Irisin Modulates Inflammatory, Angiogenic, and Osteogenic Factors during Fracture Healing
Abstract
1. Introduction
2. Results
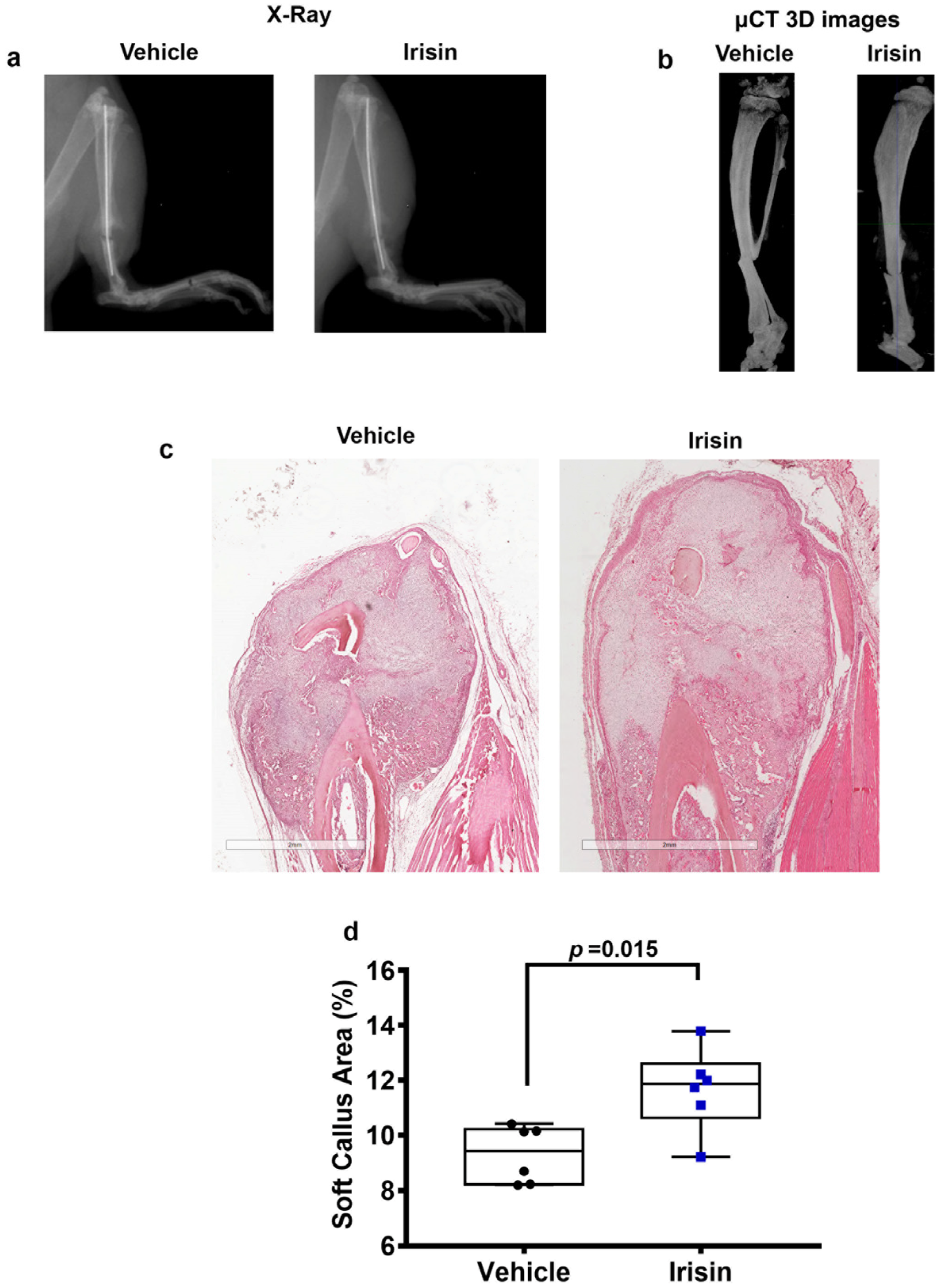
2.1. Irisin Increases Soft Callus Area at 10 Days Post-Fracture
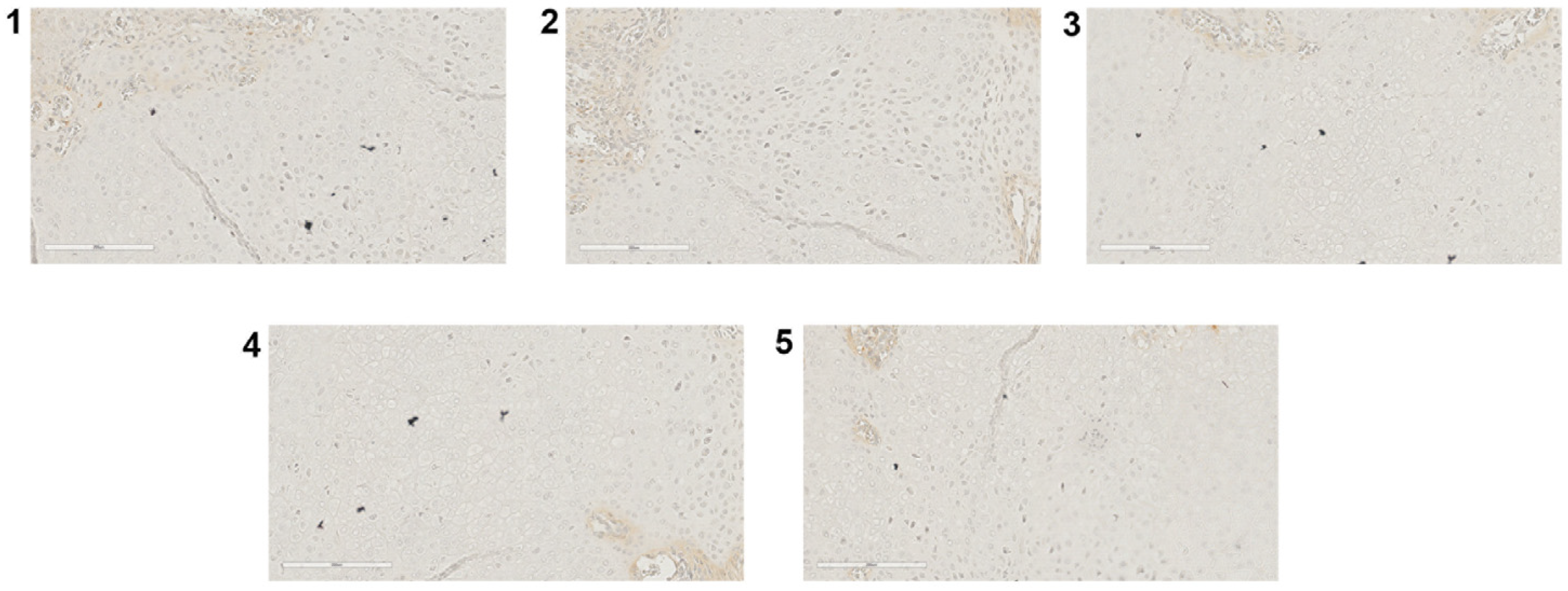
2.2. Irisin Induces Inhibition of Inflammation Markers at 10 Days Post Fracture
2.3. Irisin Promotes Angiogenesis and Matrix Degradation at 10 Days Post Fracture
2.4. Irisin Induces the Secretion of an Osteogenic Factor at 10 Days Post-Fracture
3. Discussion
4. Materials and Methods
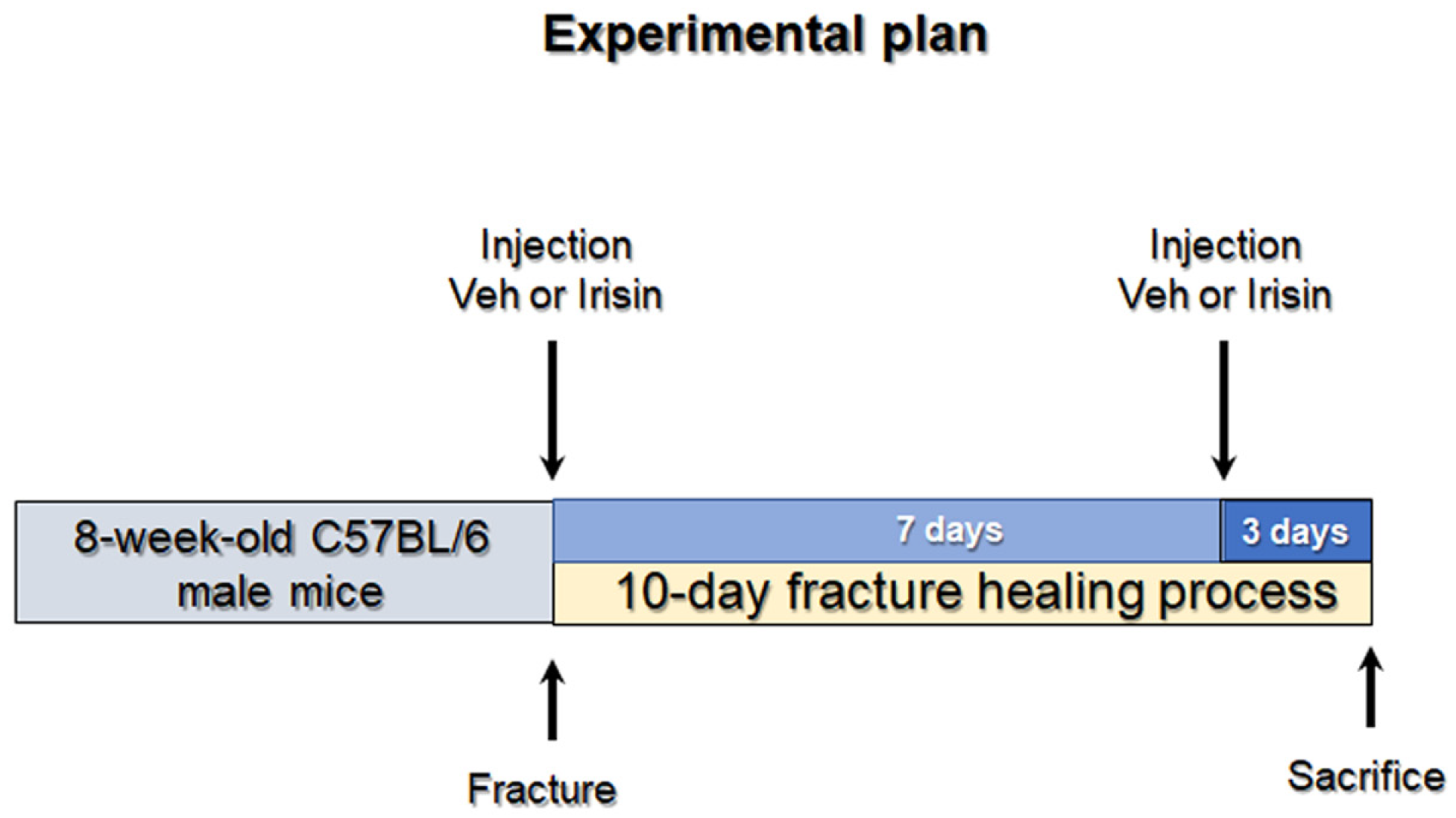
4.1. Tibial Fracture Procedure and Experimental Design
4.2. X-ray and Micro-Computed Tomography
4.3. Histological and Immunohistochemical Assays
4.4. Morphometric Analysis
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Morice, A.; Ducellier, F.; Bizot, P.; Orthopaedics and Traumatology Society of Western France. Total hip arthroplasty after failed fixation of a proximal femur fracture: Analysis of 59 cases of intra- and extra-capsular fractures. Orthop. Traumatol. Surg. Res. 2018, 104, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Hak, D.J. The biology of fracture healing in osteoporosis and in the presence of anti-osteoporotic drugs. Injury 2018, 49, 1461–1465. [Google Scholar] [CrossRef] [PubMed]
- Kodama, J.; Wilkinson, K.J.; Iwamoto, M.; Otsuru, S.; Enomoto-Iwamoto, M. The role of hypertrophic chondrocytes in regulation of the cartilage-to-bone transition in fracture healing. Bone Rep. 2022, 17, 101616. [Google Scholar] [CrossRef] [PubMed]
- Hozain, S.; Cottrell, J. CDllb+ targeted depletion of macrophages negatively affects bone fracture healing. Bone 2020, 138, 115479. [Google Scholar] [CrossRef]
- Kon, T.; Cho, T.J.; Aizawa, T.; Yamazaki, M.; Nooh, N.; Graves, D.; Gerstenfeld, L.C.; Einhorn, T.A. Expression of osteoprotegerin, receptor activator of NF-kappaB ligand (osteoprotegerin ligand) and related proinflammatory cytokines during fracture healing. J. Bone Miner. Res. 2001, 16, 1004–1014. [Google Scholar] [CrossRef]
- Einhorn, T.A. The Cell and Molecular Biology of Fracture Healing. Clin. Orthop. Relat. Res. 1998, 355, S7–S21. [Google Scholar] [CrossRef]
