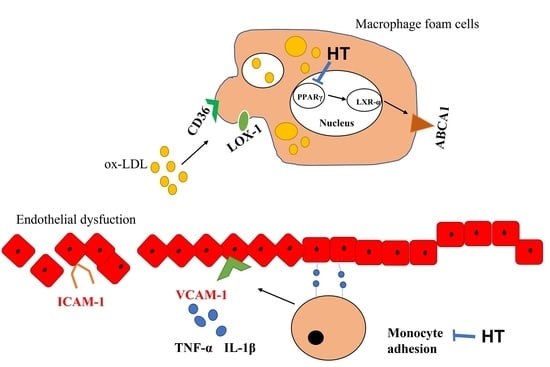
Hydroxytyrosol Reduces Foam Cell Formation and Endothelial Inflammation Regulating the PPARγ/LXRα/ABCA1 Pathway
Abstract
1. Introduction
2. Results
2.1. HT Does Not Affect the Viability and Mitigates Oxidative Stress in THP-1 Macrophage Foam Cells
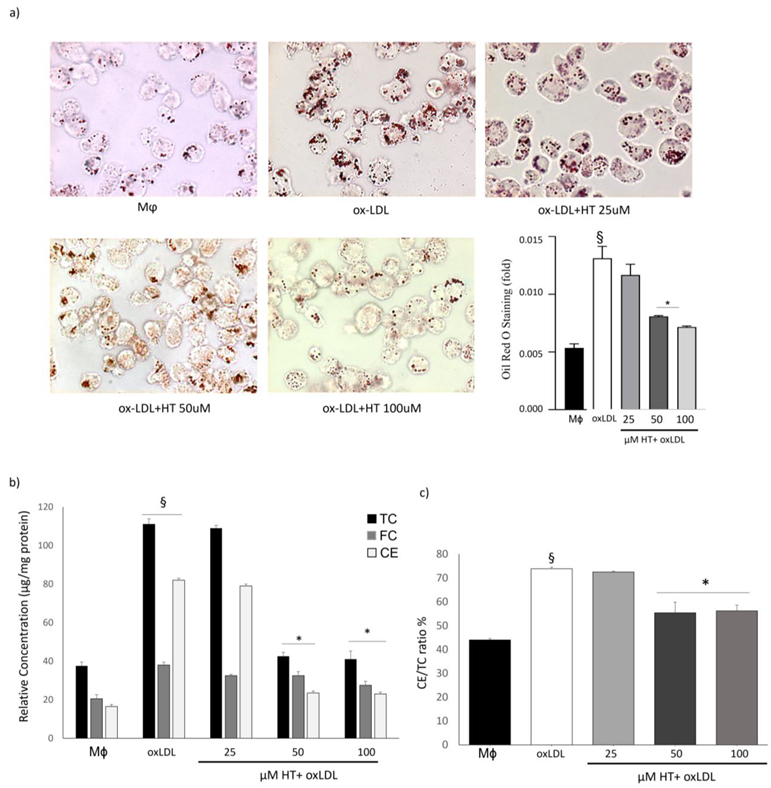
2.2. HT Decreases Lipid Over-Accumulation in THP-1 Macrophage Foam Cells
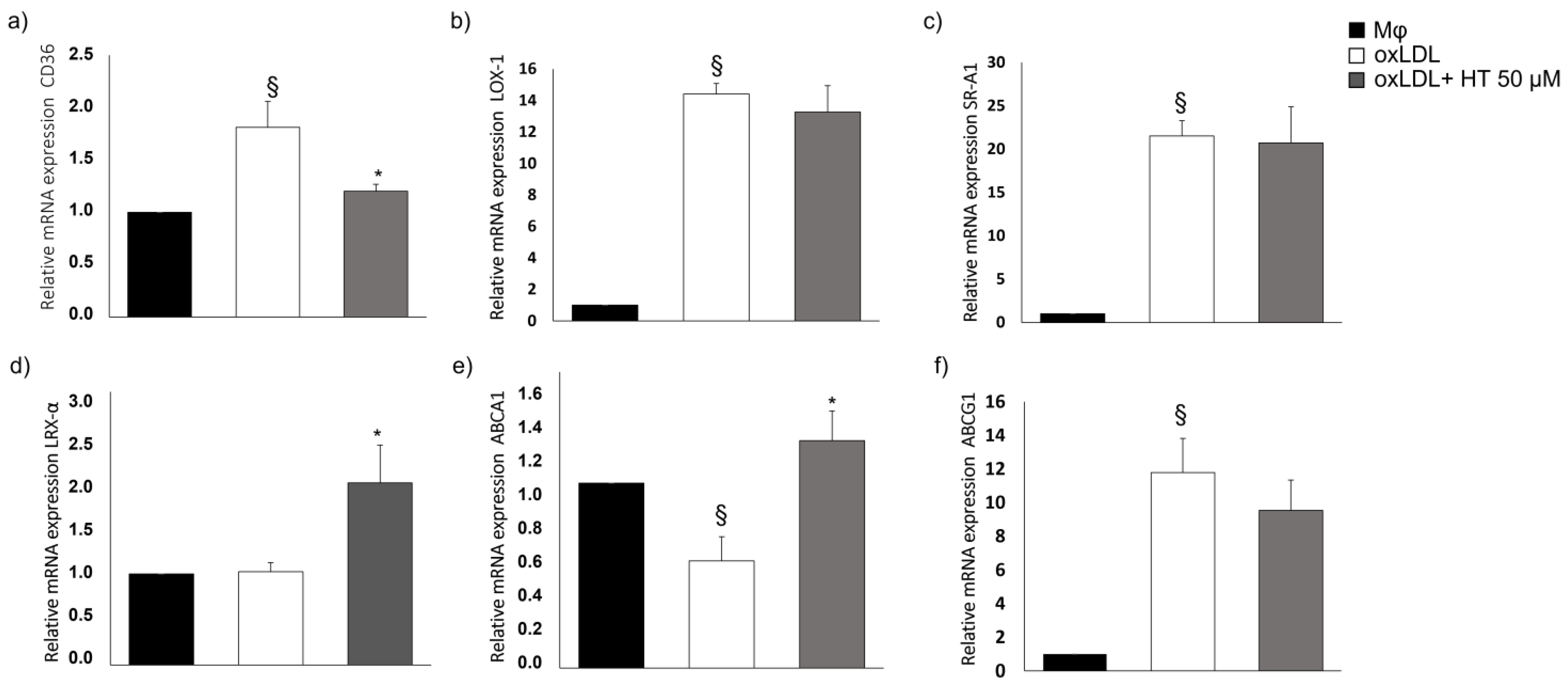
