic Pain
3. Discussion
4. Materials and Methods
4.1. Design
4.2. Patients with Chronic Pain and Healthy Participants
4.3. Genotyping and Linkage Disequilibrium Analysis
4.4. Public Database Search
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, S.; Kobayashi, F.; Nishihara, M.; Arai, Y.C.; Ikemoto, T.; Kawai, T.; Inoue, M.; Hasegawa, T.; Ushida, T. Chronic pain in the Japanese community—Prevalence, characteristics and impact on quality of life. PLoS ONE 2015, 10, e0129262. [Google Scholar] [CrossRef]
- Nishizawa, D.; Iseki, M.; Arita, H.; Hanaoka, K.; Yajima, C.; Kato, J.; Ogawa, S.; Hiranuma, A.; Kasai, S.; Hasegawa, J.; et al. Genome-wide association study identifies candidate loci associated with chronic pain and postherpetic neuralgia. Mol. Pain 2021, 17, 1744806921999924. [Google Scholar] [CrossRef]
- Aoki, Y.; Nishizawa, D.; Ohka, S.; Kasai, S.; Arita, H.; Hanaoka, K.; Yajima, C.; Iseki, M.; Kato, J.; Ogawa, S.; et al. Rs11726196 single-nucleotide polymorphism of the transient receptor potential canonical 3 (TRPC3) gene is associated with chronic pain. Int. J. Mol. Sci. 2023, 24, 1028. [Google Scholar] [CrossRef] [PubMed]
- Takei-Taniguchi, R.; Imai, Y.; Ishikawa, C.; Sakaguchi, Y.; Nakagawa, N.; Tsuda, T.; Hollenberg, M.D.; Yamanishi, K. Interleukin-17- and protease-activated receptor 2-mediated production of CXCL1 and CXCL8 modulated by cyclosporine A, vitamin D3 and glucocorticoids in human keratinocytes. J. Dermatol. 2012, 39, 625–631. [Google Scholar] [CrossRef]
- Mrozkova, P.; Palecek, J.; Spicarova, D. The role of protease-activated receptor type 2 in nociceptive signaling and pain. Physiol. Res. 2016, 65, 357–367. [Google Scholar] [CrossRef]
- Antoniak, S.; Mackman, N. Multiple roles of the coagulation protease cascade during virus infection. Blood 2014, 123, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Lin, H.; Liang, H.P.H.; McKelvey, K.; Zhao, R.; March, L.; Jackson, C. Deficiency of protease-activated receptor (PAR) 1 and PAR2 exacerbates collagen-induced arthritis in mice via differing mechanisms. Rheumatology 2021, 60, 2990–3003. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Arora, N. Serine protease allergen favours Th2 responses via PAR-2 and STAT3 activation in murine model. Allergy 2018, 73, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, C.X.; Wang, Y.P.; Ma, K.T.; Li, L.; Yin, J.W.; Dai, Z.G.; Wang, S.; Si, J.Q. The association between the expression of PAR2 and TMEM16A and neuropathic pain. Mol. Med. Rep. 2018, 17, 3744–3750. [Google Scholar] [CrossRef] [PubMed]
- Bushell, T.J.; Cunningham, M.R.; McIntosh, K.A.; Moudio, S.; Plevin, R. Protease-activated receptor 2: Are common functions in glial and immune cells linked to inflammation-related CNS disorders? Curr. Drug Targets 2016, 17, 1861–1870. [Google Scholar] [CrossRef]
- Jimenez-Vargas, N.N.; Pattison, L.A.; Zhao, P.; Lieu, T.; Latorre, R.; Jensen, D.D.; Castro, J.; Aurelio, L.; Le, G.T.; Flynn, B.; et al. Protease-activated receptor-2 in endosomes signals persistent pain of irritable bowel syndrome. Proc. Natl. Acad. Sci. USA 2018, 115, E7438–E7447. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.J.; Li, H.C.; Cowan, A.A.; Liu, S.; Zhang, Y.K.; Song, X.J. Chronic compression or acute dissociation of dorsal root ganglion induces cAMP-dependent neuronal hyperexcitability through activation of PAR2. Pain 2012, 153, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- Lam, D.K.; Dang, D.; Zhang, J.; Dolan, J.C.; Schmidt, B.L. Novel animal models of acute and chronic cancer pain: A pivotal role for PAR2. J. Neurosci. 2012, 32, 14178–14183. [Google Scholar] [CrossRef] [PubMed]
