Strong Coupling between Surface Plasmon Resonance and Exciton of Labeled Protein–Dye Complex for Immunosensing Applications
Abstract
1. Introduction
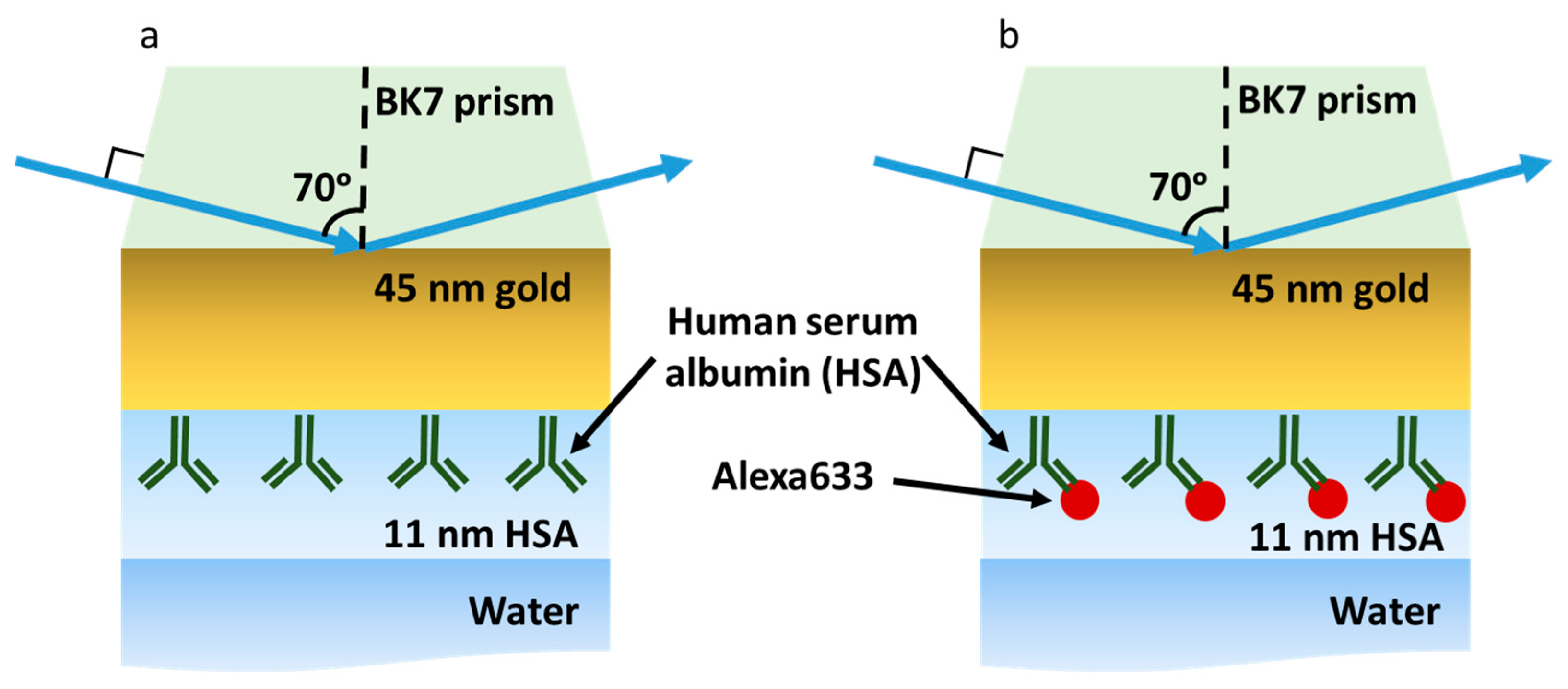
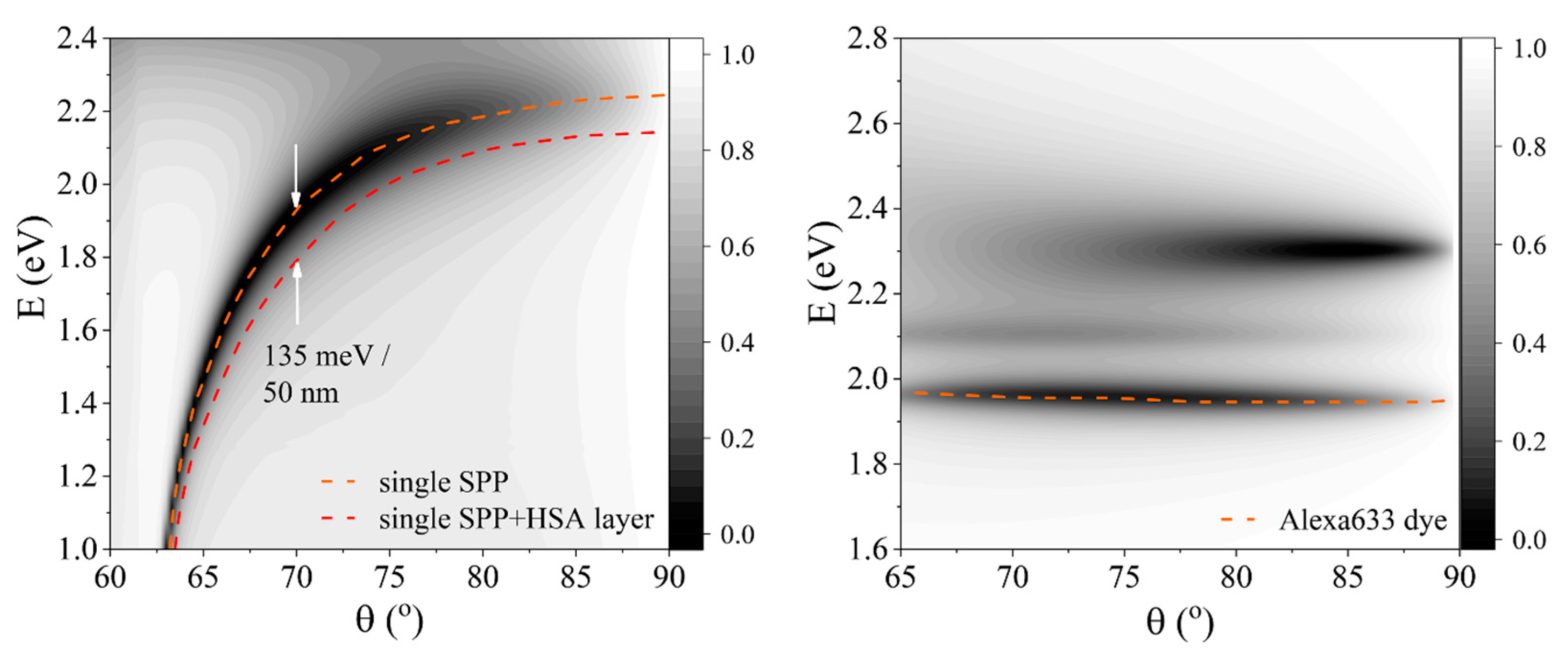
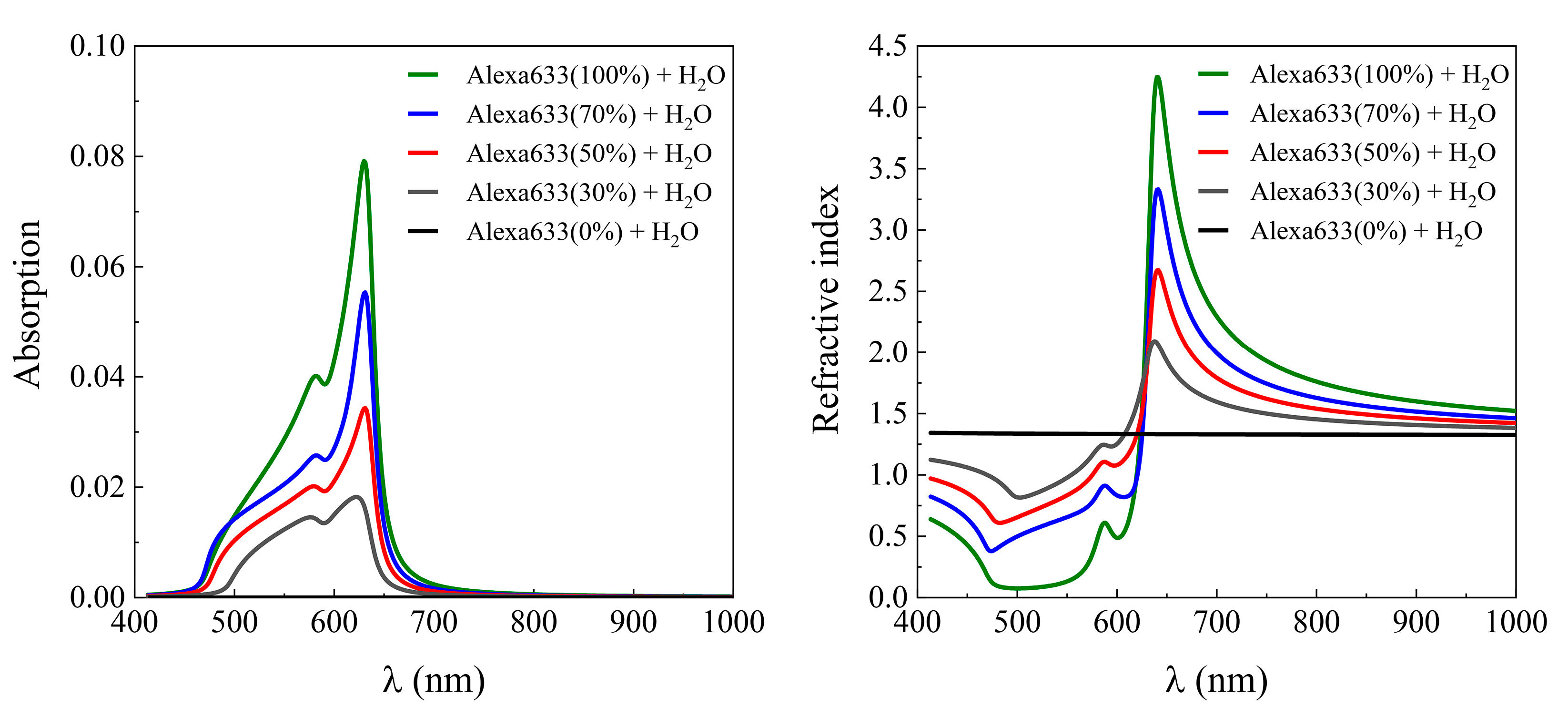
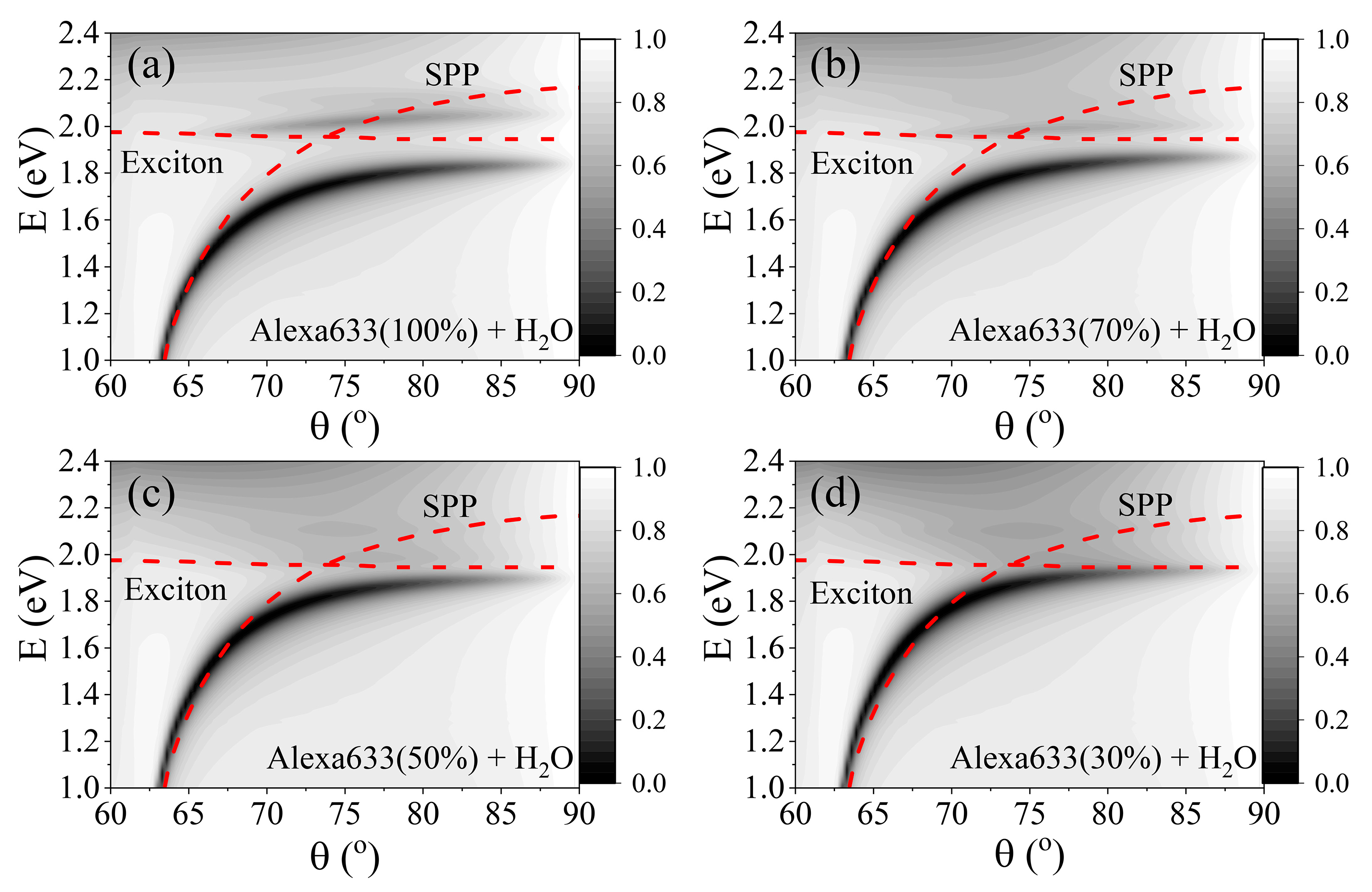
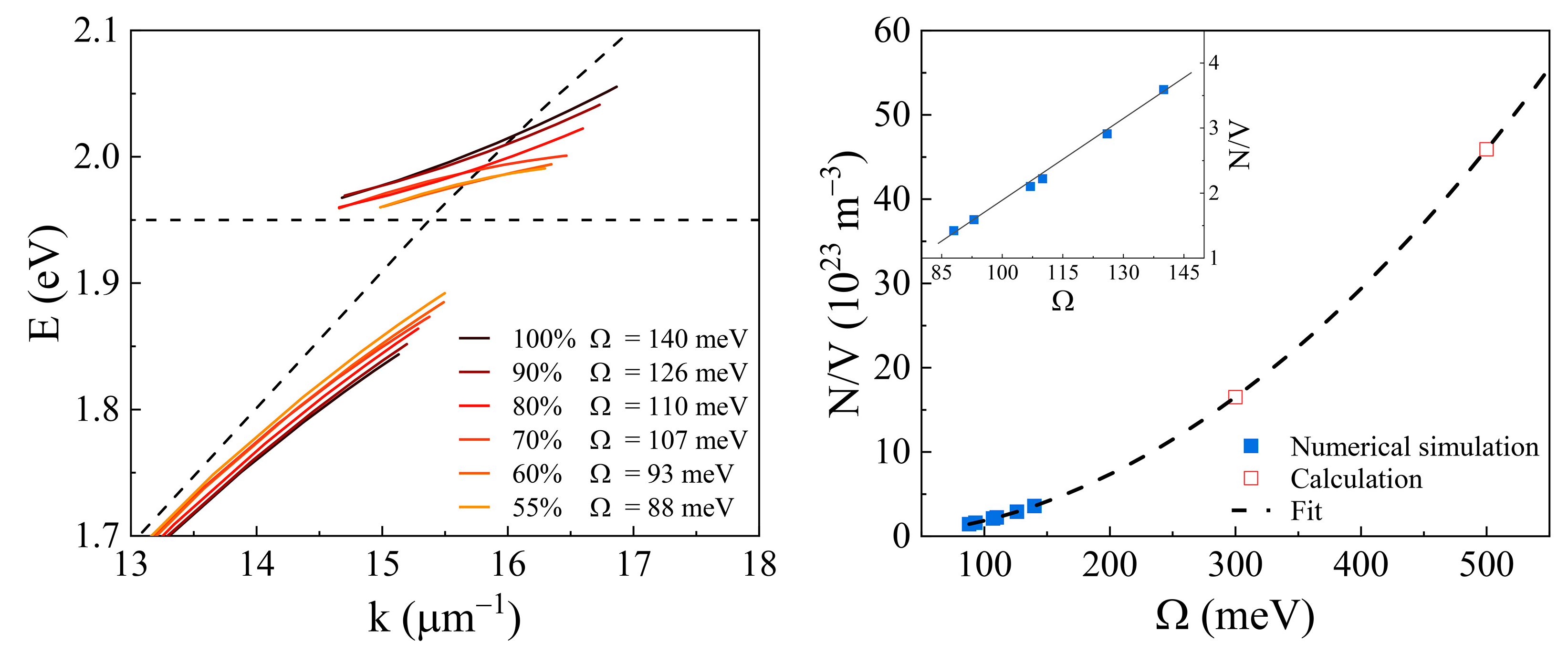
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spencer, K.R.; Wang, J.; Silk, A.W.; Ganesan, S.; Kaufman, H.L.; Mehnert, J.M. Biomarkers for Immunotherapy: Current Developments and Challenges. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, e493–e503. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, Y.; Li, R.; Zhang, P.; Wang, Y.; Gopinath, S.C.B.; Gong, K.; Wan, P. High-performance Detection of an Abdominal Aortic Aneurysm Biomarker by Immunosensing. Biotechnol. Appl. Biochem. 2020, 67, bab.1877. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.-I.; Desmulliez, M.P.Y. Lab-on-a-Chip Based Immunosensor Principles and Technologies for the Detection of Cardiac Biomarkers: A Review. Lab Chip 2011, 11, 569–595. [Google Scholar] [CrossRef]
- Li, B.; Tan, H.; Jenkins, D.; Srinivasa Raghavan, V.; Rosa, B.G.; Güder, F.; Pan, G.; Yeatman, E.; Sharp, D.J. Clinical Detection of Neurodegenerative Blood Biomarkers Using Graphene Immunosensor. Carbon 2020, 168, 144–162. [Google Scholar] [CrossRef]
