Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy
Abstract
:1. Introduction
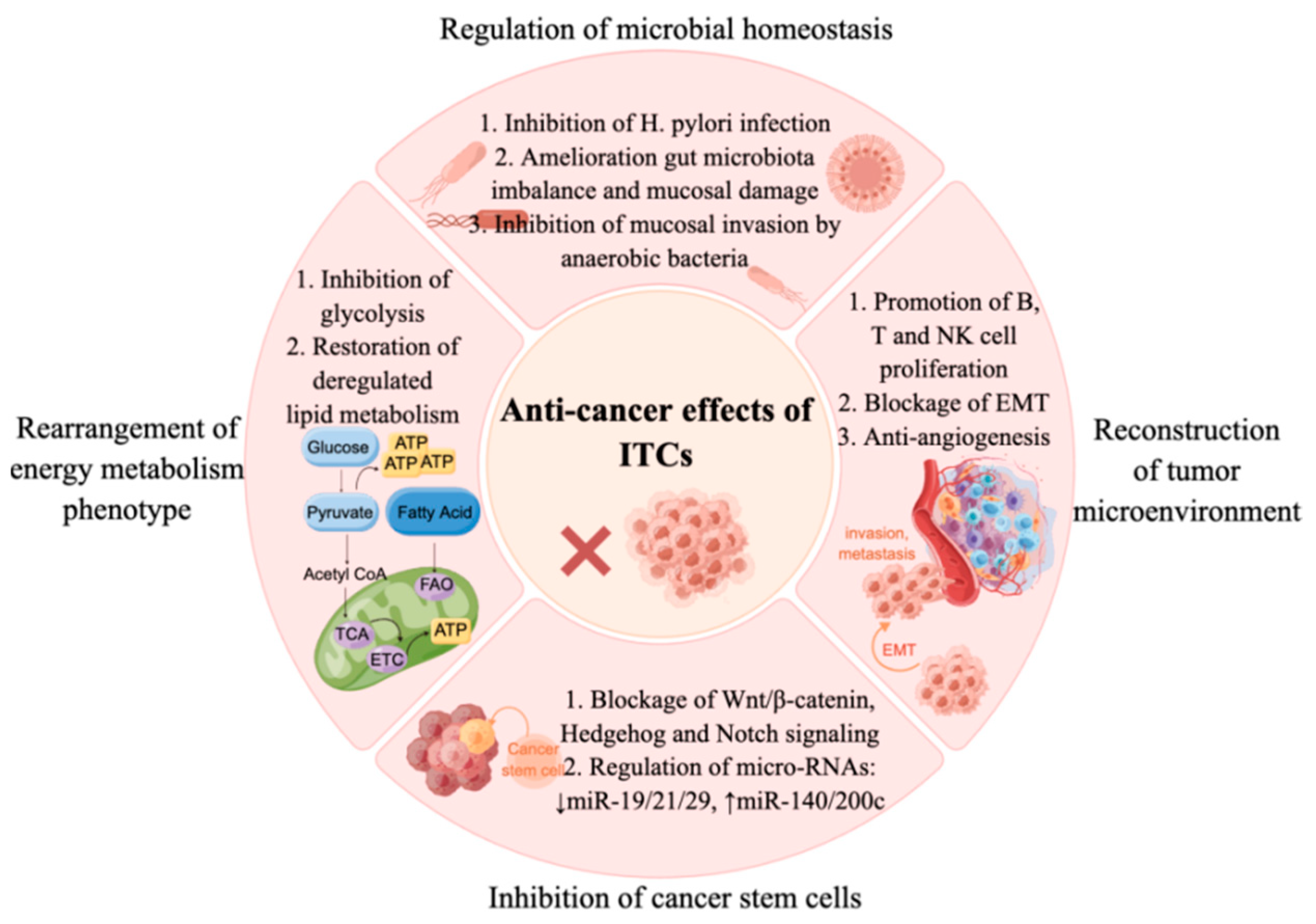
2. Microbiota and Anti-Cancer Effects of ITCs
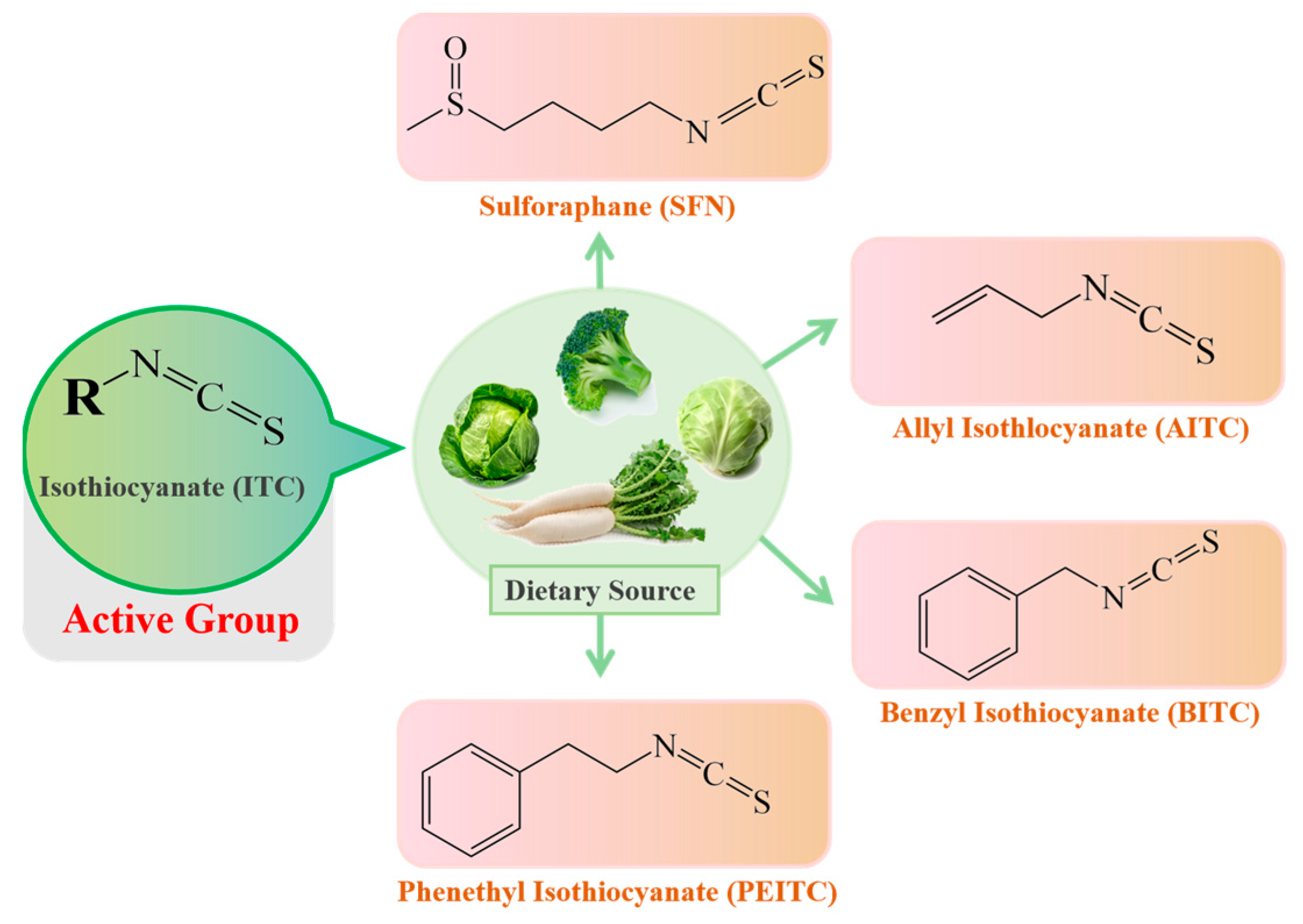
Microbiota: A Mediator Transforming Glucosinolate Precursors to the Active Isothiocyanates
3. Activity of ITCs on Helicobacter pylori Strains
4. Rearrangement of Energy Metabolism Phenotype by Sulforaphane
5. Inhibition of Cancer Stem Cells by Sulforaphane
6. Remodeling of the Tumor Microenvironment by Sulforaphane
7. The Hormetic Effects of ITCs on Cancer
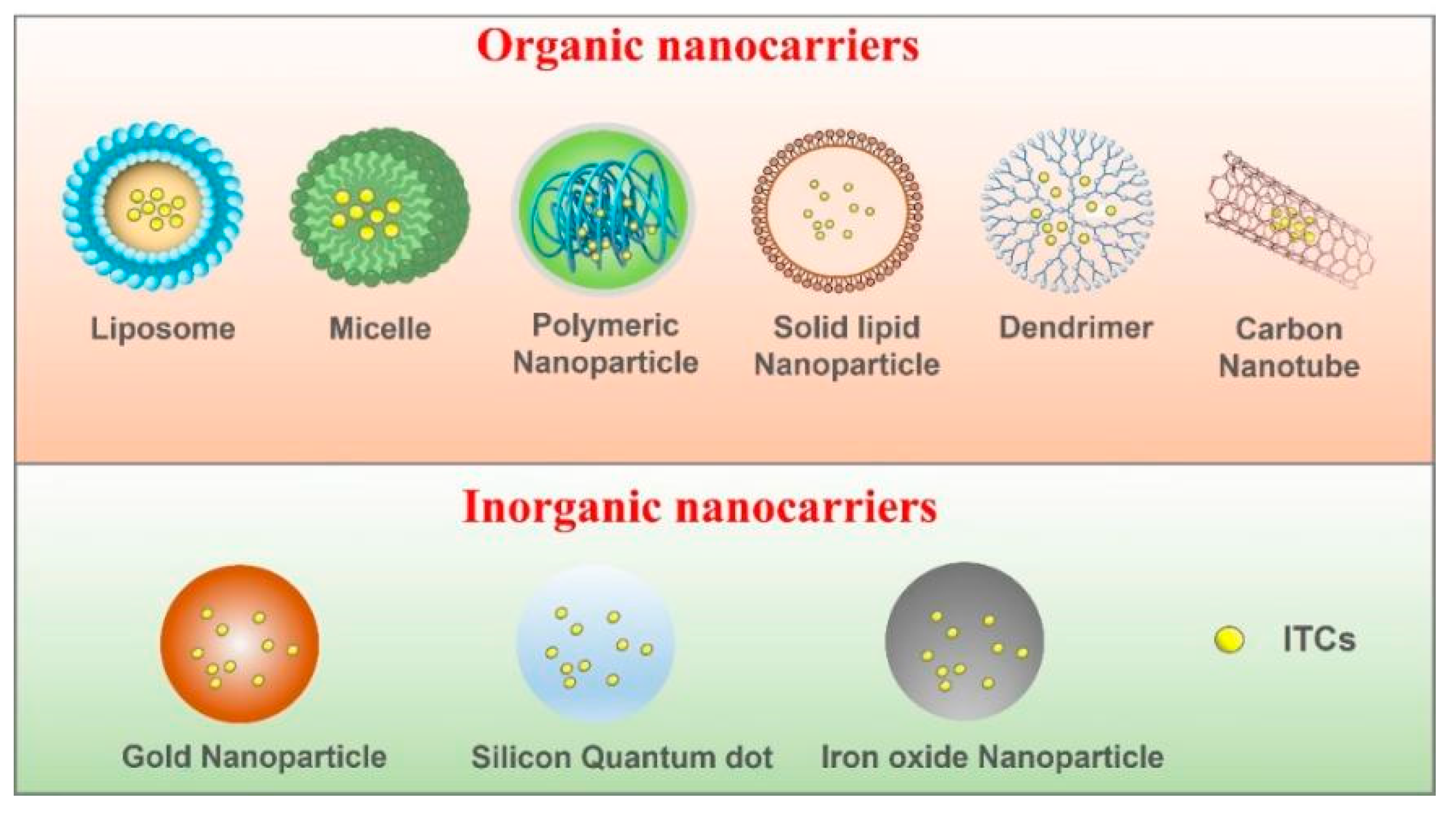
8. Nanotechnology-Based ITC-Delivery Systems
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef] [Green Version]
- Mastrangelo, L.; Cassidy, A.; Mulholland, F.; Wang, W.; Bao, Y. Serotonin receptors, novel targets of sulforaphane identified by proteomic analysis in Caco-2 cells. Cancer Res. 2008, 68, 5487–5491. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y. Chemoprevention by isothiocyanates: Molecular basis of apoptosis induction. Forum Nutr. 2009, 61, 170–181. [Google Scholar]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous vegetables, isothiocyanates, and bladder cancer prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef]
- Yeger, H.; Mokhtari, R.B. Perspective on dietary isothiocyanates in the prevention, development and treatment of cancer. J. Cancer Metastasis Treat. 2020, 6, 26. [Google Scholar] [CrossRef]
- Barrera, L.N.; Cassidy, A.; Wang, W.; Wei, T.; Belshaw, N.J.; Johnson, I.T.; Brigelius-Flohé, R.; Bao, Y. TrxR1 and GPx2 are potently induced by isothiocyanates and selenium, and mutually cooperate to protect Caco-2 cells against free radical-mediated cell death. Biochim. Biophys. Acta 2012, 1823, 1914–1924. [Google Scholar] [CrossRef] [Green Version]
- Russo, M.; Spagnuolo, C.; Russo, G.L.; Skalicka-Woźniak, K.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Nrf2 targeting by sulforaphane: A potential therapy for cancer treatment. Crit. Rev. Food Sci. Nutr. 2018, 58, 1391–1405. [Google Scholar] [CrossRef]
- Warpsinski, G.; Smith, M.J.; Srivastava, S.; Keeley, T.P.; Siow, R.C.M.; Fraser, P.A.; Mann, G.E. Nrf2-regulated redox signaling in brain endothelial cells adapted to physiological oxygen levels: Consequences for sulforaphane mediated protection against hypoxia-reoxygenation. Redox. Biol. 2020, 37, 101708. [Google Scholar] [CrossRef]
- Ishida, K.; Kaji, K.; Sato, S.; Ogawa, H.; Takagi, H.; Takaya, H.; Kawaratani, H.; Moriya, K.; Namisaki, T.; Akahane, T.; et al. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J. Nutr. Biochem. 2021, 89, 108573. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Kozumbo, W.J. The hormetic dose-response mechanism: Nrf2 activation. Pharm. Res. 2021, 167, 105526. [Google Scholar] [CrossRef]