2.3. Effect of HT on Cholesterol Metabolism-Related Molecules
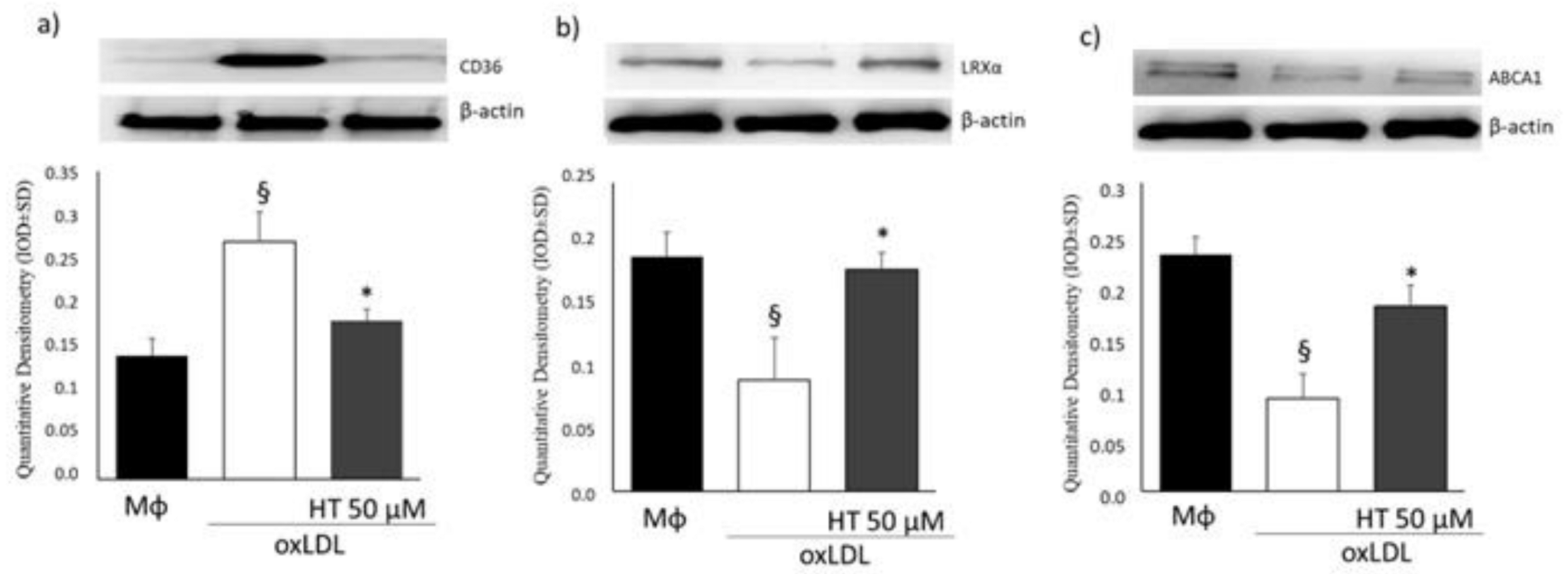
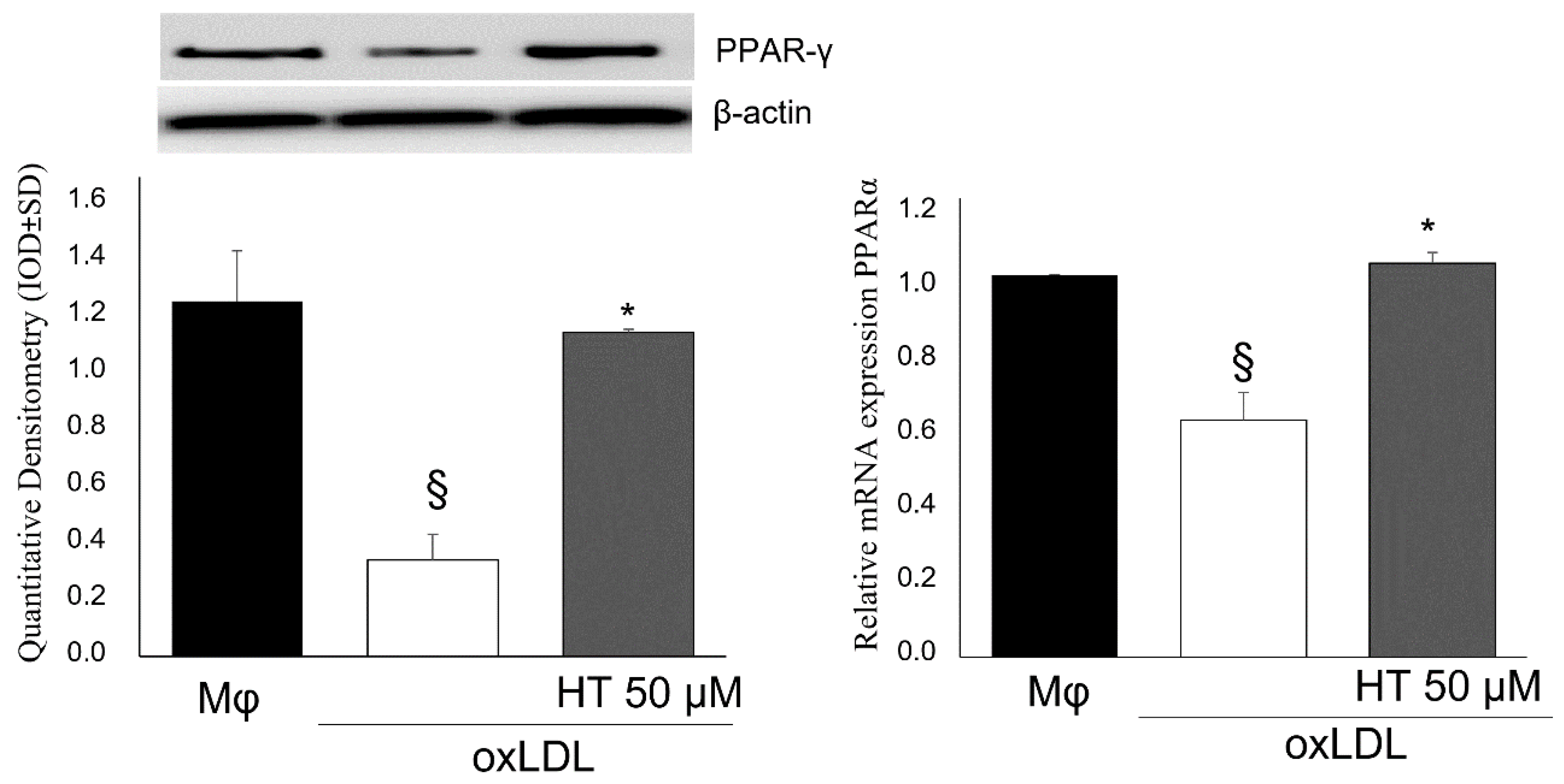
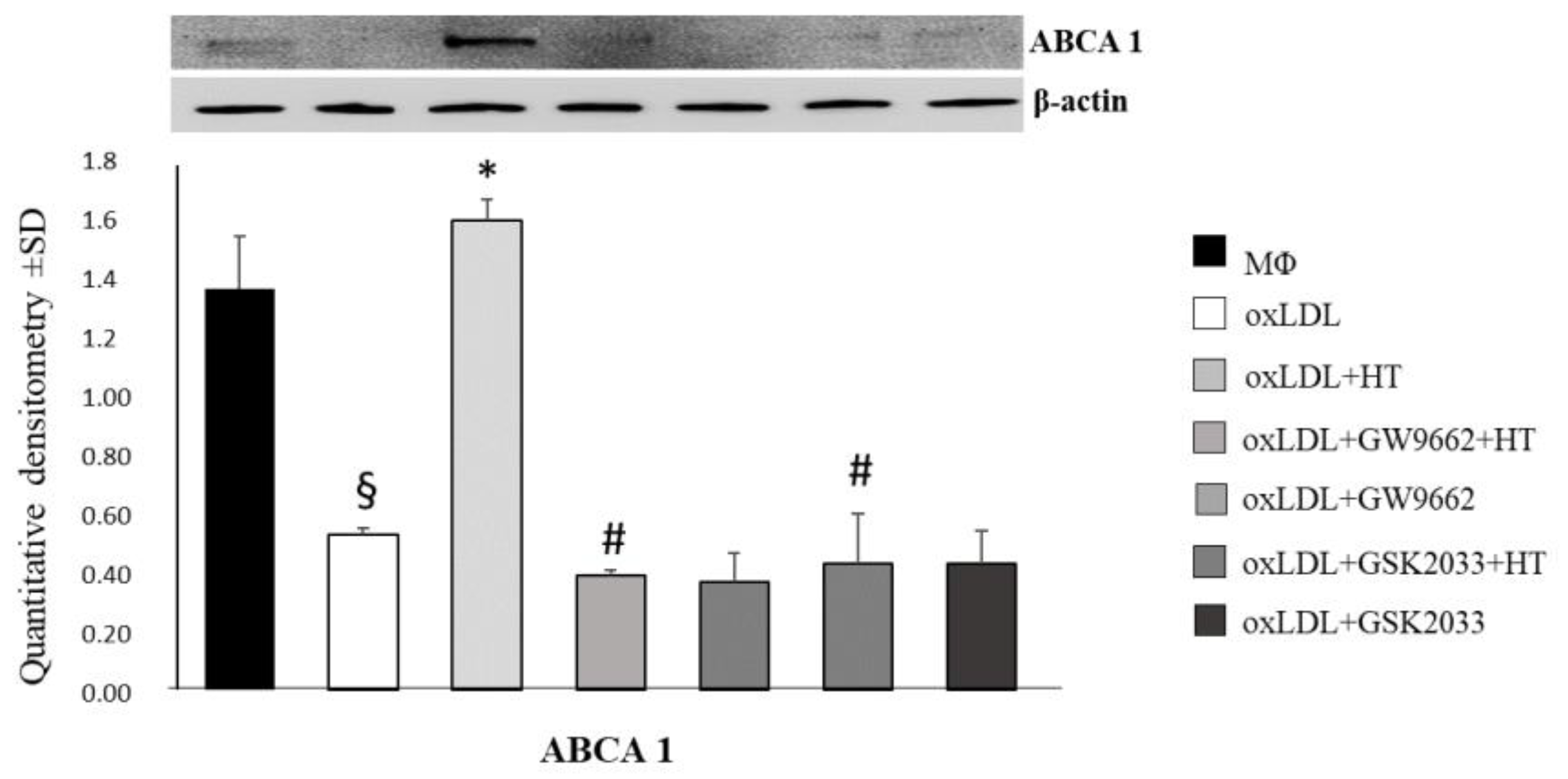
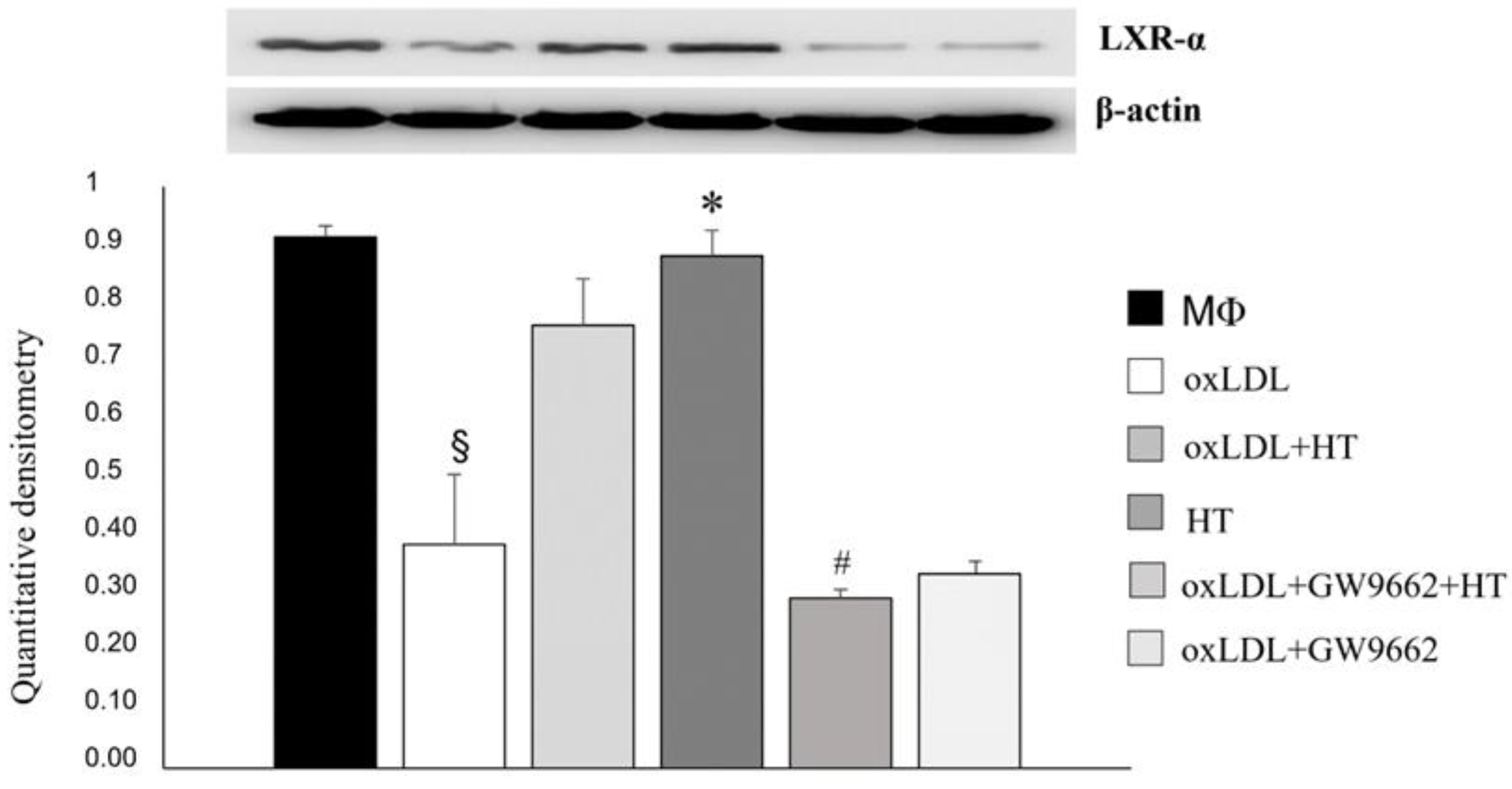
2.4. Effect of HT on PPARγ/LXRα/ABCA1 Signaling in Human THP-1 Macrophage Foam Cells
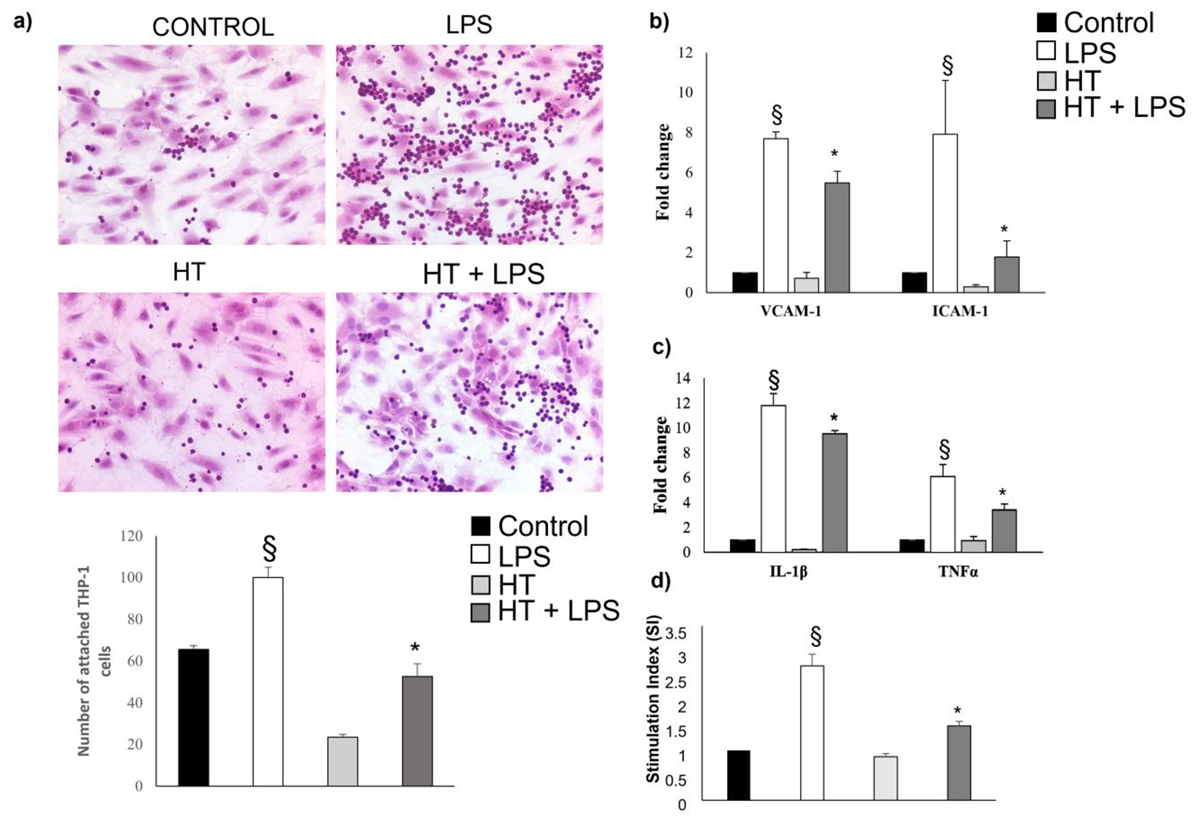
2.5. HT Decrease LPS-Induced Adhesion and Inflammatory Response in Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Monocyte-HUVEC Adhesion