- Wei, Q.; Lee, M.; Yu, X.; Lee, E.K.; Seong, G.H.; Choo, J.; Cho, Y.W. Development of an Open Sandwich Fluoroimmunoassay Based on Fluorescence Resonance Energy Transfer. Anal. Biochem. 2006, 358, 31–37. [Google Scholar] [CrossRef]
- Sabatté, G.; Keir, R.; Lawlor, M.; Black, M.; Graham, D.; Smith, W.E. Comparison of Surface-Enhanced Resonance Raman Scattering and Fluorescence for Detection of a Labeled Antibody. Anal. Chem. 2008, 80, 2351–2356. [Google Scholar] [CrossRef]
- Diaspro, A.; Chirico, G.; Usai, C.; Ramoino, P.; Dobrucki, J. Photobleaching. In Handbook of Biological Confocal Microscopy; Pawley, J.B., Ed.; Springer: Boston, MA, USA, 2006; pp. 690–702. ISBN 978-0-387-25921-5. [Google Scholar]
- Soler, M.; Estevez, M.-C.; Alvarez, M.; Otte, M.; Sepulveda, B.; Lechuga, L. Direct Detection of Protein Biomarkers in Human Fluids Using Site-Specific Antibody Immobilization Strategies. Sensors 2014, 14, 2239–2258. [Google Scholar] [CrossRef]
- Pultar, J.; Sauer, U.; Domnanich, P.; Preininger, C. Aptamer–Antibody on-Chip Sandwich Immunoassay for Detection of CRP in Spiked Serum. Biosens. Bioelectron. 2009, 24, 1456–1461. [Google Scholar] [CrossRef]
- Jun, J.V.; Chenoweth, D.M.; Petersson, E.J. Rational Design of Small Molecule Fluorescent Probes for Biological Applications. Org. Biomol. Chem. 2020, 18, 5747–5763. [Google Scholar] [CrossRef]
