Targeting Gut Microbiota in Cancer Cachexia: Towards New Treatment Options
Abstract
1. Definition and Clinical Presentation of Cancer Cachexia
2. Emerging Treatment Options of Cancer Cachexia
3. Linking Gut Microbiota to Cancer Cachexia
3.1. Putative Microbial Mechanisms Contributing to Cachexia Development
3.2. Cachexia-Related Microbiota Profiles in Animal Models
3.3. Cachexia-Related Microbiota Profiles in Humans
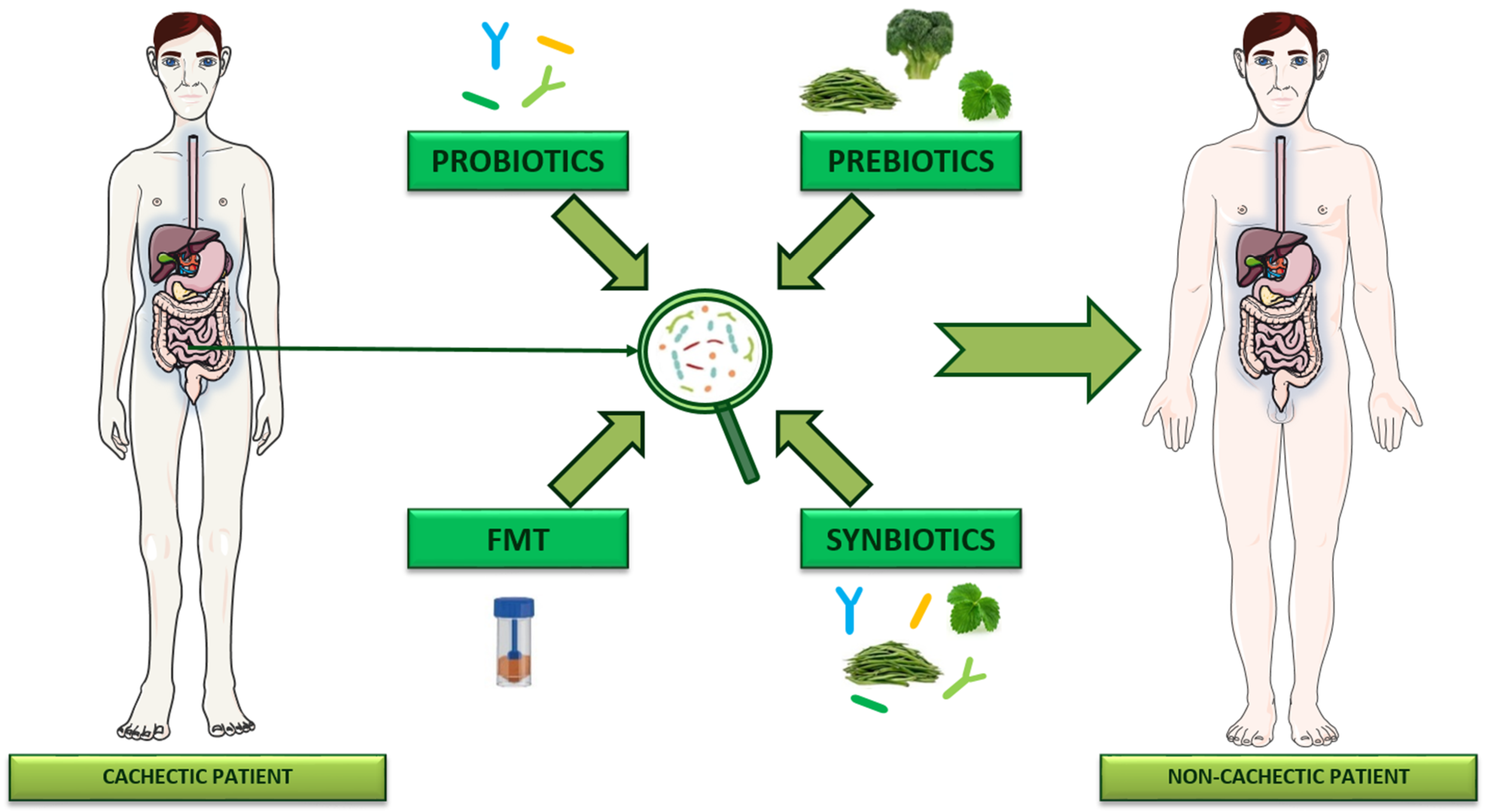
4. Targeting Gut Microbiota to Manage Cancer Cachexia
4.1. Probiotics
4.2. Prebiotics
4.3. Synbiotics
4.4. Fecal Microbiota Transplantation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Ni, J.; Zhang, L. Cancer Cachexia: Definition, Staging, and Emerging Treatments. Cancer Manag. Res. 2020, 12, 5597–5605. [Google Scholar] [CrossRef]
- Braha, A.; Albai, A.; Timar, B.; Negru, S.; Sorin, S.; Roman, D.; Popovici, D. Nutritional Interventions to Improve Cachexia Outcomes in Cancer-A Systematic Review. Medicina 2022, 58, 966. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.F.; Rohm, M.; Herzig, S.; Berriel Diaz, M. Cancer Cachexia: More Than Skeletal Muscle Wasting. Trends Cancer 2018, 4, 849–860. [Google Scholar] [CrossRef]
- Garcia, J.M.; Dunne, R.F.; Santiago, K.; Martin, L.; Birnbaum, M.J.; Crawford, J.; Hendifar, A.E.; Kochanczyk, M.; Moravek, C.; Piccinin, D.; et al. Addressing unmet needs for people with cancer cachexia: Recommendations from a multistakeholder workshop. J. Cachexia Sarcopenia Muscle 2022, 13, 1418–1425. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Kasamatsu, E.; Machii, K. Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma. Support. Care Cancer 2020, 28, 5271–5279. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Lopez-Soriano, F.J.; Stemmler, B.; Busquets, S. Novel targeted therapies for cancer cachexia. Biochem. J. 2017, 474, 2663–2678. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Dolan, R.D.; Skipworth, R.J.; Laird, B.J.; McMillan, D.C. Cancer cachexia: A nutritional or a systemic inflammatory syndrome? Br. J. Cancer 2022, 127, 379–382. [Google Scholar] [CrossRef]
- Rohm, M.; Zeigerer, A.; Machado, J.; Herzig, S. Energy metabolism in cachexia. EMBO Rep. 2019, 20, e47258. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.R. Distinguishing starvation from cachexia. Clin. Geriatr. Med. 2002, 18, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Scott, A.M.; Hoogenraad, N.J.; Osellame, L.D. Mediators and clinical treatment for cancer cachexia: A systematic review. JCSM Rapid Commun. 2021, 4, 166–186. [Google Scholar] [CrossRef]