- Mountziaris, P.M.; Tzouanas, S.N.; Mikos, A.G. Dose effect of tumor necrosis factor-alpha on in vitro osteogenic dif-ferentiation of mesenchymal stem cells on biodegradable polymeric microfiber scaffolds. Biomaterials 2010, 31, 1666–1675. [Google Scholar] [CrossRef]
- Zhou, F.H.; Foster, B.K.; Zhou, X.-F.; Cowin, A.J.; Xian, C.J. TNF-α Mediates p38 MAP Kinase Activation and Negatively Regulates Bone Formation at the Injured Growth Plate in Rats. J. Bone Miner. Res. 2006, 21, 1075–1088. [Google Scholar] [CrossRef]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef]
- D’Angelo, M.; Yan, Z.; Nooreyazdan, M.; Pacifici, M.; Sarment, D.S.; Billings, P.C.; Leboy, P.S. MMP-13 is induced during chondrocyte hypertrophy. J. Cell. Biochem. 2000, 77, 678–693. [Google Scholar] [CrossRef]
- Uusitalo, H.; Hiltunen, A.; Söderström, M.; Aro, H.T.; Vuorio, E. Expression of cathepsins B, H, K, L, and S and matrix metalloproteinases 9 and 13 during chondrocyte hypertrophy and endochondral ossification in mouse fracture callus. Calcif. Tissue Int. 2000, 67, 382–390. [Google Scholar] [CrossRef]
- Kosaki, N.; Takaishi, H.; Kamekura, S.; Kimura, T.; Okada, Y.; Minqi, L.; Amizuka, N.; Chung, U.-I.; Nakamura, K.; Kawaguchi, H.; et al. Impaired bone fracture healing in matrix metalloproteinase-13 deficient mice. Biochem. Biophys. Res. Commun. 2007, 354, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Keramaris, N.C.; Calori, G.M.; Nikolaou, V.S.; Schemitsch, E.H.; Giannoudis, F.V. Fracture vascularity and bone healing: A systematic review of the role of VEGF. Injury 2008, 39, S45–S57. [Google Scholar] [CrossRef]
- Buccoliero, C.; Oranger, A.; Colaianni, G.; Pignataro, P.; Zerlotin, R.; Lovero, R.; Errede, M.; Grano, M. The effect of Irisin on bone cells in vivo and in vitro. Biochem. Soc. Trans. 2021, 49, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Oranger, A.; Mori, G.; Brunetti, G.; Colucci, S.; Cinti, S.; Grano, M. Irisin enhances osteoblast differentiation in vitro. Int. J. Endocrinol. 2014, 2014, 902186. [Google Scholar] [CrossRef] [PubMed]