- Rasnik, I.; McKinney, S.A.; Ha, T. Nonblinking and Long-Lasting Single-Molecule Fluorescence Imaging. Nat. Methods 2006, 3, 891–893. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Wan, H.; Wang, L.; Xu, W.; Zhu, S.; Liang, Y.; Zhang, B.; Lou, J.; Dai, H.; et al. High Performance, Multiplexed Lung Cancer Biomarker Detection on a Plasmonic Gold Chip. Adv. Funct. Mater. 2016, 26, 7994–8002. [Google Scholar] [CrossRef]
- Kéna-Cohen, S.; Wiener, A.; Sivan, Y.; Stavrinou, P.N.; Bradley, D.D.C.; Horsfield, A.; Maier, S.A. Plasmonic Sinks for the Selective Removal of Long-Lived States. ACS Nano 2011, 5, 9958–9965. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Park, J.; Kang, S.; Kim, M. Surface Plasmon Resonance: A Versatile Technique for Biosensor Applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [PubMed]
- Plikusiene, I.; Balevicius, Z.; Ramanaviciene, A.; Talbot, J.; Mickiene, G.; Balevicius, S.; Stirke, A.; Tereshchenko, A.; Tamosaitis, L.; Zvirblis, G.; et al. Evaluation of Affinity Sensor Response Kinetics towards Dimeric Ligands Linked with Spacers of Different Rigidity: Immobilized Recombinant Granulocyte Colony-Stimulating Factor Based Synthetic Receptor Binding with Genetically Engineered Dimeric Analyte Derivatives. Biosens. Bioelectron. 2020, 156, 112112. [Google Scholar] [CrossRef]
