Optimization and Technical Considerations for the Dye-Exclusion Protocol Used to Assess Blood–Brain Barrier Integrity in Adult Drosophila melanogaster
Abstract
1. Introduction
2. Results
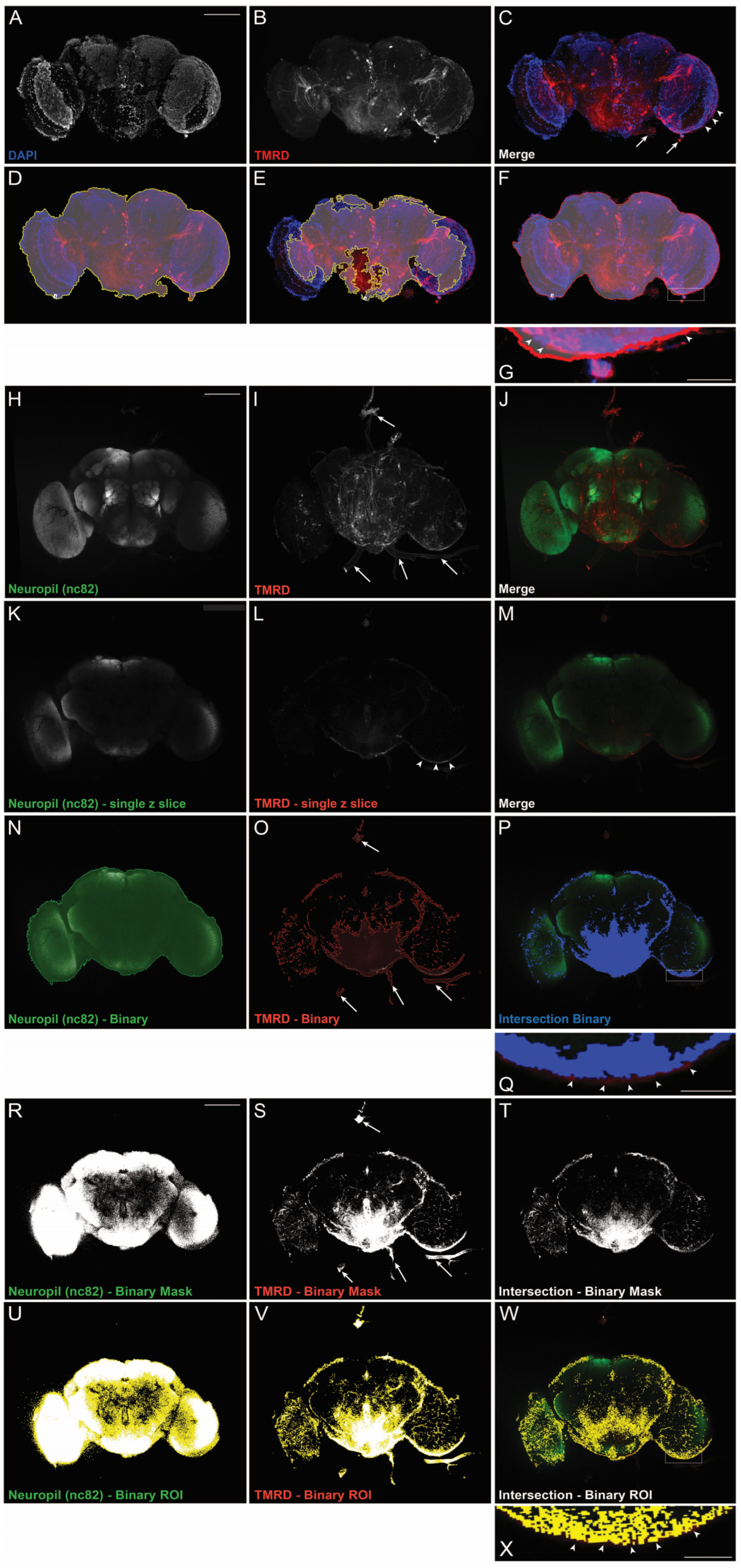
2.1. Intraparenchymal Binary Analysis More Accurately Reflects Dye Infiltration Than Single-Channel Region of Interest (ROI) Analysis
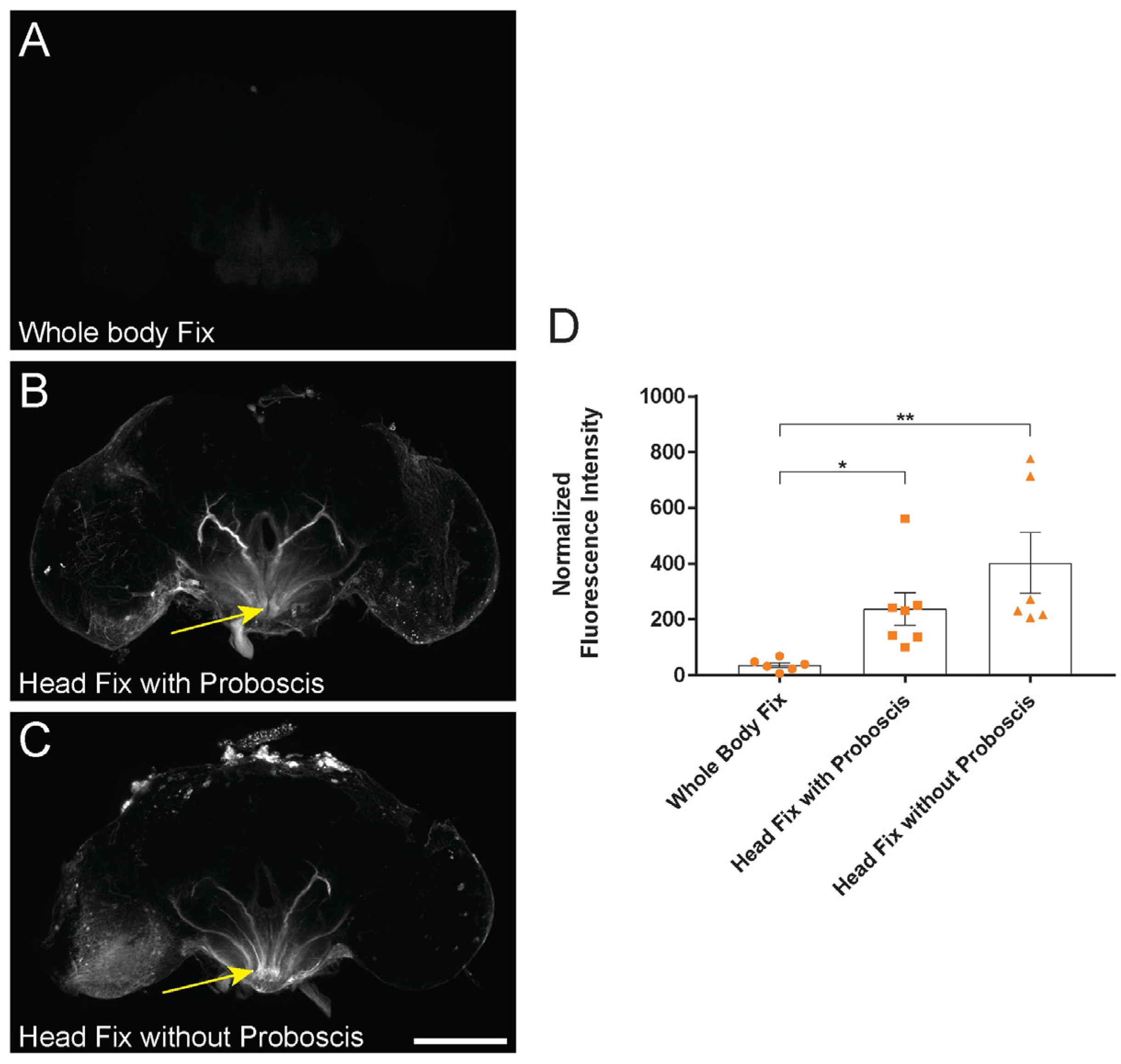
2.2. Whole-Body Pre-Fixation Reduces Variability of Dye-Infiltration Assay in Adult Drosophila
2.3. Whole-Body Fixation Better Reveals the Effect of Traumatic Injury on Barrier Integrity
3. Discussion
4. Materials and Methods
4.1. Fly Stocks
4.2. Dye Injections
4.3. Traumatic Injury
4.4. Fixation, Brain Dissection, and Immunofluorescence
4.5. Imaging and Analysis
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Alluri, H.; Wiggins-Dohlvik, K.; Davis, M.L.; Huang, J.H.; Tharakan, B. Blood–Brain Barrier Dysfunction Following Traumatic Brain Injury. Metab. Brain Dis. 2015, 30, 1093–1104. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-Brain Barrier Breakdown in Alzheimer Disease and Other Neurodegenerative Disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef]
- Mason, H.D.; Johnson, A.M.; Mihelson, N.A.; Mastorakos, P.; McGavern, D.B. Glia Limitans Superficialis Oxidation and Breakdown Promote Cortical Cell Death after Repetitive Head Injury. JCI Insight 2021, 6, e149229. [Google Scholar] [CrossRef]
