Interactions between Dental MSCs and Biomimetic Composite Scaffold during Bone Remodeling Followed by In Vivo Real-Time Bioimaging
Abstract
:1. Introduction
2. Results
2.1. Mechanical and Rheological Behavior
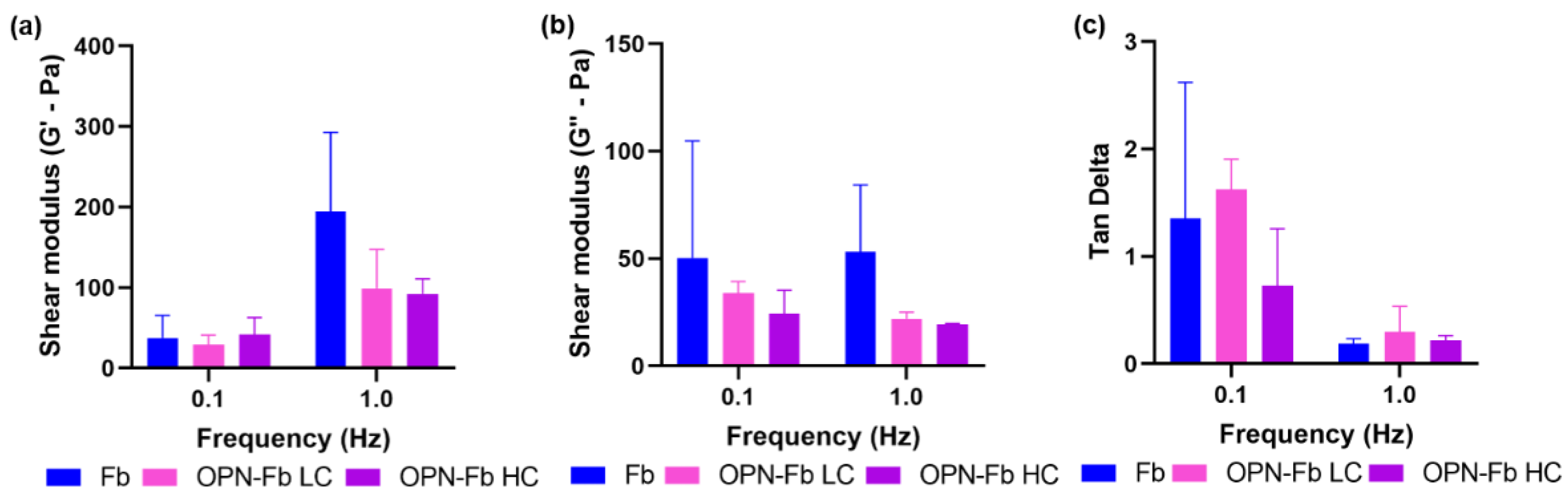
2.1.1. Viscoelastic Analysis of Osteopontin–Fibrin Hydrogel (OPN-Fb)
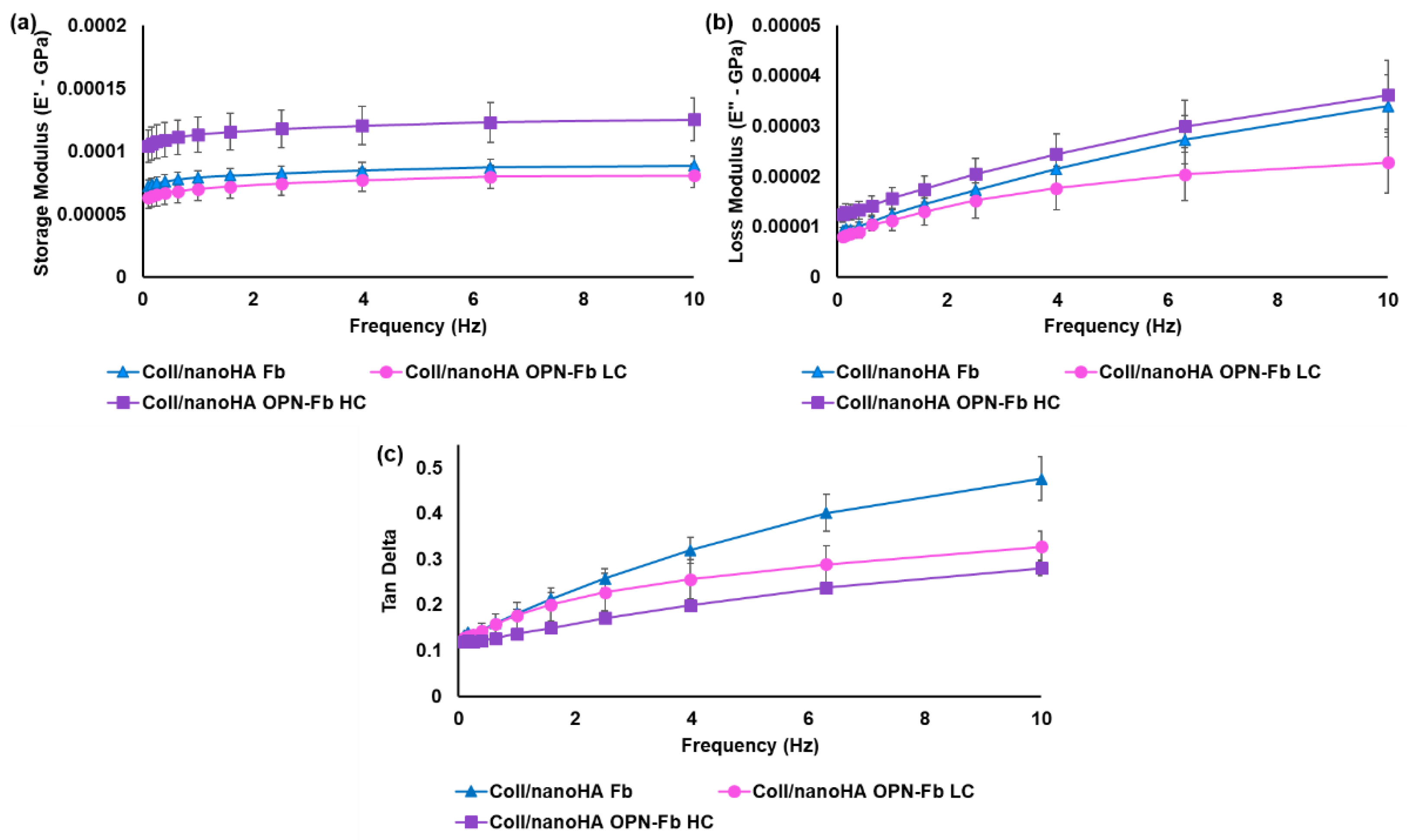
2.1.2. Mechanical Characterization of the Coll/nanoHA Scaffold Loaded with OPN-Fibrin Gel
2.2. In Vitro Biological Studies
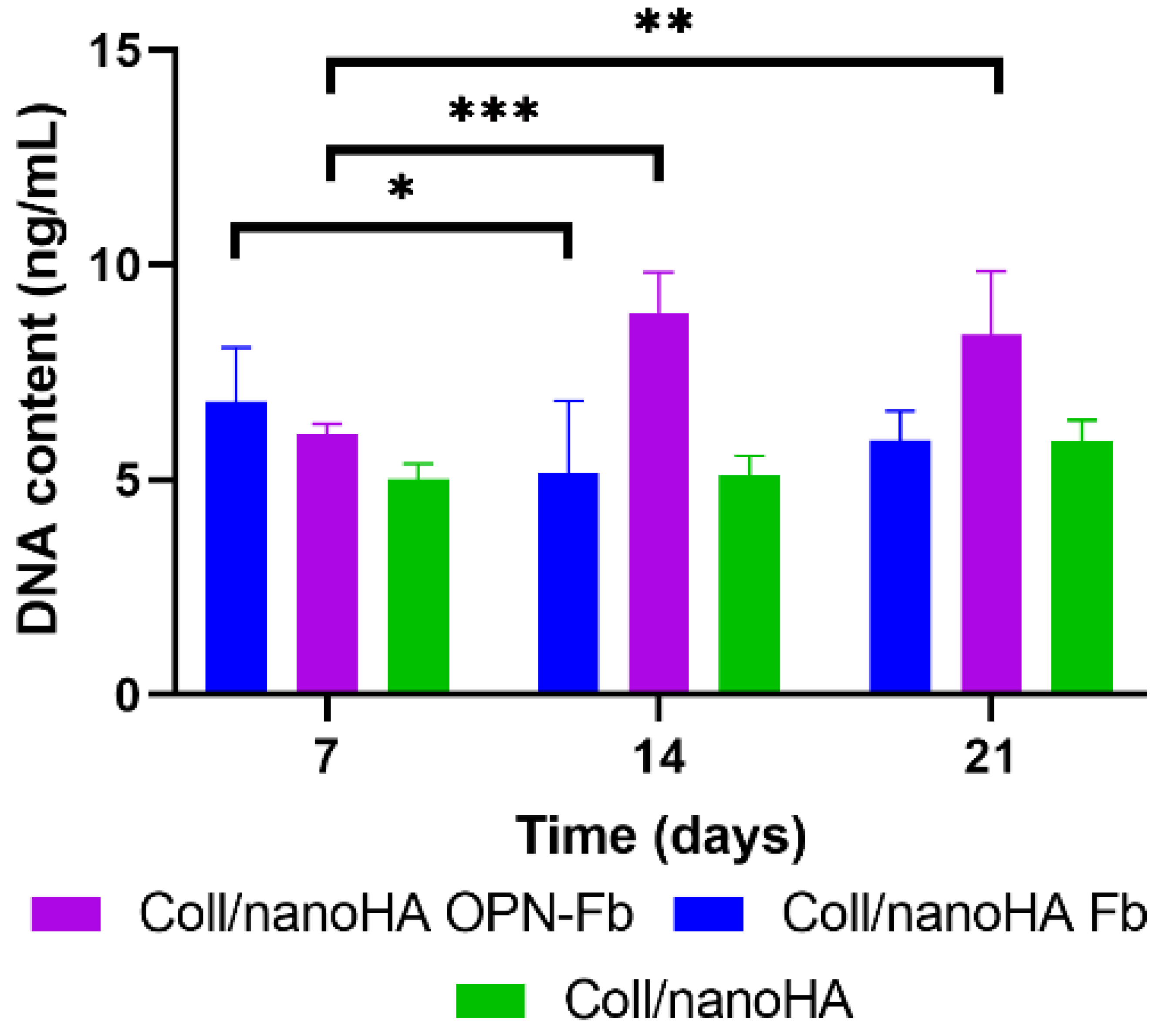
2.2.1. DNA Quantification Assay
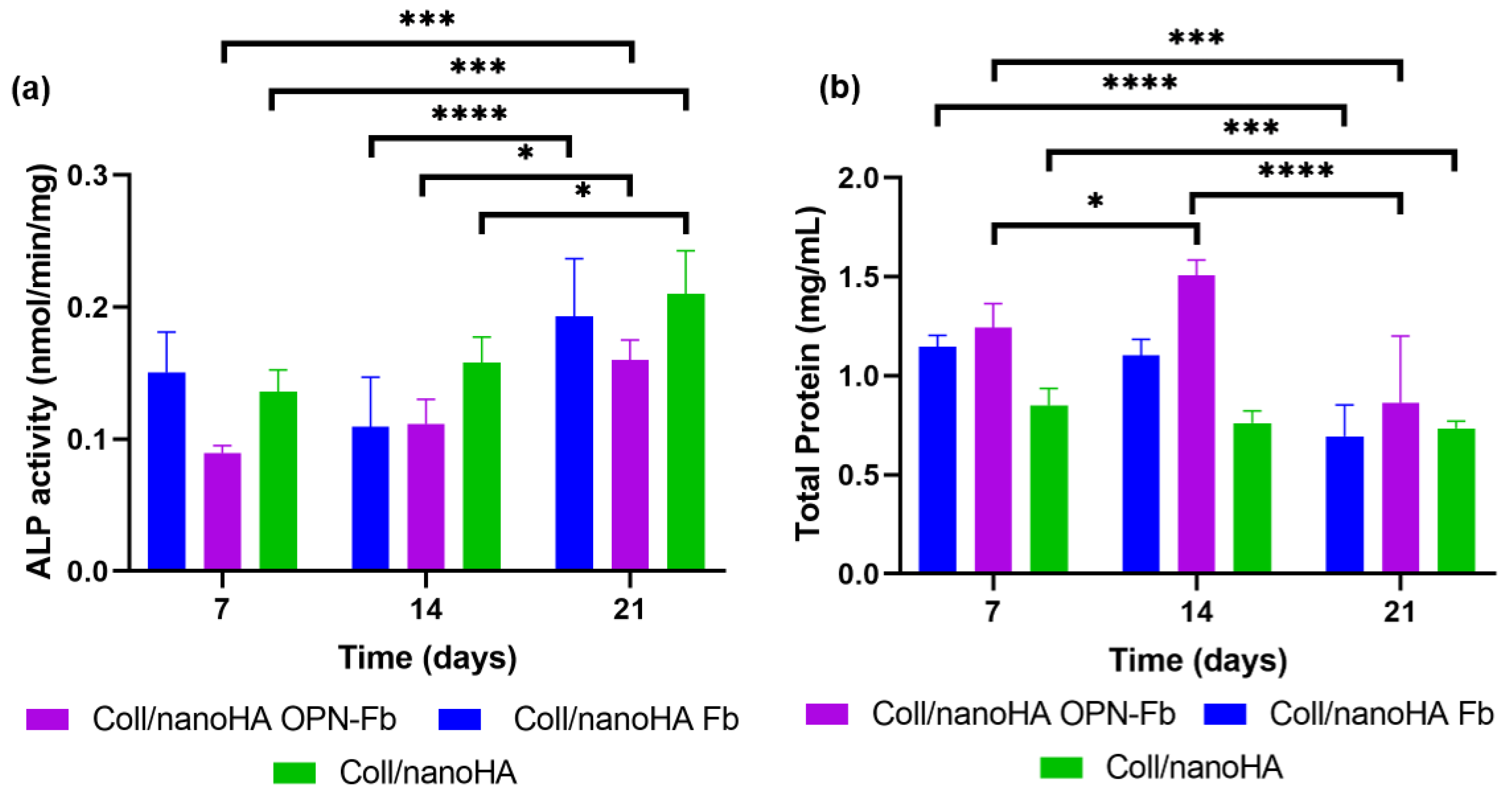
2.2.2. Cellular Differentiation Assay
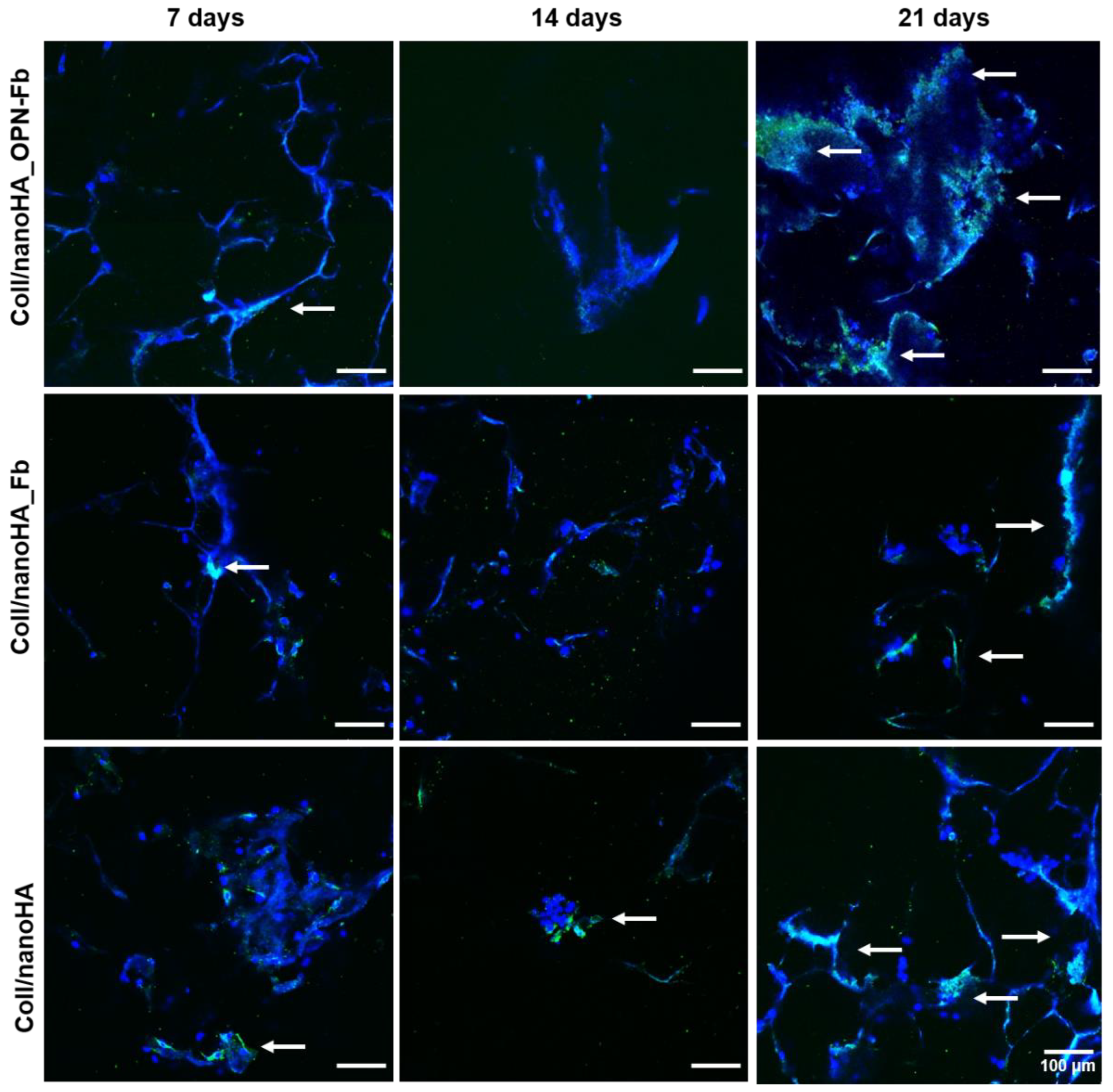
2.2.3. Confocal Laser Scanning Microscopy
2.2.4. Assessment of Immune Cell Activation (Macrophages Polarization—M1 and M2)
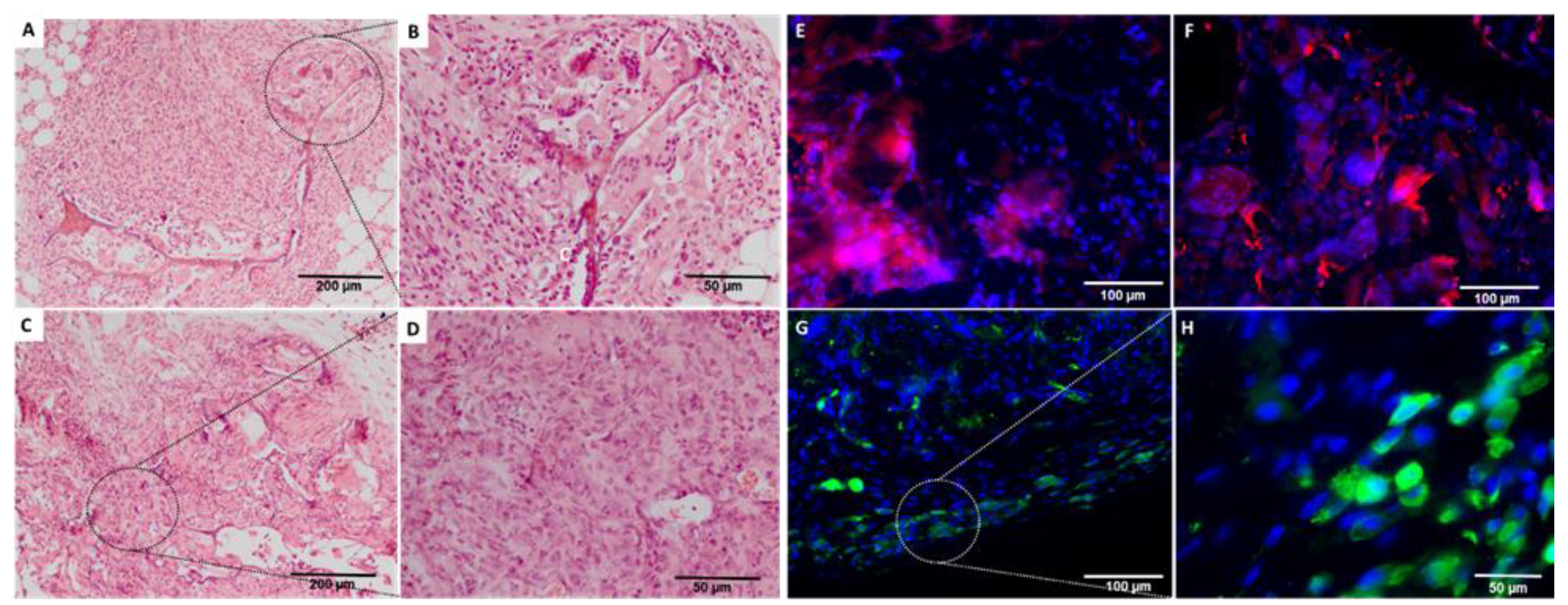
2.3. In Vivo Biological Evaluation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Biomimetic Composite Production
4.2.1. Preparation of Collagen/Nanohydroxyapatite Scaffolds
4.2.2. Preparation of OPN–Fibrin Hydrogel
4.3. Mechanical Characterization
4.3.1. Rheometer Analysis of OPN–Fibrin Hydrogel
4.3.2. Dynamic Mechanical Analysis of Coll/nanoHA Scaffold Loaded with OPN–Fibrin Hydrogel
4.4. In Vitro Biological Evaluation
4.4.1. Establishment of Stem Cell Cultures from the Human Dental Follicle (hDFMSC)
4.4.2. Cellular Delivery within OPN–Fibrin Hydrogel
4.4.3. Cellular Proliferation Assay
4.4.4. Cellular Differentiation Assay
4.4.5. Confocal Laser Scanning Microscopy (CLSM)
4.4.6. Assessing Immune Cell Activation (Macrophages—M1 and M2) in Direct Contact with hDFMSC within Biomimetic Scaffold or Indirect Contact with Exposure to Soluble Factors (Secretome)
4.5. In Vivo Evaluation
4.5.1. Animal Model of Ectopic Intramembranous Ossification (IMO)
4.5.2. Histology Analysis