- De Feijter, J.A.; Benjamins, J.; Veer, F.A. Ellipsometry as a Tool to Study the Adsorption Behavior of Synthetic and Biopolymers at the Air-Water Interface. Biopolymers 1978, 17, 1759–1772. [Google Scholar] [CrossRef]
- Anulytė, J.; Bužavaitė-Vertelienė, E.; Vertelis, V.; Stankevičius, E.; Vilkevičius, K.; Balevičius, Z. Influence of a Gold Nano-Bumps Surface Lattice Array on the Propagation Length of Strongly Coupled Tamm and Surface Plasmon Polaritons. J. Mater. Chem. C 2022, 10, 13234–13241. [Google Scholar] [CrossRef]
- Barnes, W.L. Surface Plasmon–Polariton Length Scales: A Route to Sub-Wavelength Optics. J. Opt. Pure Appl. Opt. 2006, 8, S87–S93. [Google Scholar] [CrossRef]
- Buzavaite-Verteliene, E.; Plikusiene, I.; Tolenis, T.; Valavicius, A.; Anulyte, J.; Ramanavicius, A.; Balevicius, Z. Hybrid Tamm-Surface Plasmon Polariton Mode for Highly Sensitive Detection of Protein Interactions. Opt. Express 2020, 28, 29033. [Google Scholar] [CrossRef]
- Paulauskas, A.; Tumenas, S.; Selskis, A.; Tolenis, T.; Valavicius, A.; Balevicius, Z. Hybrid Tamm-Surface Plasmon Polaritons Mode for Detection of Mercury Adsorption on 1D Photonic Crystal/Gold Nanostructures by Total Internal Reflection Ellipsometry. Opt. Express 2018, 26, 30400. [Google Scholar] [CrossRef]