- Carlson, S.D.; Juang, J.L.; Hilgers, S.L.; Garment, M.B. Blood Barriers of the Insect. Annu. Rev. Entomol. 2000, 45, 151–174. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R.; Prat, A. The Blood–Brain Barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The Role of Brain Vasculature in Neurodegenerative Disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Limmer, S.; Weiler, A.; Volkenhoff, A.; Babatz, F.; Klämbt, C. The Drosophila Blood-Brain Barrier: Development and Function of a Glial Endothelium. Front. Neurosci. 2014, 8, 365. [Google Scholar] [CrossRef]
- Saunders, N.R.; Dreifuss, J.J.; Dziegielewska, K.M.; Johansson, P.A.; Habgood, M.D.; Møllgård, K.; Bauer, H.C. The Rights and Wrongs of Blood-Brain Barrier Permeability Studies: A Walk through 100 Years of History. Front. Neurosci. 2014, 8, 404. [Google Scholar] [CrossRef] [PubMed]
- Reese, T.S.; Karnovsky, M.J. Fine Structural Localization of a Blood-Brain Barrier to Exogenous Peroxidase. J. Cell Biol. 1967, 34, 207–217. [Google Scholar] [CrossRef]
- Mayer, F.; Mayer, N.; Chinn, L.; Pinsonneault, R.L.; Kroetz, D.; Bainton, R.J. Evolutionary Conservation of Vertebrate Blood-Brain Barrier Chemoprotective Mechanisms in Drosophila. J. Neurosci. 2009, 29, 3538–3550. [Google Scholar] [CrossRef] [PubMed]
- Pinsonneault, R.L.; Mayer, N.; Mayer, F.; Tegegn, N.; Bainton, R.J. Novel Models for Studying the Blood-Brain and Blood-Eye Barriers in Drosophila. Methods Mol. Biol. 2011, 686, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Stork, T.; Engelen, D.; Krudewig, A.; Silies, M.; Bainton, R.J.; Klambt, C. Organization and Function of the Blood Brain Barrier in Drosophila. J. Neurosci. 2008, 28, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, T.; Bainton, R.J.; Fetter, R.D.; Heberlein, U.; Gaul, U. GPCR Signaling Is Required for Blood-Brain Barrier Formation in Drosophila. Cell 2005, 123, 133–144. [Google Scholar] [CrossRef]