- Lee, J.; Han, Y.; Wang, W.; Jo, H.; Kim, H.; Kim, S.; Yang, K.M.; Kim, S.J.; Dhanasekaran, D.N.; Song, Y.S. Phytochemicals in cancer immune checkpoint inhibitor therapy. Biomolecules 2021, 11, 1107. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.K.; Yoo, D.R.; Jang, Y.H.; Jang, S.Y.; Nam, M.J. Sulforaphane induces apoptosis in human hepatic cancer cells through inhibition of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase4, mediated by hypoxia inducible factor-1-dependent pathway. Biochim. Biophys. Acta 2011, 1814, 1340–1348. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Kim, S.H.; Hahm, E.R.; Pore, S.K.; Jacobs, B.L.; Singh, S.V. Prostate cancer chemoprevention by sulforaphane in a preclinical mouse model is associated with inhibition of fatty acid metabolism. Carcinogenesis 2018, 39, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Haristoy, X.; Fahey, J.W.; Scholtus, I.; Lozniewski, A. Evaluation of the antimicrobial effects of several isothiocyanates on Helicobacter pylori. Planta Med. 2005, 71, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Travers-Martin, N.; Kuhlmann, F.; Müller, C. Revised determination of free and complexed myrosinase activities in plant extracts. Plant Physiol. Biochem. 2008, 46, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Vyas, D. Myrosinase: Insights on structural, catalytic, regulatory, and environmental interactions. Crit. Rev. Biotechnol. 2019, 39, 508–523. [Google Scholar] [CrossRef]
- Angelino, D.; Jeffery, E. Glucosinolate hydrolysis and bioavailability of resulting isothiocyanates: Focus on glucoraphanin. J. Funct. Foods 2014, 7, 67–76. [Google Scholar] [CrossRef]
- Palop, M.L.; Smiths, J.P.; Brink, B.T. Degradation of sinigrin by Lactobacillus agilis strain R16. Int. J. Food Microbiol. 1995, 26, 219–229. [Google Scholar] [CrossRef]
- Li, F.; Hullar, M.A.; Beresford, S.A.; Lampe, J.W. Variation of glucoraphanin metabolism in vivo and ex vivo by human gut bacteria. Br. J. Nutr. 2011, 106, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Mullaney, J.A.; Kelly, W.J.; McGhie, T.K.; Ansell, J.; Heyes, J.A. Lactic acid bacteria convert glucosinolates to nitriles efficiently yet differently from enterobacteriaceae. J. Agric. Food Chem. 2013, 61, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Elfoul, L.; Rabot, S.; Khelifa, N.; Quinsac, A.; Duguay, A.; Rimbault, A. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of Bacteroides thetaiotaomicron. FEMS Microbiol. Lett. 2001, 197, 99–103. [Google Scholar] [CrossRef] [PubMed]
- McColl, K.E. Clinical practice. Helicobacter pylori infection. N. Engl. J. Med. 2010, 362, 1597–1604. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Haristoy, X.; Dolan, P.M.; Kensler, T.W.; Scholtus, I.; Stephenson, K.K.; Talalay, P.; Lozniewski, A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc. Natl. Acad. Sci. USA 2002, 99, 7610–7615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keenan, J.I.; Salm, N.; Wallace, A.J.; Hampton, M.B. Using food to reduce H. pylori-associated inflammation. Phytother. Res. 2012, 26, 1620–1625. [Google Scholar] [CrossRef]