- Anulytė, J.; Bužavaitė-Vertelienė, E.; Stankevičius, E.; Vilkevičius, K.; Balevičius, Z. High Spectral Sensitivity of Strongly Coupled Hybrid Tamm-Plasmonic Resonances for Biosensing Application. Sensors 2022, 22, 9453. [Google Scholar] [CrossRef]
- Vasa, P.; Wang, W.; Pomraenke, R.; Lammers, M.; Maiuri, M.; Manzoni, C.; Cerullo, G.; Lienau, C. Real-Time Observation of Ultrafast Rabi Oscillations between Excitons and Plasmons in Metal Nanostructures with J-Aggregates. Nat. Photonics 2013, 7, 128–132. [Google Scholar] [CrossRef]
- Bužavaitė-Vertelienė, E.; Vertelis, V.; Balevičius, Z. The Experimental Evidence of a Strong Coupling Regime in the Hybrid Tamm Plasmon-Surface Plasmon Polariton Mode. Nanophotonics 2021, 10, 1565–1571. [Google Scholar] [CrossRef]
- Herrera, F.; Spano, F.C. Cavity-Controlled Chemistry in Molecular Ensembles. Phys. Rev. Lett. 2016, 116, 238301. [Google Scholar] [CrossRef]
- Galego, J.; Garcia-Vidal, F.J.; Feist, J. Suppressing Photochemical Reactions with Quantized Light Fields. Nat. Commun. 2016, 7, 13841. [Google Scholar] [CrossRef] [PubMed]
- Urbanavičiūtė, I.; Višniakova, S.; Dirsytė, J.; Juška, G.; Lenkevičiūtė, B.; Bužavaitė, E.; Žilinskas, A.; Arlauskas, K. A Series of New Luminescent Non-Planar 1,8-Naphthyridine Derivatives Giving Coloured and Close-to-White Electroluminescence Spectra. J. Lumin. 2017, 181, 299–309. [Google Scholar] [CrossRef]