4.5.3. Immunohistochemical Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Yanamoto, S.; Umeda, M.; Otsuru, M.; Ota, Y.; Kurita, H.; Kamata, T.; Kirita, T.; Yamakawa, N.; Yamashita, T.; et al. Prognostic and staging implications of mandibular canal invasion in lower gingival squamous cell carcinoma. Cancer Med. 2016, 5, 3378–3385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakrabarti, S.; Chakrabarti, P.R.; Desai, S.M.; Agrawal, D.; Mehta, D.Y.; Pancholi, M. Reconstruction in oral malignancy: Factors affecting morbidity of various procedures. Ann. Maxillofac. Surg. 2015, 5, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for Bone Regeneration: From Graft to Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef]
- Girón, J.; Kerstner, E.; Medeiros, T.; Oliveira, L.; Machado, G.M.; Malfatti, C.F.; Pranke, P. Biomaterials for bone regeneration: An orthopedic and dentistry overview. Braz. J. Med. Biol. Res. 2021, 54, e11055. [Google Scholar] [CrossRef]
- Thiebot, N.; Hamdani, A.; Blanchet, F.; Dame, M.; Tawfik, S.; Mbapou, E.; Kaddouh, A.A.; Alantar, A. Implant failure rate and the prevalence of associated risk factors: A 6-year retrospective observational survey. J. Oral Med. Oral Surg. 2022, 28, 19. [Google Scholar] [CrossRef]
- Alvarez, K.; Nakajima, H. Metallic Scaffolds for Bone Regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.T.J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smojver, I.; Katalinić, I.; Bjelica, R.; Gabrić, D.; Matišić, V.; Molnar, V.; Primorac, D. Mesenchymal Stem Cells Based Treatment in Dental Medicine: A Narrative Review. Int. J. Mol. Sci. 2022, 23, 1662. [Google Scholar] [CrossRef]