- Galan, M.V.; Kishan, A.A.; Silverman, A.L. Oral broccoli sprouts for the treatment of Helicobacter pylori infection: A preliminary report. Dig Dis. Sci. 2004, 49, 1088–1090. [Google Scholar] [CrossRef]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, I.; Yamamoto, M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev. Res. 2009, 2, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Yanaka, A. Role of Sulforaphane in Protection of Gastrointestinal Tract Against H. pylori and NSAID-induced oxidative stress. Curr. Pharm. Des. 2017, 23, 4066–4075. [Google Scholar] [CrossRef] [Green Version]
- Yanaka, A.; Sato, J.; Ohmori, S. Sulforaphane protects small intestinal mucosa from aspirin/NSAID-induced injury by enhancing host defense systems against oxidative stress and by inhibiting mucosal invasion of anaerobic enterobacteria. Curr. Pharm. Des. 2013, 19, 157–162. [Google Scholar]
- He, C.; Huang, L.; Lei, P.; Liu, X.; Li, B.; Shan, Y. Sulforaphane normalizes intestinal flora and enhances gut barrier in mice with BBN-induced bladder cancer. Mol. Nutr. Food Res. 2018, 62, e1800427. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Tan, K.N.; Rodriguez, T.; Avery, V.M. The molecular effects of sulforaphane and capsaicin on metabolism upon androgen and Tip60 activation of androgen receptor. Int. J. Mol. Sci. 2019, 20, 5384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, L.; He, C.; Zheng, S.; Wu, C.; Ren, M.; Shan, Y. AKT1/HK2 Axis-mediated glucose metabolism: A novel therapeutic target of sulforaphane in bladder cancer. Mol. Nutr. Food Res. 2022, 66, e2100738. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.B.; Hahm, E.R.; Alumkal, J.J.; Foley, L.M.; Hitchens, T.K.; Shiva, S.S.; Parikh, R.A.; Jacobs, B.L.; Singh, S.V. Reversal of the Warburg phenomenon in chemoprevention of prostate cancer by sulforaphane. Carcinogenesis 2019, 40, 1545–1556. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, Y.; Li, J.; Zheng, Z.; Hu, Y.; Li, L.; Wu, W. Sulforaphane downregulated fatty acid synthase and inhibited microtubule-mediated mitophagy leading to apoptosis. Cell Death Dis. 2021, 12, 917. [Google Scholar] [CrossRef]
- Najafi, M.; Farhood, B.; Mortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell Physiol. 2019, 234, 8381–8395. [Google Scholar] [CrossRef]
- Rodova, M.; Fu, J.; Watkins, D.N.; Srivastava, R.K.; Shankar, S. Sonic hedgehog signaling inhibition provides opportunities for targeted therapy by sulforaphane in regulating pancreatic cancer stem cell self-renewal. PLoS ONE 2012, 7, e46083. [Google Scholar] [CrossRef]
- Li, S.H.; Fu, J.; Watkins, D.N.; Srivastava, R.K.; Shankar, S. Sulforaphane regulates self-renewal of pancreatic cancer stem cells through the modulation of Sonic hedgehog-GLI pathway. Mol. Cell Biochem. 2013, 373, 217–227. [Google Scholar] [CrossRef]
- Fisher, M.L.; Ciavattone, N.; Grun, D.; Adhikary, G.; Eckert, R.L. Sulforaphane reduces YAP/Np63alpha signaling to reduce cancer stem cell survival and tumor formation. Oncotarget 2017, 8, 73407–73418. [Google Scholar] [CrossRef] [Green Version]