- Pelton, M.; Storm, S.D.; Leng, H. Strong Coupling of Emitters to Single Plasmonic Nanoparticles: Exciton-Induced Transparency and Rabi Splitting. Nanoscale 2019, 11, 14540–14552. [Google Scholar] [CrossRef]
- Kongsuwan, N.; Xiong, X.; Bai, P.; You, J.-B.; Png, C.E.; Wu, L.; Hess, O. Quantum Plasmonic Immunoassay Sensing. Nano Lett. 2019, 19, 5853–5861. [Google Scholar] [CrossRef]
- Munkhbat, B.; Wersäll, M.; Baranov, D.G.; Antosiewicz, T.J.; Shegai, T. Suppression of Photo-Oxidation of Organic Chromophores by Strong Coupling to Plasmonic Nanoantennas. Sci. Adv. 2018, 4, eaas9552. [Google Scholar] [CrossRef]
- Nooke, A.; Beck, U.; Hertwig, A.; Krause, A.; Krüger, H.; Lohse, V.; Negendank, D.; Steinbach, J. On the Application of Gold Based SPR Sensors for the Detection of Hazardous Gases. Sens. Actuators B Chem. 2010, 149, 194–198. [Google Scholar] [CrossRef]
- Balevicius, Z.; Makaraviciute, A.; Babonas, G.-J.; Tumenas, S.; Bukauskas, V.; Ramanaviciene, A.; Ramanavicius, A. Study of Optical Anisotropy in Thin Molecular Layers by Total Internal Reflection Ellipsometry. Sens. Actuators B Chem. 2013, 181, 119–124. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific Alexa Fluor® 633 (Catalog Num. A20005). Available online: https://www.thermofisher.com/order/catalog/product/A20005 (accessed on 20 December 2022).