- Naito, T. Emerging Treatment Options For Cancer-Associated Cachexia: A Literature Review. Ther. Clin. Risk Manag. 2019, 15, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.F.; An, Z.Y.; Lin, D.H.; Jin, W.L. Targeting cancer cachexia: Molecular mechanisms and clinical study. MedComm 2022, 3, e164. [Google Scholar] [CrossRef] [PubMed]
- Madeddu, C.; Maccio, A.; Mantovani, G. Multitargeted treatment of cancer cachexia. Crit. Rev. Oncog. 2012, 17, 305–314. [Google Scholar] [CrossRef]
- Loprinzi, C.L.; Michalak, J.C.; Schaid, D.J.; Mailliard, J.A.; Athmann, L.M.; Goldberg, R.M.; Tschetter, L.K.; Hatfield, A.K.; Morton, R.F. Phase III evaluation of four doses of megestrol acetate as therapy for patients with cancer anorexia and/or cachexia. J. Clin. Oncol. 1993, 11, 762–767. [Google Scholar] [CrossRef]
- Ferrara, M.; Samaden, M.; Ruggieri, E.; Venereau, E. Cancer cachexia as a multiorgan failure: Reconstruction of the crime scene. Front. Cell Dev. Biol. 2022, 10, 960341. [Google Scholar] [CrossRef]
- Herremans, K.M.; Riner, A.N.; Cameron, M.E.; Trevino, J.G. The Microbiota and Cancer Cachexia. Int. J. Mol. Sci. 2019, 20, 6267. [Google Scholar] [CrossRef]
- Ruusunen, A.; Rocks, T.; Jacka, F.; Loughman, A. The gut microbiome in anorexia nervosa: Relevance for nutritional rehabilitation. Psychopharmacology 2019, 236, 1545–1558. [Google Scholar] [CrossRef]
- Kang, L.; Li, P.; Wang, D.; Wang, T.; Hao, D.; Qu, X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci. Rep. 2021, 11, 4628. [Google Scholar] [CrossRef]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J. Cachexia Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef] [PubMed]
- Przewlocka, K.; Folwarski, M.; Kazmierczak-Siedlecka, K.; Skonieczna-Zydecka, K.; Kaczor, J.J. Gut-Muscle AxisExists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef]
- Genton, L.; Mareschal, J.; Charretier, Y.; Lazarevic, V.; Bindels, L.B.; Schrenzel, J. Targeting the Gut Microbiota to Treat Cachexia. Front. Cell. Infect. Microbiol. 2019, 9, 305. [Google Scholar] [CrossRef]
- Lin, R.; Liu, W.; Piao, M.; Zhu, H. A review of the relationship between the gut microbiota and amino acid metabolism. Amino Acids 2017, 49, 2083–2090. [Google Scholar] [CrossRef]
- Ziemons, J.; Smidt, M.L.; Damink, S.O.; Rensen, S.S. Gut microbiota and metabolic aspects of cancer cachexia. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101508. [Google Scholar] [CrossRef]
- Ni, Y.; Lohinai, Z.; Heshiki, Y.; Dome, B.; Moldvay, J.; Dulka, E.; Galffy, G.; Berta, J.; Weiss, G.J.; Sommer, M.O.A.; et al. Distinct composition and metabolic functions of human gut microbiota are associated with cachexia in lung cancer patients. ISME J. 2021, 15, 3207–3220. [Google Scholar] [CrossRef]