- Galvin, D.A.; McCrory, C. The role of T-lymphocytes in neuropathic pain initiation, development of chronicity and treatment. Brain Behav. Immun. Health 2021, 18, 100371. [Google Scholar] [CrossRef]
- Waisman, A.; Hauptmann, J.; Regen, T. The role of IL-17 in CNS diseases. Acta Neuropathol. 2015, 129, 625–637. [Google Scholar] [CrossRef]
- Huppler, A.R.; Bishu, S.; Gaffen, S.L. Mucocutaneous candidiasis: The IL-17 pathway and implications for targeted immunotherapy. Arthritis Res. Ther. 2012, 14, 217. [Google Scholar] [CrossRef]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef]
- Liu, H.; Dolkas, J.; Hoang, K.; Angert, M.; Chernov, A.V.; Remacle, A.G.; Shiryaev, S.A.; Strongin, A.Y.; Nishihara, T.; Shubayev, V.I. The alternatively spliced fibronectin CS1 isoform regulates IL-17A levels and mechanical allodynia after peripheral nerve injury. J. Neuroinflamm. 2015, 12, 158. [Google Scholar] [CrossRef]
- Heyn, J.; Luchting, B.; Azad, S.C. Smoking associated T-cell imbalance in patients with chronic pain. Nicotine Tob. Res. 2020, 22, 111–117. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Zhong, Y. Interleukin-17A: The key cytokine in neurodegenerative diseases. Front. Aging Neurosci. 2020, 12, 566922. [Google Scholar] [CrossRef]
- Xue, M.; Lin, H.; Zhao, R.; Fryer, C.; March, L.; Jackson, C.J. Activated protein C protects against murine contact dermatitis by suppressing protease-activated receptor 2. Int. J. Mol. Sci. 2022, 23, 516. [Google Scholar] [CrossRef]
- Nadeem, A.; Al-Harbi, N.O.; Ahmad, S.F.; Ibrahim, K.E.; Alotaibi, M.R.; Siddiqui, N.; Alsharari, S.D.; Attia, S.M.; Al-Harbi, M.M. Protease activated receptor-2 mediated upregulation of IL-17 receptor signaling on airway epithelial cells is responsible for neutrophilic infiltration during acute exposure of house dust mite allergens in mice. Chem. Biol. Interact. 2019, 304, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef]
- Okada, H.; Khoury, S.J. Type17 T-cells in central nervous system autoimmunity and tumors. J. Clin. Immunol. 2012, 32, 802–808. [Google Scholar] [CrossRef][Green Version]
- Prajeeth, C.K.; Kronisch, J.; Khorooshi, R.; Knier, B.; Toft-Hansen, H.; Gudi, V.; Floess, S.; Huehn, J.; Owens, T.; Korn, T.; et al. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J. Neuroinflamm. 2017, 14, 204. [Google Scholar] [CrossRef]
- Radulovic, M.; Yoon, H.; Wu, J.; Mustafa, K.; Fehlings, M.G.; Scarisbrick, I.A. Genetic targeting of protease activated receptor 2 reduces inflammatory astrogliosis and improves recovery of function after spinal cord injury. Neurobiol. Dis. 2015, 83, 75–89. [Google Scholar] [CrossRef]
- Soeda, M.; Ohka, S.; Nishizawa, D.; Hasegawa, J.; Nakayama, K.; Ebata, Y.; Ichinohe, T.; Fukuda, K.I.; Ikeda, K. Cold pain sensitivity is associated with single-nucleotide polymorphisms of PAR2/F2RL1 and TRPM8. Mol. Pain 2021, 17, 17448069211002009. [Google Scholar] [CrossRef]
- Yan, N.; Yu, Y.L.; Yang, J.; Qin, Q.; Zhu, Y.F.; Wang, X.; Song, R.H.; Zhang, J.A. Association of interleukin-17A and -17F gene single-nucleotide polymorphisms with autoimmune thyroid diseases. Autoimmunity 2012, 45, 533–539. [Google Scholar] [CrossRef] [PubMed]
- GTEx. Portal: rs2243057 of PAR2/F2RL1 Gene, Multi-Tissue eQTL Comparison, Variant Page. Available online: https://www.gtexportal.org/home/snp/rs2243057 (accessed on 3 July 2023).
- ZENBU: PAR2/F2RL1, [FANTOM CAT] v1.0.0 Main View v1 (Modified). Available online: https://fantom.gsc.riken.jp/zenbu/gLyphs/index.html#config=9O1caUYdJRqglKrQ1IUVu;loc=hg19::chr5:76110662..76135236+ (accessed on 3 July 2023).
- ZENBU: IL-17A, [FANTOM CAT] v1.0.0 Main View v1 (Modified). Available online: https://fantom.gsc.riken.jp/zenbu/gLyphs/index.html#config=9O1caUYdJRqglKrQ1IUVu;loc=hg19::chr6:52050122..52056499 (accessed on 3 July 2023).