- Salgado, C.L.; Teixeira, B.I.B.; Monteiro, F.J.M. Biomimetic Composite Scaffold With Phosphoserine Signaling for Bone Tissue Engineering Application. Front. Bioeng. Biotechnol. 2019, 7, 206. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, S.C.; Salgado, C.L.; Sahu, A.; Garcia, M.P.; Fernandes, M.H.; Monteiro, F.J. Preparation and characterization of collagen-nanohydroxyapatite biocomposite scaffolds by cryogelation method for bone tissue engineering applications. J. Biomed. Mater. Res. Part A 2013, 101A, 1080–1094. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, A.; Berman, J.S. Osteopontin: A key cytokine in cell-mediated and granulomatous inflammation. Int. J. Exp. Pathol. 2000, 81, 373–390. [Google Scholar] [CrossRef]
- Iline-Vul, T.; Nanda, R.; Mateos, B.; Hazan, S.; Matlahov, I.; Perelshtein, I.; Keinan-Adamsky, K.; Althoff-Ospelt, G.; Konrat, R.; Goobes, G. Osteopontin regulates biomimetic calcium phosphate crystallization from disordered mineral layers covering apatite crystallites. Sci. Rep. 2020, 10, 15722. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Veis, A. Phosphorylated proteins and control over apatite nucleation, crystal growth, and inhibition. Chem. Rev. 2008, 108, 4670–4693. [Google Scholar] [CrossRef] [Green Version]
- Gorski, J.P.; Hankenson, K.D. Chapter 15—Secreted noncollagenous proteins of bone. In Principles of Bone Biology, 4th ed.; Bilezikian, J.P., Martin, T.J., Clemens, T.L., Rosen, C.J., Eds.; Academic Press: Amsterdam, The Netherlands, 2020; pp. 359–378. [Google Scholar]
- Hunter, G.K.; Kyle, C.L.; Goldberg, H.A. Modulation of crystal formation by bone phosphoproteins: Structural specificity of the osteopontin-mediated inhibition of hydroxyapatite formation. Biochem. J. 1994, 300, 723–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Zeng, X.; Ma, C.; Yi, H.; Ali, Z.; Mou, X.; Li, S.; Deng, Y.; He, N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017, 5, 17014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Nan, D.; Jin, H.; Qu, X. Recent advances of injectable hydrogels for drug delivery and tissue engineering applications. Polym. Test. 2020, 81, 106283. [Google Scholar] [CrossRef]