- Storlino, G.; Colaianni, G.; Sanesi, L.; Lippo, L.; Brunetti, G.; Errede, M.; Colucci, S.; Passeri, G.; Grano, M. Irisin Prevents Disuse-Induced Osteocyte Apoptosis. J. Bone Miner. Res. 2020, 35, 766–775. [Google Scholar] [CrossRef]
- Estell, E.G.; Le, P.T.; Vegting, Y.; Kim, H.; Wrann, C.; Bouxsein, M.L.; Nagano, K.; Baron, R.; Spiegelman, B.M.; Rosen, C.J.; et al. Irisin directly stimulates osteoclastogenesis and bone resorption in vitro and in vivo. eLife 2020, 9, e58172. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Valverde, P.; Zhu, X.; Murray, D.; Wu, Y.; Yu, L.; Jiang, H.; Dard, M.M.; Huang, J.; Xu, Z.; et al. Exercise-induced irisin in bone and systemic irisin administration reveal new regulatory mechanisms of bone metabolism. Bone Res. 2017, 5, 16056. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Qiao, X.; Zeng, R.; Cheng, R.; Zhang, J.; Luo, Y.; Nie, Y.; Hu, Y.; Yang, Z.; Zhang, J.; et al. Irisin promotes proliferation but inhibits differentiation in osteoclast precursor cells. FASEB J. 2018, 32, 5813–5823. [Google Scholar] [CrossRef]
- Colucci, S.; Colaianni, G.; Brunetti, G.; Ferranti, F.; Mascetti, G.; Mori, G.; Grano, M. Irisin prevents microgravity-induced impairment of osteoblast differentiation in vitro during the space flight CRS-14 mission. FASEB J. 2020, 34, 10096–10106. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Cuscito, C.; Mongelli, T.; Pignataro, P.; Buccoliero, C.; Liu, P.; Lu, P.; Sartini, L.; Di Comite, M.; Mori, G.; et al. The myokine irisin increases cortical bone mass. Proc. Natl. Acad. Sci. USA 2015, 112, 12157–12162. [Google Scholar] [CrossRef]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Mantzoros, C.S. An update on the validity of irisin assays and the link between irisin and hepatic me-tabolism. Metabolism 2015, 64, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A.; et al. Irisin—A myth rather than an exercise-inducible myokine. Sci. Rep. 2015, 5, 8889. [Google Scholar] [CrossRef]