- Bainton, R.J.; Tsai, L.T.Y.; Schwabe, T.; DeSalvo, M.; Gaul, U.; Heberlein, U. Moody Encodes Two GPCRs That Regulate Cocaine Behaviors and Blood-Brain Barrier Permeability in Drosophila. Cell 2005, 123, 145–156. [Google Scholar] [CrossRef]
- Katzenberger, R.J.; Chtarbanova, S.; Rimkus, S.A.; Fischer, J.A.; Kaur, G.; Seppala, J.M.; Swanson, L.C.; Zajac, J.E.; Ganetzky, B.; Wassarman, D.A. Death Following Traumatic Brain Injury in Drosophila Is Associated with Intestinal Barrier Dysfunction. Elife 2015, 4, e04790. [Google Scholar] [CrossRef]
- Saikumar, J.; Byrns, C.N.; Hemphill, M.; Meaney, D.F.; Bonini, N.M. Dynamic Neural and Glial Responses of a Head-Specific Model for Traumatic Brain Injury in Drosophila. Proc. Natl. Acad. Sci. USA 2020, 117, 17269–17277. [Google Scholar] [CrossRef]
- Katzenberger, R.J.; Loewen, C.A.; Wassarman, D.R.; Petersen, A.J.; Ganetzky, B.; Wassarman, D.A. A Drosophila Model of Closed Head Traumatic Brain Injury. Proc. Natl. Acad. Sci. USA 2013, 110, E4152–E4159. [Google Scholar] [CrossRef]
- Wagh, D.A.; Rasse, T.M.; Asan, E.; Hofbauer, A.; Schwenkert, I.; Dürrbeck, H.; Buchner, S.; Dabauvalle, M.C.; Schmidt, M.; Qin, G.; et al. Bruchpilot, a Protein with Homology to ELKS/CAST, Is Required for Structural Integrity and Function of Synaptic Active Zones in Drosophila. Neuron 2006, 49, 833–844. [Google Scholar] [CrossRef]
- Van Alphen, B.; Semenza, E.R.; Yap, M.; Van Swinderen, B.; Allada, R. A Deep Sleep Stage in Drosophila with a Functional Role in Waste Clearance. Sci. Adv. 2021, 7, eabc2999. [Google Scholar] [CrossRef]