- Naujokat, C.; McKee, D.L. The “Big Five” phytochemicals targeting cancer stem cells: Curcumin, EGCG, sulforaphane, resveratrol and genistein. Curr. Med. Chem. 2021, 28, 4321–4342. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Chen, Y.; Li, X.; Jiang, Y.; Yang, X.; Li, Y.; Wang, X.; Meng, Y.; Zhu, M.; et al. miR-19 targeting of GSK3beta mediates sulforaphane suppression of lung cancer stem cells. J. Nutr. Biochem. 2017, 44, 80–91. [Google Scholar] [CrossRef]
- Li, Q.; Eades, G.; Yao, Y.; Zhang, Y.; Zhou, Q. Characterization of a stem-like subpopulation in basal-like ductal carcinoma in situ (DCIS) lesions. J. Biol. Chem. 2014, 289, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Yao, Y.; Eades, G.; Liu, Z.; Zhang, Y.; Zhou, Q. Downregulation of miR-140 promotes cancer stem cell formation in basal-like early stage breast cancer. Oncogene 2014, 33, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Peng, C.Y.; Liao, Y.W.; Lu, M.Y.; Tsai, M.L.; Yeh, J.C.; Yu, C.H.; Yu, C.C. Sulforaphane targets cancer stemness and tumor initiating properties in oral squamous cell carcinomas via miR-200c induction. J. Med. Assoc. 2017, 116, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zang, X.; Loke, P.; Kim, J.; Murphy, K.; Waitz, R.; Allison, J.P. B7x: A widely expressed B7 family member that inhibits T cell activation. Proc. Natl. Acad. Sci. USA 2003, 100, 10388–10392. [Google Scholar] [CrossRef] [Green Version]
- Quatrini, L.; Mariotti, F.R.; Munari, E.; Tumino, N.; Vacca, P.; Moretta, L. The Immune Checkpoint PD-1 in Natural Killer Cells: Expression, Function and Targeting in Tumour Immunotherapy. Cancers 2020, 12, 3285. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, A.H.; Wherry, E.J.; Ahmed, R.; Freeman, G.J. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007, 8, 239–245. [Google Scholar] [CrossRef]
- Kumar, R.; De Mooij, T.; Peterson, T.E.; Kaptzan, T.; Johnson, A.J.; Daniels, D.J.; Parney, I.F. Modulating glioma-mediated myeloid-derived suppressor cell development with sulforaphane. PLoS ONE 2017, 12, e0179012. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Petrikova, E.; Gross, W.; Sticht, C.; Gretz, N.; Herr, I.; Karakhanova, S. Sulforaphane promotes dendritic cell stimulatory capacity through modulation of regulatory molecules, JAK/STAT3- and microRNA-signaling. Front. Immunol. 2020, 11, 589818. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. Emt: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [Green Version]
- Rai, R.; Gong Essel, K.; Mangiaracina Benbrook, D.; Garland, J.; Daniel Zhao, Y.; Chandra, V. Preclinical efficacy and involvement of AKT, mTOR, and ERK Kinases in the mechanism of sulforaphane against endometrial cancer. Cancers 2020, 12, 1273. [Google Scholar] [CrossRef]
- Gerhauser, C. Epigenetic impact of dietary isothiocyanates in cancer chemoprevention. Curr. Opin. Clin. Nutr. Metab. Care 2013, 16, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Gaur, A.B.; Lengyel, E.; Peter, M.E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008, 22, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Kyung, S.Y.; Kim, D.Y.; Yoon, J.Y.; Son, E.S.; Kim, Y.J.; Park, J.W.; Jeong, S.H. Sulforaphane attenuates pulmonary fibrosis by inhibiting the epithelial-mesenchymal transition. BMC Pharm. Toxicol. 