- Potgens, S.A.; Thibaut, M.M.; Joudiou, N.; Sboarina, M.; Neyrinck, A.M.; Cani, P.D.; Claus, S.P.; Delzenne, N.M.; Bindels, L.B. Multi-compartment metabolomics and metagenomics reveal major hepatic and intestinal disturbances in cancer cachectic mice. J. Cachexia Sarcopenia Muscle 2021, 12, 456–475. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Soons, Z.; Aarnoutse, R.; van Dijk, D.P.J.; Penders, J.; van Helvoort, A.; Smidt, M.L.; Kruitwagen, R.; Baade-Corpelijn, L.; et al. Gut microbiota and short-chain fatty acid alterations in cachectic cancer patients. J. Cachexia Sarcopenia Muscle 2021, 12, 2007–2021. [Google Scholar] [CrossRef]
- Boscaini, S.; Leigh, S.J.; Lavelle, A.; Garcia-Cabrerizo, R.; Lipuma, T.; Clarke, G.; Schellekens, H.; Cryan, J.F. Microbiota and body weight control: Weight watchers within? Mol. Metab. 2021, 57, 101427. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yu, J.; Li, Y.; Yang, F.; Yu, H.; Xue, M.; Zhang, F.; Jiang, X.; Ji, X.; Bao, Z. Depletion of gut microbiota induces skeletal muscle atrophy by FXR-FGF15/19 signalling. Ann. Med. 2021, 53, 508–522. [Google Scholar] [CrossRef]
- Bindels, L.B.; Beck, R.; Schakman, O.; Martin, J.C.; De Backer, F.; Sohet, F.M.; Dewulf, E.M.; Pachikian, B.D.; Neyrinck, A.M.; Thissen, J.P.; et al. Restoring specific lactobacilli levels decreases inflammation and muscle atrophy markers in an acute leukemia mouse model. PLoS ONE 2012, 7, e37971. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Claus, S.P.; Le Roy, C.I.; Grangette, C.; Pot, B.; Martinez, I.; Walter, J.; Cani, P.D.; Delzenne, N.M. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 2016, 10, 1456–1470. [Google Scholar] [CrossRef]
- Potgens, S.A.; Brossel, H.; Sboarina, M.; Catry, E.; Cani, P.D.; Neyrinck, A.M.; Delzenne, N.M.; Bindels, L.B. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci. Rep. 2018, 8, 12321. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, W.; Shen, Q.; Miao, C.; Chen, L.; Li, Y.; Gu, X.; Fan, M.; Ma, Y.; Wang, H.; et al. Bile acid metabolism dysregulation associates with cancer cachexia: Roles of liver and gut microbiome. J. Cachexia Sarcopenia Muscle 2021, 12, 1553–1569. [Google Scholar] [CrossRef]
- Castellani, C.; Singer, G.; Kaiser, M.; Kaiser, T.; Huang, J.; Sperl, D.; Kashofer, K.; Fauler, G.; Guertl-Lackner, B.; Hofler, G.; et al. Neuroblastoma causes alterations of the intestinal microbiome, gut hormones, inflammatory cytokines, and bile acid composition. Pediatr. Blood Cancer 2017, 64, e26425. [Google Scholar] [CrossRef]
- de Maria, Y.; Aciole Barbosa, D.; Menegidio, F.B.; Santos, K.; Humberto, A.C.; Alencar, V.C.; Silva, J.F.S.; Costa de Oliveira, R.; Batista, M.L., Jr.; Nunes, L.R.; et al. Analysis of mouse faecal dysbiosis, during the development of cachexia, induced by transplantation with Lewis lung carcinoma cells. Microbiology 2021, 167, 001088. [Google Scholar] [CrossRef]