- Noori, A.; Ashrafi, S.J.; Vaez-Ghaemi, R.; Hatamian-Zaremi, A.; Webster, T.J. A review of fibrin and fibrin composites for bone tissue engineering. Int. J. Nanomed. 2017, 12, 4937–4961. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, H.; Shi, Q. Macrophages and bone inflammation. J. Orthop. Transl. 2017, 10, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, A.T.; Hanjani, N.A.; Movahed, E.; Doroudian, M. The role of macrophage subtypes and exosomes in immunomodulation. Cell. Mol. Biol. Lett. 2022, 27, 83. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Hu, C.; Feng, Y.; Li, D.; Ai, T.; Huang, Y.; Chen, X.; Huang, L.; Tan, J. Osteoimmunomodulatory effects of biomaterial modification strategies on macrophage polarization and bone regeneration. Regen. Biomater. 2020, 7, 233–245. [Google Scholar] [CrossRef]
- Chang, H.-I.; Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering-Cells and Biomaterials; InTechOpen: London, UK, 2011. [Google Scholar]
- Leahy, M.; Thompson, K.; Zafar, H.; Alexandrov, S.; Foley, M.; O’Flatharta, C.; Dockery, P. Functional imaging for regenerative medicine. Stem Cell Res. Ther. 2016, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Tung, J.K.; Berglund, K.; Gutekunst, C.-A.; Hochgeschwender, U.; Gross, R.E. Bioluminescence imaging in live cells and animals. Neurophotonics 2016, 3, 025001. [Google Scholar] [CrossRef]
- Christensen, J.; Vonwil, D.; Shastri, V.P. Non-Invasive In Vivo Imaging and Quantification of Tumor Growth and Metastasis in Rats Using Cells Expressing Far-Red Fluorescence Protein. PLoS ONE 2015, 10, e0132725. [Google Scholar] [CrossRef]