- Törmä, P.; Barnes, W.L. Strong Coupling between Surface Plasmon Polaritons and Emitters: A Review. Rep. Prog. Phys. 2015, 78, 013901. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurkšaitis, P.; Bužavaitė-Vertelienė, E.; Balevičius, Z. Strong Coupling between Surface Plasmon Resonance and Exciton of Labeled Protein–Dye Complex for Immunosensing Applications. Int. J. Mol. Sci. 2023, 24, 2029. https://doi.org/10.3390/ijms24032029
Jurkšaitis P, Bužavaitė-Vertelienė E, Balevičius Z. Strong Coupling between Surface Plasmon Resonance and Exciton of Labeled Protein–Dye Complex for Immunosensing Applications. International Journal of Molecular Sciences. 2023; 24(3):2029. https://doi.org/10.3390/ijms24032029
Chicago/Turabian StyleJurkšaitis, Povilas, Ernesta Bužavaitė-Vertelienė, and Zigmas Balevičius. 2023. "Strong Coupling between Surface Plasmon Resonance and Exciton of Labeled Protein–Dye Complex for Immunosensing Applications" International Journal of Molecular Sciences 24, no. 3: 2029. https://doi.org/10.3390/ijms24032029
APA StyleJurkšaitis, P., Bužavaitė-Vertelienė, E., & Balevičius, Z. (2023). Strong Coupling between Surface Plasmon Resonance and Exciton of Labeled Protein–Dye Complex for Immunosensing Applications. International Journal of Molecular Sciences, 24(3), 2029. https://doi.org/10.3390/ijms24032029