- Choi, E.; Carruthers, K.; Zhang, L.; Thomas, N.; Battaglino, R.A.; Morse, L.R.; Widrick, J.J. Concurrent muscle and bone deterioration in a murine model of cancer cachexia. Physiol. Rep. 2013, 1, e00144. [Google Scholar] [CrossRef]
- Deboer, M.D. Animal models of anorexia and cachexia. Expert Opin. Drug Discov. 2009, 4, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Jabes, D.L.; de Maria, Y.; Aciole Barbosa, D.; Santos, K.; Carvalho, L.M.; Humberto, A.C.; Alencar, V.C.; Costa de Oliveira, R.; Batista, M.L., Jr.; Menegidio, F.B.; et al. Fungal Dysbiosis Correlates with the Development of Tumor-Induced Cachexia in Mice. J. Fungi 2020, 6, 364. [Google Scholar] [CrossRef] [PubMed]
- Hakozaki, T.; Nolin-Lapalme, A.; Kogawa, M.; Okuma, Y.; Nakamura, S.; Moreau-Amaru, D.; Tamura, T.; Hosomi, Y.; Takeyama, H.; Richard, C.; et al. Cancer Cachexia among Patients with Advanced Non-Small-Cell Lung Cancer on Immunotherapy: An Observational Study with Exploratory Gut Microbiota Analysis. Cancers 2022, 14, 5405. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.K.; Gudmundsdottir, V.; Nielsen, H.B.; Hyotylainen, T.; Nielsen, T.; Jensen, B.A.; Forslund, K.; Hildebrand, F.; Prifti, E.; Falony, G.; et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 2016, 535, 376–381. [Google Scholar] [CrossRef]
- Mielcarek, M.; Smolenski, R.T.; Isalan, M. Transcriptional Signature of an Altered Purine Metabolism in the Skeletal Muscle of a Huntington’s Disease Mouse Model. Front. Physiol. 2017, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.G.; Hafen, P.S.; Brault, J.J. Increased Adenine Nucleotide Degradation in Skeletal Muscle Atrophy. Int. J. Mol. Sci. 2020, 21, 88. [Google Scholar] [CrossRef] [PubMed]
- Laverdure, R.; Mezouari, A.; Carson, M.A.; Basiliko, N.; Gagnon, J. A role for methanogens and methane in the regulation of GLP-1. Endocrinol. Diabetes Metab. 2018, 1, e00006. [Google Scholar] [CrossRef]
- Massier, L.; Bluher, M.; Kovacs, P.; Chakaroun, R.M. Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front. Endocrinol. 2021, 12, 616506. [Google Scholar] [CrossRef]
- An, J.M.; Kang, E.A.; Han, Y.M.; Oh, J.Y.; Lee, D.Y.; Choi, S.H.; Kim, D.H.; Hahm, K.B. Dietary intake of probiotic kimchi ameliorated IL-6-driven cancer cachexia. J. Clin. Biochem. Nutr. 2019, 65, 109–117. [Google Scholar] [CrossRef]
- Bindels, L.B.; Neyrinck, A.M.; Salazar, N.; Taminiau, B.; Druart, C.; Muccioli, G.G.; Francois, E.; Blecker, C.; Richel, A.; Daube, G.; et al. Non Digestible Oligosaccharides Modulate the Gut Microbiota to Control the Development of Leukemia and Associated Cachexia in Mice. PLoS ONE 2015, 10, e0131009. [Google Scholar] [CrossRef]