2018, 19, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calabrese, E.J.; Blain, R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: An overview. Toxicol. Appl. Pharm. 2005, 202, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Blain, R.B. The hormesis database: The occurrence of hormetic dose responses in the toxicological literature. Regul. Toxicol. Pharm. 2011, 61, 73–81. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis mediates acquired resilience: Using plant-derived chemicals to enhance health. Annu. Rev. Food Sci. Technol. 2021, 12, 355–381. [Google Scholar] [CrossRef]
- Jodynis-Liebert, J.; Kujawska, M. Biphasic dose-response induced by phytochemicals: Experimental evidence. J. Clin. Med. 2020, 9, 718. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.; Wang, W.; Zhou, Z.; Sun, C. Benefits and risks of the hormetic effects of dietary isothiocyanates on cancer prevention. PLoS ONE 2014, 9, e114764. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Atkinson, S.J.; Akbareian, S.E.; Zhou, Z.; Munsterberg, A.; Robinson, S.D.; Bao, Y. Sulforaphane exerts anti-angiogenesis effects against hepatocellular carcinoma through inhibition of STAT3/HIF-1alpha/VEGF signalling. Sci. Rep. 2017, 7, 12651. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Wang, W.; Shan, Y.; Barrera, L.N.; Howie, A.F.; Beckett, G.J.; Wu, K.; Bao, Y. Synergy between sulforaphane and selenium in the up-regulation of thioredoxin reductase and protection against hydrogen peroxide-induced cell death in human hepatocytes. Food Chem. 2012, 133, 300–307. [Google Scholar] [CrossRef]
- Liu, P.; Behray, M.; Wang, Q.; Wang, W.; Zhou, Z.; Chao, Y.; Bao, Y. Anti-cancer activities of allyl isothiocyanate and its conjugated silicon quantum dots. Sci. Rep. 2018, 8, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misiewicz, I.; Skupińska, K.; Kowalska, E.; Lubiński, J.; Kasprzycka-Guttman, T. Sulforaphane-mediated induction of a phase 2 detoxifying enzyme NAD(P)H:quinone reductase and apoptosis in human lymphoblastoid cells. Acta Biochim. Pol. 2004, 51, 711–721. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [PubMed] [Green Version]
- Wang, Y.; Chen, F.; Zhang, Y.; Zheng, X.; Liu, S.; Tang, M.; Wang, Z.; Wang, P.; Bao, Y.; Li, D. Biphasic effect of sulforaphane on angiogenesis in hypoxia via modulation of both Nrf2 and mitochondrial dynamics. Food Funct. 2022, 13, 2884–2898. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Nie, J.H.; Bao, Y.; Yang, X. Sulforaphane rescues ethanol-suppressed angiogenesis through oxidative and endoplasmic reticulum stress in chick embryos. J. Agric. Food Chem. 2018, 66, 9522–9533. [Google Scholar] [CrossRef] [Green Version]
- Gasper, A.V.; Al-Janobi, A.; Smith, J.A.; Bacon, J.R.; Fortun, P.; Atherton, C.; Taylor, M.A.; Hawkey, C.J.; Barrett, D.A.; Mithen, R.F. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am. J. Clin. Nutr. 2005, 82, 1283–1291. [Google Scholar] [CrossRef] [Green Version]