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Sreekumaran Nair, K.; Gygi, S.P.; Spiegelman, B.M. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell Metab. 2015, 22, 734–740. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Bisbinas, I.; Katsarou, A.; Filippaios, A.; Mantzoros, C.S. Circulating irisin is associated with osteoporotic fractures in postmenopausal women with low bone mass but is not af-fected by either teriparatide or denosumab treatment for 3 months. Osteoporos. Int. 2014, 25, 1633–1642. [Google Scholar] [CrossRef]
- Palermo, A.; Strollo, R.; Maddaloni, E.; Tuccinardi, D.; D’Onofrio, L.; Briganti, S.I.; Defeudis, G.; De Pascalis, M.; Lazzaro, M.C.; Colleluori, G.; et al. Irisin is associated with osteoporotic fractures independently of bone mineral density, body composition or daily physical activity. Clin. Endocrinol. 2015, 82, 615–619. [Google Scholar] [CrossRef]
- Yan, J.; Liu, H.-J.; Guo, W.-C.; Yang, J. Low serum concentrations of Irisin are associated with increased risk of hip fracture in Chinese older women. Jt. Bone Spine 2018, 85, 353–358. [Google Scholar] [CrossRef]
- Faienza, M.F.; Brunetti, G.; Sanesi, L.; Colaianni, G.; Celi, M.; Piacente, L.; D’Amato, G.; Schipani, E.; Colucci, S.; Grano, M. High irisin levels are associated with better glycemic control and bone health in children with Type 1 diabetes. Diabetes Res. Clin. Pract. 2018, 141, 10–17. [Google Scholar] [CrossRef]
- Singhal, V.; Lawson, E.; Ackerman, K.E.; Fazeli, P.K.; Clarke, H.; Lee, H.; Eddy, K.; Marengi, D.A.; Derrico, N.P.; Bouxsein, M.L.; et al. Irisin Levels Are Lower in Young Amenorrheic Athletes Compared with Eumenorrheic Athletes and Non-Athletes and Are Associated with Bone Density and Strength Estimates. PLoS ONE 2014, 9, e100218. [Google Scholar] [CrossRef] [PubMed]
- Vadalà, G.; Di Giacomo, G.; Ambrosio, L.; Cannata, F.; Cicione, C.; Papalia, R.; Denaro, V. Irisin Recovers Osteoarthritic Chondrocytes In Vitro. Cells 2020, 9, 1478. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-S.; Kuo, C.-W.; Ko, J.-Y.; Chen, Y.-S.; Wang, S.-Y.; Ke, H.-J.; Kuo, P.-C.; Lee, C.-H.; Wu, J.-C.; Lu, W.-B.; et al. Irisin Mitigates Oxidative Stress, Chondrocyte Dysfunction and Osteoarthritis Development through Regulating Mitochondrial Integrity and Autophagy. Antioxidants 2020, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Colucci, S.C.; Buccoliero, C.; Sanesi, L.; Errede, M.; Colaianni, G.; Annese, T.; Khan, M.P.; Zerlotin, R.; Dicarlo, M.; Schipani, E.; et al. Systemic Administration of Recombinant Irisin Accelerates Fracture Healing in Mice. Int. J. Mol. Sci. 2021, 22, 10863. [Google Scholar] [CrossRef]