- Huang, G.; Khan, I.; Li, X.; Chen, L.; Leong, W.; Ho, L.T.; Hsiao, W.L.W. Ginsenosides Rb3 and Rd reduce polyps formation while reinstate the dysbiotic gut microbiota and the intestinal microenvironment in Apc(Min/+) mice. Sci. Rep. 2017, 7, 12552. [Google Scholar] [CrossRef] [PubMed]
- Sakakida, T.; Ishikawa, T.; Doi, T.; Morita, R.; Endo, Y.; Matsumura, S.; Ota, T.; Yoshida, J.; Hirai, Y.; Mizushima, K.; et al. Water-soluble dietary fiber alleviates cancer-induced muscle wasting through changes in gut microenvironment in mice. Cancer Sci. 2022, 113, 1789–1800. [Google Scholar] [CrossRef] [PubMed]
- Varian, B.J.; Gourishetti, S.; Poutahidis, T.; Lakritz, J.R.; Levkovich, T.; Kwok, C.; Teliousis, K.; Ibrahim, Y.M.; Mirabal, S.; Erdman, S.E. Beneficial bacteria inhibit cachexia. Oncotarget 2016, 7, 11803–11816. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, J.G.; Laino, J.E.; del Valle, M.J.; Vannini, V.; van Sinderen, D.; Taranto, M.P.; de Valdez, G.F.; de Giori, G.S.; Sesma, F. B-group vitamin production by lactic acid bacteria--current knowledge and potential applications. J. Appl. Microbiol. 2011, 111, 1297–1309. [Google Scholar] [CrossRef]
- Rudzki, L.; Stone, T.W.; Maes, M.; Misiak, B.; Samochowiec, J.; Szulc, A. Gut microbiota-derived vitamins-underrated powers of a multipotent ally in psychiatric health and disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 107, 110240. [Google Scholar] [CrossRef]
- Roobab, U.; Batool, Z.; Manzoor, M.F.; Shabbir, M.A.; Khan, M.R.; Aadil, R.M. Sources, formulations, advanced delivery and health benefits of probiotics. Curr. Opin. Food Sci. 2020, 32, 17–28. [Google Scholar] [CrossRef]
- Silva, D.R.; de Cassia Orlandi Sardi, J.; de Souza Pitangui, N.; Magri Roque, S.; Barbosa da Silva, A.C.; Rosalen, P.L. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. J. Funct. Foods 2020, 73, 104080. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhaes Junior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications-A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.H.; Cho, Y.S. Fecal Microbiota Transplantation: Current Applications, Effectiveness, and Future Perspectives. Clin. Endosc. 2016, 49, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, W.P.D.W.; Langille, M.G.I.; Rupasinghe, H.P.V. Polyphenol-based prebiotics and synbiotics: Potential for cancer chemoprevention. Curr. Opin. Food Sci. 2018, 20, 51–57. [Google Scholar] [CrossRef]
- Kim, K.O.; Gluck, M. Fecal Microbiota Transplantation: An Update on Clinical Practice. Clin. Endosc. 2019, 52, 137–143. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2017, 67, 634–643. [Google Scholar]