- Mielczarek, L.; Krug, P.; Mazur, M.; Milczarek, M.; Chilmonczyk, Z.; Wiktorska, K. In the triple-negative breast cancer MDA-MB-231 cell line, sulforaphane enhances the intracellular accumulation and anticancer action of doxorubicin encapsulated in liposomes. Int. J. Pharm. 2019, 558, 311–318. [Google Scholar] [CrossRef]
- Encinas-Basurto, D.; Ibarra, J.; Juarez, J.; Burboa, M.G.; Barbosa, S.; Taboada, P.; Troncoso-Rojas, R.; Valdez, M.A. Poly (lactic-co-glycolic acid) nanoparticles for sustained release of allyl isothiocyanate: Characterization, in vitro release and biological activity. J. Microencapsul. 2017, 34, 231–242. [Google Scholar] [CrossRef]
- Krug, P.; Mielczarek, L.; Wiktorska, K.; Kaczyńska, K.; Wojciechowski, P.; Andrzejewski, K.; Ofiara, K.; Szterk, A.; Mazur, M. Sulforaphane-conjugated selenium nanoparticles: Towards a synergistic anticancer effect. Nanotechnology 2019, 30, 65101. [Google Scholar] [CrossRef]
- Soni, K.; Kohli, K. Sulforaphane-decorated gold nanoparticle for anti-cancer activity: In vitro and in vivo studies. Pharm. Dev. Technol. 2019, 24, 427–438. [Google Scholar] [CrossRef]
- Uppal, S.; Aashima; Kumar, R.; Sareen, S.; Mehta, S.K. Biofabrication of cerium oxide nanoparticles using emulsification for an efficient delivery of benzyl isothiocyanate. Appl. Surf. Sci. 2020, 510, 145011. [Google Scholar] [CrossRef]
- Sun, M.; Shi, Y.; Dang, U.; Di Pasqua, A. Phenethyl isothiocyanate and cisplatin co-encapsulated in a liposomal nanoparticle for treatment of non-small cell lung cancer. Molecules 2019, 24, 801. [Google Scholar] [CrossRef] [PubMed]
- Pulliero, A.; Wu, Y.; Fenoglio, D.; Parodi, A.; Romani, M.; Soares, C.P.; Filaci, G.; Lee, J.L.; Sinkam, P.N.; Lzzotti, A. Nanoparticles increase the efficacy of cancer chemopreventive agents in cells exposed to cigarette smoke condensate. Carcinogenesis 2015, 36, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danafar, H.; Sharafi, A.; Kheiri Manjili, H.; Andalib, S. Sulforaphane delivery using mPEG–PCL co-polymer nanoparticles to breast cancer cells. Pharm. Dev. Technol. 2017, 22, 642–651. [Google Scholar] [CrossRef]
- Manjili, H.K.; Sharafi, A.; Attari, E.; Danafar, H. Pharmacokinetics and in vitro and in vivo delivery of sulforaphane by PCL-PEG-PCL copolymeric-based micelles. Artif. Cell Nanomed. B 2017, 45, 1728–1739. [Google Scholar] [CrossRef] [Green Version]
- Encinas-Basurto, D.; Juarez, J.; Valdez, M.A.; Burboa, M.G.; Barbosa, S.; Taboada, P. Targeted drug delivery via human epidermal growth factor receptor for sustained release of allyl isothiocyanate. Curr. Top. Med. Chem. 2018, 18, 1252–1260. [Google Scholar] [CrossRef]
- Huang, J.; Tao, C.; Yu, Y.; Yu, F.; Zhang, H.; Gao, J.; Wang, D.; Chen, Y.; Gao, J.; Zhang, G. Simultaneous targeting of differentiated breast cancer cells and breast cancer stem cells by combination of docetaxel- and sulforaphane-loaded self-assembled poly(D, L-lactide-co-glycolide)/hyaluronic acid block copolymer-based nanoparticles. J. Biomed. Nanotechnol. 2016, 12, 1463–1477. [Google Scholar] [CrossRef]