- Tang, H.; Pei, H.; Xia, Q.; Tang, Y.; Huang, J.; Huang, J.; Pei, F. Role of gene polymorphisms/haplotypes and serum levels of interleukin-17A in susceptibility to viral myocarditis. Exp. Mol. Pathol. 2018, 104, 140–145. [Google Scholar] [CrossRef]
- Nishizawa, D.; Fukuda, K.; Kasai, S.; Hasegawa, J.; Aoki, Y.; Nishi, A.; Saita, N.; Koukita, Y.; Nagashima, M.; Katoh, R.; et al. Genome-wide association study identifies a potent locus associated with human opioid sensitivity. Mol. Psychiatry 2014, 19, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, D.; Fukuda, K.; Kasai, S.; Ogai, Y.; Hasegawa, J.; Sato, N.; Yamada, H.; Tanioka, F.; Sugimura, H.; Hayashida, M.; et al. Association between KCNJ6 (GIRK2) gene polymorphism rs2835859 and post-operative analgesia, pain sensitivity, and nicotine dependence. J. Pharmacol. Sci. 2014, 126, 253–263. [Google Scholar] [CrossRef]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.B.; Schaffner, S.F.; Nguyen, H.; Moore, J.M.; Roy, J.; Blumenstiel, B.; Higgins, J.; DeFelice, M.; Lochner, A.; Faggart, M.; et al. The structure of haplotype blocks in the human genome. Science 2002, 296, 2225–2229. [Google Scholar] [CrossRef] [PubMed]
- de Bakker, P.I.; Yelensky, R.; Pe’er, I.; Gabriel, S.B.; Daly, M.J.; Altshuler, D. Efficiency and power in genetic association studies. Nat. Genet. 2005, 37, 1217–1223. [Google Scholar] [CrossRef]

| Genotype Groups | Attribution | n | n (%) | p |
|---|---|---|---|---|
| AA/AG/GG | Chronic pain | 9/80/102 | 4.7/41.9/53.4 | 0.079 |
| Healthy subjects | 28/101/153 | 9.9/35.8/54.3 | ||
| AA/AG + GG | Chronic pain | 9/182 | 4.7/95.3 | 0.038 * |
| Healthy subjects | 28/254 | 9.9/90.1 | ||
| AA + AG/GG | Chronic pain | 89/102 | 46.6/53.4 | 0.85 |
| Healthy subjects | 129/153 | 45.7/54.3 |
| Genotype Groups | Attribution | n | n (%) | p |
|---|---|---|---|---|
| AA/AG/GG | Chronic pain | 9/65/117 | 4.7/34.0/61.3 | 0.016 * |
| Healthy subjects | 29/113/140 | 10.3/40.1/49.6 | ||
| AA/AG + GG | Chronic pain | 9/182 | 4.7/95.3 | 0.029 * |
| Healthy subjects | 29/253 | 10.3/89.7 | ||
| AA + AG/GG | Chronic pain | 74/117 | 38.7/61.3 | 0.013 * |
| Healthy subjects | 142/140 | 50.4/49.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soeda, M.; Ohka, S.; Nishizawa, D.; Iseki, M.; Yamaguchi, K.; Arita, H.; Hanaoka, K.; Kato, J.; Ogawa, S.; Hiranuma, A.; et al. Single-Nucleotide Polymorphisms of the PAR2 and IL-17A Genes Are Significantly Associated with Chronic Pain. Int. J. Mol. Sci. 2023, 24, 17627. https://doi.org/10.3390/ijms242417627
Soeda M, Ohka S, Nishizawa D, Iseki M, Yamaguchi K, Arita H, Hanaoka K, Kato J, Ogawa S, Hiranuma A, et al. Single-Nucleotide Polymorphisms of the PAR2 and IL-17A Genes Are Significantly Associated with Chronic Pain. International Journal of Molecular Sciences. 2023; 24(24):17627. https://doi.org/10.3390/ijms242417627
Chicago/Turabian StyleSoeda, Moe, Seii Ohka, Daisuke Nishizawa, Masako Iseki, Keisuke Yamaguchi, Hideko Arita, Kazuo Hanaoka, Jitsu Kato, Setsuro Ogawa, Ayako Hiranuma, and et al. 2023. "Single-Nucleotide Polymorphisms of the PAR2 and IL-17A Genes Are Significantly Associated with Chronic Pain" International Journal of Molecular Sciences 24, no. 24: 17627. https://doi.org/10.3390/ijms242417627
APA StyleSoeda, M., Ohka, S., Nishizawa, D., Iseki, M., Yamaguchi, K., Arita, H., Hanaoka, K., Kato, J., Ogawa, S., Hiranuma, A., Hasegawa, J., Nakayama, K., Ebata, Y., Hayashida, M., Ichinohe, T., Fukuda, K.-i., & Ikeda, K. (2023). Single-Nucleotide Polymorphisms of the PAR2 and IL-17A Genes Are Significantly Associated with Chronic Pain. International Journal of Molecular Sciences, 24(24), 17627. https://doi.org/10.3390/ijms242417627