- Schwarz, O.; Bohra, A.A.; Liu, X.; Reichert, H.; Vijayraghavan, K.; Pielage, J. Motor Control of Drosophila Feeding Behavior. Elife 2017, 6, e19892. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.C.; Jung, C.; Batelli, S.; Rubin, G.M.; Gaul, U. The Glia of the Adult Drosophila Nervous System. Glia 2017, 65, 606–638. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhasiin, K.; Heintz, O.; Colodner, K.J. Optimization and Technical Considerations for the Dye-Exclusion Protocol Used to Assess Blood–Brain Barrier Integrity in Adult Drosophila melanogaster. Int. J. Mol. Sci. 2023, 24, 1886. https://doi.org/10.3390/ijms24031886
Bhasiin K, Heintz O, Colodner KJ. Optimization and Technical Considerations for the Dye-Exclusion Protocol Used to Assess Blood–Brain Barrier Integrity in Adult Drosophila melanogaster. International Journal of Molecular Sciences. 2023; 24(3):1886. https://doi.org/10.3390/ijms24031886
Chicago/Turabian StyleBhasiin, Kesshni, Olivia Heintz, and Kenneth J. Colodner. 2023. "Optimization and Technical Considerations for the Dye-Exclusion Protocol Used to Assess Blood–Brain Barrier Integrity in Adult Drosophila melanogaster" International Journal of Molecular Sciences 24, no. 3: 1886. https://doi.org/10.3390/ijms24031886
APA StyleBhasiin, K., Heintz, O., & Colodner, K. J. (2023). Optimization and Technical Considerations for the Dye-Exclusion Protocol Used to Assess Blood–Brain Barrier Integrity in Adult Drosophila melanogaster. International Journal of Molecular Sciences, 24(3), 1886. https://doi.org/10.3390/ijms24031886