- Danafar, H.; Sharafi, A.; Kheiri, S.; Kheiri Manjili, H. Co-delivery of sulforaphane and curcumin with PEGylated iron oxide-gold core shell nanoparticles for delivery to breast cancer cell line. Iran J. Pharm. Res. 2018, 17, 480–494. [Google Scholar]
- Yang, Y.; Shi, Y.; Jay, M.; Di Pasqua, A. Enhanced toxicity of cisplatin with chemosensitizer phenethyl isothiocyanate toward non-small cell lung cancer cells when delivered in liposomal nanoparticles. Chem. Res. Toxicol. 2014, 27, 946–948. [Google Scholar] [CrossRef]
- Xu, Y.; Han, X.; Li, Y.; Min, H.; Zhao, X.; Zhang, Y.; Qi, Y.; Shi, J.; Qi, S.; Bao, Y.; et al. Sulforaphane mediates glutathione depletion via polymeric nanoparticles to restore cisplatin chemosensitivity. ACS Nano 2019, 13, 13445–13455. [Google Scholar] [CrossRef]
- Lu, W.; Du, F.; Zhao, X.; Shi, L.; Shuang, S.; Cui, X.; Dong, C. Sulforaphane-conjugated carbon dots: A versatile nanosystem for targeted imaging and inhibition of EGFR-overexpressing cancer cells. ACS Biomater. Sci. Eng. 2019, 5, 4692–4699. [Google Scholar] [CrossRef] [PubMed]
- Hamidreza, K.M.; Leila, M.; Sharareh, T.; Maedeh, M.; Abbas, S.; Hossein, N.M.; Baptista, P.V. D, L-sulforaphane loaded Fe3O4@ gold core shell nanoparticles: A potential sulforaphane delivery system. PLoS ONE 2016, 11, e0151344. [Google Scholar]
- Li, Y.Z.; Yang, Z.Y.; Gong, T.T.; Liu, Y.S.; Liu, F.H.; Wen, Z.Y.; Li, X.Y.; Gao, C.; Luan, M.; Zhao, Y.H.; et al. Cruciferous vegetable consumption and multiple health outcomes: An umbrella review of 41 systematic reviews and meta-analyses of 303 observational studies. Food Funct. 2022, 13, 4247–4259. [Google Scholar] [CrossRef] [PubMed]
| Isothiocyanate | Disorders | Research Type | Information/Results | Reference |
|---|---|---|---|---|
| SFN | Breast cancer | In vitro | ALDH positive CSCs was reduced by 65–80% by SFN and breast CSCs can be effectively eliminated. | [12] |
| SFN | Liver cancer | In vitro | SFN is a potent inducer of apoptosis in hepatocellular carcinoma cells via PFKFB4 inhibition pathways. | [13] |
| broccoli sprouts/SFN | Helicobacter acceleratum/ gastric cancer | In vitro | H. pylori-associated inflammation was inhibited by reducing IL-8 releasing. | [25] |
| broccoli sprouts/SFN | Helicobacter pylori infection | Clinical trial | Helicobacter pylori infections were reduced significantly. | [26] |
| broccoli sprouts/SFN | Helicobacter pylori infection | Clinical trial | Urease and serum proproteinase I and II levels were significantly reduced. | [27] |
| broccoli sprouts/SFN | Helicobacter pylori infection | Clinical trial | Helicobacter pylori stool antigen (HpSA) values became negative after 8-week Treatment. | [28] |
| SFN | Bladder cancer | C57BL mice | Bladder carcinogenesis was effectively prevented by SFN via balancing of intestinal flora. | [30] |
| SFN | Prostate cancer | In vitro and in vivo | The expressions of hexokinase II, pyruvate kinase M2 and/or lactate dehydrogenase A were significantly down-regulated and glycolysis was inhibited. | [33] |