- Feehley, T.; Plunkett, C.H.; Bao, R.; Choi Hong, S.M.; Culleen, E.; Belda-Ferre, P.; Campbell, E.; Aitoro, R.; Nocerino, R.; Paparo, L.; et al. Healthy infants harbor intestinal bacteria that protect against food allergy. Nat. Med. 2019, 25, 448–453. [Google Scholar] [CrossRef]
- Wardill, H.R.; Secombe, K.R.; Bryant, R.V.; Hazenberg, M.D.; Costello, S.P. Adjunctive fecal microbiota transplantation in supportive oncology: Emerging indications and considerations in immunocompromised patients. EBioMedicine 2019, 44, 730–740. [Google Scholar] [CrossRef]
- Kelly, C.R.; Ihunnah, C.; Fischer, M.; Khoruts, A.; Surawicz, C.; Afzali, A.; Aroniadis, O.; Barto, A.; Borody, T.; Giovanelli, A.; et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am. J. Gastroenterol. 2014, 109, 1065–1071. [Google Scholar] [CrossRef]
- Suchman, K.; Luo, Y.; Grinspan, A. Fecal Microbiota Transplant for Clostridioides Difficile Infection Is Safe and Efficacious in an Immunocompromised Cohort. Dig. Dis. Sci. 2022, 67, 4866–4873. [Google Scholar] [CrossRef]
- Agrawal, M.; Aroniadis, O.C.; Brandt, L.J.; Kelly, C.; Freeman, S.; Surawicz, C.; Broussard, E.; Stollman, N.; Giovanelli, A.; Smith, B.; et al. The Long-term Efficacy and Safety of Fecal Microbiota Transplant for Recurrent, Severe, and Complicated Clostridium difficile Infection in 146 Elderly Individuals. J. Clin. Gastroenterol. 2016, 50, 403–407. [Google Scholar] [CrossRef]
- Nigam, M.; Panwar, A.S.; Singh, R.K. Orchestrating the fecal microbiota transplantation: Current technological advancements and potential biomedical application. Front. Med. Technol. 2022, 4, 961569. [Google Scholar] [CrossRef] [PubMed]
- Servetas, S.L.; Daschner, P.J.; Guyard, C.; Thomas, V.; Affagard, H.; Sergaki, C.; Sokol, H.; Wargo, J.A.; Wu, G.D.; Sabot, P. Evolution of FMT-From early clinical to standardized treatments. Biologicals 2022, 76, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Bindels, L.B.; Neyrinck, A.M.; Loumaye, A.; Catry, E.; Walgrave, H.; Cherbuy, C.; Leclercq, S.; Van Hul, M.; Plovier, H.; Pachikian, B.; et al. Increased gut permeability in cancer cachexia: Mechanisms and clinical relevance. Oncotarget 2018, 9, 18224–18238. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Miquel, S.; Chain, F.; Natividad, J.M.; Jury, J.; Lu, J.; Sokol, H.; Theodorou, V.; Bercik, P.; Verdu, E.F.; et al. Faecalibacterium prausnitzii prevents physiological damages in a chronic low-grade inflammation murine model. BMC Microbiol. 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, A.H.; Yakymenko, O.; Olivier, I.; Hakansson, F.; Postma, E.; Keita, A.V.; Soderholm, J.D. Faecalibacterium prausnitzii supernatant improves intestinal barrier function in mice DSS colitis. Scand. J. Gastroenterol. 