- Studwell, A.J.; Kotton, D.N. A shift from cell cultures to creatures: In vivo imaging of small animals in experimental regenerative medicine. Mol. Ther. 2011, 19, 1933–1941. [Google Scholar] [CrossRef] [Green Version]
- Sproul, E.; Nandi, S.; Brown, A. 6—Fibrin biomaterials for tissue regeneration and repair. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Barbosa, M.A., Martins, M.C.L., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 151–173. [Google Scholar]
- Gordjestani, M.; Dermaut, L.; De Ridder, L.; De Waele, P. Osteopontin and bone repair in rabbit tibial defect. Eur. J. Orthop. Surg. Traumatol. 2007, 17, 139–145. [Google Scholar] [CrossRef]
- Thurner, P.J.; Chen, C.G.; Ionova-Martin, S.; Sun, L.; Harman, A.; Porter, A.; Ager, J.W., 3rd; Ritchie, R.O.; Alliston, T. Osteopontin deficiency increases bone fragility but preserves bone mass. Bone 2010, 46, 1564–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKee, M.D.; Pedraza, C.E.; Kaartinen, M.T. Osteopontin and wound healing in bone. Cells Tissues Organs 2011, 194, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Gill, G.; Kaur, H.; Amhmed, M.; Jakhu, H. Role of osteopontin in bone remodeling and orthodontic tooth movement: A review. Prog. Orthod. 2018, 19, 18. [Google Scholar] [CrossRef] [PubMed]
- Menard, K.P. Dynamic Mechanical Analysis: A Practical Introduction; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Jiang, B.; Waller, T.M.; Larson, J.C.; Appel, A.A.; Brey, E.M. Fibrin-loaded porous poly(ethylene glycol) hydrogels as scaffold materials for vascularized tissue formation. Tissue Eng. Part A 2013, 19, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Brougham, C.M.; Levingstone, T.J.; Jockenhoevel, S.; Flanagan, T.C.; O’Brien, F.J. Incorporation of fibrin into a collagen-glycosaminoglycan matrix results in a scaffold with improved mechanical properties and enhanced capacity to resist cell-mediated contraction. Acta Biomater. 