| SFN | Non-small-cell lung cancer | In vitro | SREBP1, FASN, ACACA, and ACLY were down-regulated, and the amount of intracellular fatty acids were reduced, leading to apoptosis of human non-small cell lung cancer cells. | [34] |
| SFN | Pancreatic cancer | In vitro | SFN probably downregulated the sonic hedgehog signaling pathway to prevent pancreatic cancer. | [36] |
| SFN | Pancreatic cancer | In vitro and in vivo | The stemness of pancreatic CSCs were inhibited by SFN. | [37] |
| SFN | Epidermal squamous cell carcinoma cancer | In vitro | SFN inhibited ECS formation by reducing levels of YAP1 and ∆Np63α. | [38] |
| SFN | Breast cancer | In vitro | The expression of exosome miR-140 was increased, and miR-21 and miR-29 were decreased, leading to a decrease in ALDH1 levels and mammosphere formation. | [41] |
| SFN | Breast cancer | In vitro | The tumorigenicity of MCF10DCIS stem-like cells was inhibited by SFN. | [42] |
| SFN | Oral squamous cell carcinoma | In vitro and in vivo | SFN intervention resulted in a dose-dependent increase in the levels of tumor suppressive miR-200c. | [43] |
| SFN | Glioblastoma | In vitro | SFN suppressed PD-L1 expression in a dose-dependent manner. | [47] |
| SFN | Endometrial cancer | In vitro | SFN inhibited AKT, mTOR, and induced downstream signaling changes in ERK to reduce cancer cell activity. | [50] |
| SFN | Bladder cancer, hepatoma and colon cancer | In vitro | SFN (1–5 µM) promoted cell growth by 20–43% compared to the control, whereas SFN (10–40 µM) inhibited cell growth in numerous tumor cell models. | [58] |
| SFN | Liver cancer | In vitro | SFN played an anti-hepatocellular carcinoma role by inhibiting the STAT3/HIF-1alpha/VEGF pathway. | [59] |
| AITC | Hepatoma | In vitro | AITC (2.5 µM) decreased the DNA damage from 21.57 to 12.30%, while AITC (10 and 20 µM) increased it to 36.12% and 47.48%. DNA repair protein Ku70 was involved in this biphasic effect of AITC on DNA integrity. | [59] |
| SFN combined with selenium | Hepatoma | In vitro | SFN and selenium have a synergistic effect on the upregulation of thioredoxin reductase1. | [60] |
| AITC | Hepatoma | In vitro | AITC (2.5 µM) promoted cell migration and DNA damage, which were depended on Nrf2 or glutathione synthesis. | [61] |
| SFN | Angiogenesis | In vitro and in vivo | Low doses of SFN (2.5–10 μM) alone increased angiogenesis and high concentrations of SFN (20–40 μM) inhibited angiogenesis. | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, G.; He, C.; Zhang, S.; Tian, S.; Bao, Y.; Shan, Y. Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 1962. https://doi.org/10.3390/ijms24031962
Na G, He C, Zhang S, Tian S, Bao Y, Shan Y. Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy. International Journal of Molecular Sciences. 2023; 24(3):1962. https://doi.org/10.3390/ijms24031962
Chicago/Turabian StyleNa, Guanqiong, Canxia He, Shunxi Zhang, Sicong Tian, Yongping Bao, and Yujuan Shan. 2023. "Dietary Isothiocyanates: Novel Insights into the Potential for Cancer Prevention and Therapy" International Journal of Molecular Sciences 24, no. 3: 1962. https://doi.org/10.3390/ijms24031962