- Kan, T.; He, Z.; Du, J.; Xu, M.; Cui, J.; Han, X.; Tong, D.; Li, H.; Yan, M.; Yu, Z. Irisin promotes fracture healing by improving osteogenesis and angiogenesis. J. Orthop. Transl. 2022, 37, 37–45. [Google Scholar] [CrossRef]
- Narayanan, S.A.; Metzger, C.E.; Bloomfield, S.A.; Zawieja, D.C. Inflammation-induced lymphatic architecture and bone turnover changes are ameliorated by irisin treatment in chronic inflammatory bowel disease. FASEB J. 2018, 32, 4848–4861. [Google Scholar] [CrossRef]
- Wahl, E.C.; Aronson, J.; Liu, L.; Fowlkes, J.L.; Thrailkill, K.M.; Bunn, R.C.; Skinner, R.A.; Miller, M.J.; Cockrell, G.E.; Clark, L.M.; et al. Restoration of regenerative osteoblastogenesis in aged mice: Modulation of TNF. J. Bone Miner. Res. 2010, 25, 114–123. [Google Scholar] [CrossRef]
- Gerstenfeld, L.C.; Cho, T.-J.; Kon, T.; Aizawa, T.; Tsay, A.; Fitch, J.; Barnes, G.L.; Graves, D.T.; Einhorn, T. Impaired Fracture Healing in the Absence of TNF-α Signaling: The Role of TNF-α in Endochondral Cartilage Resorption. J. Bone Miner. Res. 2003, 18, 1584–1592. [Google Scholar] [CrossRef]
- Rundle, C.H.; Mohan, S.; Edderkaoui, B. Duffy Antigen Receptor for Chemokines Regulates Post-Fracture Inflammation. PLoS ONE 2013, 8, e77362. [Google Scholar] [CrossRef]
- Hoff, P.; Gaber, T.; Strehl, C.; Schmidt-Bleek, K.; Lang, A.; Huscher, D.; Burmester, G.R.; Schmidmaier, G.; Perka, C.; Duda, G.N.; et al. Immunological characterization of the early human fracture hematoma. Immunol. Res. 2016, 64, 1195–1206. [Google Scholar] [CrossRef]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J.; Nanchahal, J. TNF-α promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, E.; Glotzer, D.J.; Hartmann, C.; Thomas, D.; Fukai, N.; Soker, S.; Olsen, B.R. Tissue specific regulation of VEGF expression during bone development requires Cbfa1/Runx2. Mech. Dev. 2001, 106, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Stickens, D.; Behonick, D.; Ortega, N.; Heyer, B.; Hartenstein, B.; Yu, Y.; Fosang, A.; Schorpp-Kistner, M.; Angel, P.; Werb, Z. Altered endochondral bone development in matrix metalloproteinase 13-deficient mice. Development 2004, 131, 5883–5895. [Google Scholar] [CrossRef]
- Mi, M.; Jin, H.; Wang, B.; Yukata, K.; Sheu, T.-J.; Ke, Q.H.; Tong, P.; Im, H.-J.; Xiao, G.; Chen, D. Chondrocyte BMP2 signaling plays an essential role in bone fracture healing. Gene 2013, 512, 211–218. [Google Scholar] [CrossRef]
- Haffner-Luntzer, M.; Kovtun, A.; Rapp, A.E.; Ignatius, A. Mouse Models in Bone Fracture Healing Research. Curr. Mol. Biol. Rep. 2016, 2, 101–111. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oranger, A.; Zerlotin, R.; Buccoliero, C.; Sanesi, L.; Storlino, G.; Schipani, E.; Kozloff, K.M.; Mori, G.; Colaianni, G.; Colucci, S.; et al. Irisin Modulates Inflammatory, Angiogenic, and Osteogenic Factors during Fracture Healing. Int. J. Mol. Sci. 2023, 24, 1809. https://doi.org/10.3390/ijms24031809
Oranger A, Zerlotin R, Buccoliero C, Sanesi L, Storlino G, Schipani E, Kozloff KM, Mori G, Colaianni G, Colucci S, et al. Irisin Modulates Inflammatory, Angiogenic, and Osteogenic Factors during Fracture Healing. International Journal of Molecular Sciences. 2023; 24(3):1809. https://doi.org/10.3390/ijms24031809
Chicago/Turabian StyleOranger, Angela, Roberta Zerlotin, Cinzia Buccoliero, Lorenzo Sanesi, Giuseppina Storlino, Ernestina Schipani, Kenneth Michael Kozloff, Giorgio Mori, Graziana Colaianni, Silvia Colucci, and et al. 2023. "Irisin Modulates Inflammatory, Angiogenic, and Osteogenic Factors during Fracture Healing" International Journal of Molecular Sciences 24, no. 3: 1809. https://doi.org/10.3390/ijms24031809
APA StyleOranger, A., Zerlotin, R., Buccoliero, C., Sanesi, L., Storlino, G., Schipani, E., Kozloff, K. M., Mori, G., Colaianni, G., Colucci, S., & Grano, M. (2023). Irisin Modulates Inflammatory, Angiogenic, and Osteogenic Factors during Fracture Healing. International Journal of Molecular Sciences, 24(3), 1809. https://doi.org/10.3390/ijms24031809