2013, 48, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Brar, M.S.; Leung, F.C.; Hsiao, W.L. Triterpenoid herbal saponins enhance beneficial bacteria, decrease sulfate-reducing bacteria, modulate inflammatory intestinal microenvironment and exert cancer preventive effects in ApcMin/+ mice. Oncotarget 2016, 7, 31226–31242. [Google Scholar] [CrossRef]
- Obermuller, B.; Singer, G.; Kienesberger, B.; Klymiuk, I.; Sperl, D.; Stadlbauer, V.; Horvath, A.; Miekisch, W.; Gierschner, P.; Grabherr, R.; et al. The Effects of Prebiotic Supplementation with OMNi-LOGiC((R)) FIBRE on Fecal Microbiome, Fecal Volatile Organic Compounds, and Gut Permeability in Murine Neuroblastoma-Induced Tumor-Associated Cachexia. Nutrients 2020, 12, 2029. [Google Scholar] [CrossRef]
- Feuerstadt, P.; Louie, T.J.; Lashner, B.; Wang, E.E.L.; Diao, L.; Bryant, J.A.; Sims, M.; Kraft, C.S.; Cohen, S.H.; Berenson, C.S.; et al. SER-109, an Oral Microbiome Therapy for Recurrent Clostridioides difficile Infection. N. Engl. J. Med. 2022, 386, 220–229. [Google Scholar] [CrossRef]
- de Clercq, N.C.; van den Ende, T.; Prodan, A.; Hemke, R.; Davids, M.; Pedersen, H.K.; Nielsen, H.B.; Groen, A.K.; de Vos, W.M.; van Laarhoven, H.W.M.; et al. Fecal Microbiota Transplantation from Overweight or Obese Donors in Cachectic Patients with Advanced Gastroesophageal Cancer: A Randomized, Double-blind, Placebo-Controlled, Phase II Study. Clin. Cancer Res. 2021, 27, 3784–3792. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panebianco, C.; Villani, A.; Potenza, A.; Favaro, E.; Finocchiaro, C.; Perri, F.; Pazienza, V. Targeting Gut Microbiota in Cancer Cachexia: Towards New Treatment Options. Int. J. Mol. Sci. 2023, 24, 1849. https://doi.org/10.3390/ijms24031849
Panebianco C, Villani A, Potenza A, Favaro E, Finocchiaro C, Perri F, Pazienza V. Targeting Gut Microbiota in Cancer Cachexia: Towards New Treatment Options. International Journal of Molecular Sciences. 2023; 24(3):1849. https://doi.org/10.3390/ijms24031849
Chicago/Turabian StylePanebianco, Concetta, Annacandida Villani, Adele Potenza, Enrica Favaro, Concetta Finocchiaro, Francesco Perri, and Valerio Pazienza. 2023. "Targeting Gut Microbiota in Cancer Cachexia: Towards New Treatment Options" International Journal of Molecular Sciences 24, no. 3: 1849. https://doi.org/10.3390/ijms24031849
APA StylePanebianco, C., Villani, A., Potenza, A., Favaro, E., Finocchiaro, C., Perri, F., & Pazienza, V. (2023). Targeting Gut Microbiota in Cancer Cachexia: Towards New Treatment Options. International Journal of Molecular Sciences, 24(3), 1849. https://doi.org/10.3390/ijms24031849