2015, 26, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Foroushani, Z.H.; Mahdavi, S.S.; Abdekhodaie, M.J.; Baradaran-Rafii, A.; Tabatabei, M.R.; Mehrvar, M. A hybrid scaffold of gelatin glycosaminoglycan matrix and fibrin as a carrier of human corneal fibroblast cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111430. [Google Scholar] [CrossRef]
- Salgado, C.L.; Barrias, C.C.; Monteiro, F.J.M. Clarifying the Tooth-Derived Stem Cells Behavior in a 3D Biomimetic Scaffold for Bone Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 724. [Google Scholar] [CrossRef]
- Rezai-Rad, M.; Bova, J.F.; Orooji, M.; Pepping, J.; Qureshi, A.; Del Piero, F.; Hayes, D.; Yao, S. Evaluation of bone regeneration potential of dental follicle stem cells for treatment of craniofacial defects. Cytotherapy 2015, 17, 1572–1581. [Google Scholar] [CrossRef] [Green Version]
- Bi, R.; Lyu, P.; Song, Y.; Li, P.; Song, D.; Cui, C.; Fan, Y. Function of Dental Follicle Progenitor/Stem Cells and Their Potential in Regenerative Medicine: From Mechanisms to Applications. Biomolecules 2021, 11, 997. [Google Scholar] [CrossRef]
- Mori, G.; Ballini, A.; Carbone, C.; Oranger, A.; Brunetti, G.; Di Benedetto, A.; Rapone, B.; Cantore, S.; Di Comite, M.; Colucci, S.; et al. Osteogenic differentiation of dental follicle stem cells. Int. J. Med. Sci. 2012, 9, 480–487. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, H.; Liu, X.; Sheng, Y.; Liu, X.; Jiang, C. Dental Follicle Stem Cells: Tissue Engineering and Immunomodulation. Stem Cells Dev. 2019, 28, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Graziano, A.; d’Aquino, R.; Laino, G.; Papaccio, G. Dental pulp stem cells: A promising tool for bone regeneration. Stem Cell Rev. 2008, 4, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granz, C.L.; Gorji, A. Dental stem cells: The role of biomaterials and scaffolds in developing novel therapeutic strategies. World J. Stem Cells 2020, 12, 897–921. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, M.M.; Burke, G.A.; Meenan, B.J. Calcium phosphate thin films enhance the response of human mesenchymal stem cells to nanostructured titanium surfaces. J. Tissue Eng. 2014, 5, 2041731414537513. [Google Scholar] [CrossRef] [PubMed]
- Salgado, C.L.; Grenho, L.; Fernandes, M.H.; Colaço, B.J.; Monteiro, F.J. Biodegradation, biocompatibility, and osteoconduction evaluation of collagen-nanohydroxyapatite cryogels for bone tissue regeneration. J. Biomed. Mater. Res. A 2016, 104, 57–70. [Google Scholar] [CrossRef]
- Carvalho, M.S.; Poundarik, A.A.; Cabral, J.M.S.; da Silva, C.L.; Vashishth, D. Biomimetic matrices for rapidly forming mineralized bone tissue based on stem cell-mediated osteogenesis. Sci. Rep. 2018, 8, 14388. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, Z.; Lohmann, C.H.; Oefinger, J.; Bonewald, L.F.; Dean, D.D.; Boyan, B.D. Implant surface characteristics modulate differentiation behavior of cells in the osteoblastic lineage. Adv. Dent. Res. 1999, 13, 38–48. [Google Scholar] [CrossRef]
- Brodbeck, W.G.; Nakayama, Y.; Matsuda, T.; Colton, E.; Ziats, N.P.; Anderson, J.M. Biomaterial surface chemistry dictates adherent monocyte/macrophage cytokine expression in vitro. Cytokine 2002, 18, 311–319. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Koziol, M.; Przekora, A. The Chitosan/Agarose/NanoHA Bone Scaffold-Induced M2 Macrophage Polarization and Its Effect on Osteogenic Differentiation In Vitro. Int. J. Mol. Sci. 2021, 22, 1109. [Google Scholar] [CrossRef]
- Elefteriou, F.; Yang, X. Genetic mouse models for bone studies—Strengths and limitations. Bone 2011, 49, 1242–1254. [Google Scholar] [CrossRef]


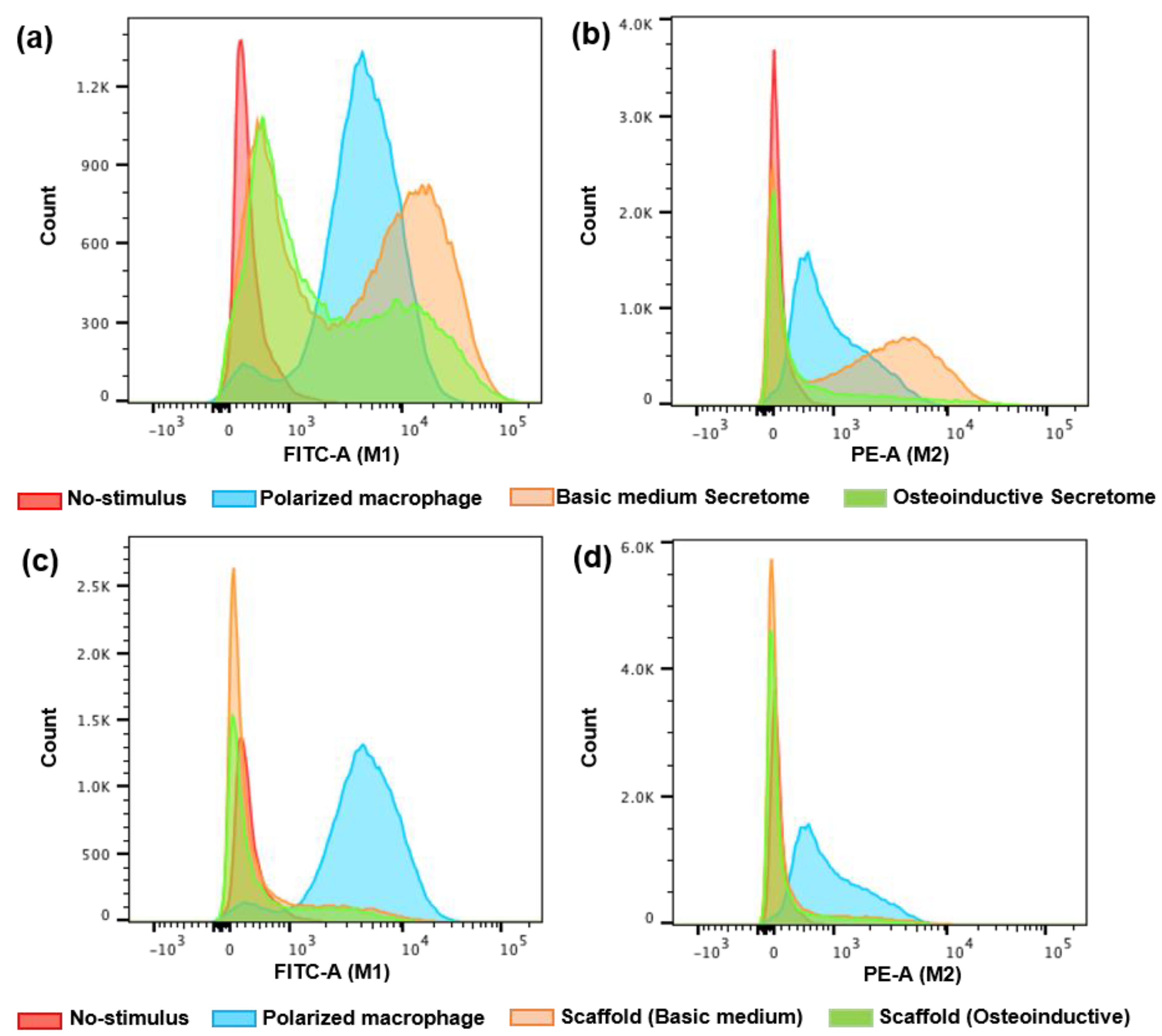

| Treatment | Macrophage Polarization (%) | ||||
|---|---|---|---|---|---|
| M0 | M1 | M0 | M2 | ||
| Control | INF-γ | 5.7 | 94 | x | x |
| No-stimulus | 98 | 2 | x | x | |
| IL-10 | x | x | 34 | 66 | |
| No-stimulus | x | x | 99 | 0.8 | |
| Indirect contact (secretome) | Basic medium | 71.7 | 28 | 51 | 50 |
| Osteoinductive medium | 82.8 | 17 | 81 | 19 | |
| Direct contact (scaffold) | Basic medium | 83.8 | 16 | 86 | 16 |
| Osteoinductive medium | 82.3 | 18 | 87 | 15 | |
| Gene | Primer Sequence (Forward) | Primer Sequence (Reverse) |
|---|---|---|
| GAPDH | 5′-TAACTGGTAAAGTGGATATTG-3′ | 5′-GAAGATGGTAGATGGATTTC-3′ |
| OPN | 5′-ACTCGAACGACTCTGATGATGT-3′ | 5′-GTCAGGTCTGCGAAACTTCTTA-3′ |
| BMP-2 | 5′-GACGAGGTCCTGAGCGAGTT-3′ | 5′-GCAATGGCCTTATCTGTGAC-3′ |
| Oct 3/4 | 5′-AGGAGTCCCAGGACATCAAAG-3′ | 5′-TCGTTTGGCTGAATACCTTC-3′ |
| DMP-1 | 5′-GAGCAGTGAGTCATCAGAAGGC-3′ | 5′-GAGAAGCCACCAGCTAGCCTAT-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.C.; Alves, P.M.; Monteiro, F.J.; Salgado, C. Interactions between Dental MSCs and Biomimetic Composite Scaffold during Bone Remodeling Followed by In Vivo Real-Time Bioimaging. Int. J. Mol. Sci. 2023, 24, 1827. https://doi.org/10.3390/ijms24031827
Costa AC, Alves PM, Monteiro FJ, Salgado C. Interactions between Dental MSCs and Biomimetic Composite Scaffold during Bone Remodeling Followed by In Vivo Real-Time Bioimaging. International Journal of Molecular Sciences. 2023; 24(3):1827. https://doi.org/10.3390/ijms24031827
Chicago/Turabian StyleCosta, Ana Catarina, Patrícia Mafalda Alves, Fernando Jorge Monteiro, and Christiane Salgado. 2023. "Interactions between Dental MSCs and Biomimetic Composite Scaffold during Bone Remodeling Followed by In Vivo Real-Time Bioimaging" International Journal of Molecular Sciences 24, no. 3: 1827. https://doi.org/10.3390/ijms24031827