4.3. Cytotoxicity Assay and ROS Detection
4.4. Foam Cell Formation Assay and Lipid Content Detection
4.5. Cholesterol Quantitation Assay
4.6. RNA Extraction, Reverse Transcription and Real-Time PCR (qPCR)
4.7. Hematoxylin–Eosin Stain
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raggi, P.; Genest, J.; Giles, J.T.; Rayner, K.J.; Dwivedi, G.; Beanlands, R.S.; Gupta, M. Role of inflammation in the pathogenesis of atherosclerosis and therapeutic interventions. Atherosclerosis 2018, 276, 98–108. [Google Scholar] [CrossRef]
- Gencer, S.; Evans, B.; van der Vorst, E.; Döring, Y.; Weber, C. Inflammatory Chemokines in Atherosclerosis. Cells 2021, 10, 226. [Google Scholar] [CrossRef]
- Zhang, J.; Alcaide, P.; Liu, L.; Sun, J.; He, A.; Luscinskas, F.; Shi, G.-P. Regulation of Endothelial Cell Adhesion Molecule Expression by Mast Cells, Macrophages, and Neutrophils. PLoS ONE 2011, 6, e14525. [Google Scholar] [CrossRef]
- Moore, K.J.; Sheedy, F.J.; Fisher, E.A. Macrophages in atherosclerosis: A dynamic balance. Nat. Rev. Immunol. 2013, 13, 709–721. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Bezsonov, E.E.; Orekhova, V.A.; Popkova, T.V.; Starodubova, A.V.; Orekhov, A.N. Recognition of Oxidized Lipids by Macrophages and Its Role in Atherosclerosis Development. Biomedicines 2021, 9, 915. [Google Scholar] [CrossRef]
- Syväranta, S.; Alanne-Kinnunen, M.; Öörni, K.; Oksjoki, R.; Kupari, M.; Kovanen, P.T.; Helske-Suihko, S. Potential pathological roles for oxidized low-density lipoprotein and scavenger receptors SR-AI, CD36, and LOX-1 in aortic valve stenosis. Atherosclerosis 2014, 235, 398–407. [Google Scholar] [CrossRef]
- Ouimet, M.; Barrett, T.J.; Fisher, E.A. HDL and Reverse Cholesterol Transport. Circ. Res. 2019, 124, 1505–1518. [Google Scholar] [CrossRef]
- Yvan-Charvet, L.; Ranalletta, M.; Wang, N.; Han, S.; Terasaka, N.; Li, R.; Welch, C.; Tall, A.R. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J. Clin. Investig. 2007, 117, 3900–3908. [Google Scholar] [CrossRef]
- Barish, G.D. Peroxisome Proliferator-Activated Receptors and Liver X Receptors in Atherosclerosis and Immunity. J. Nutr. 2006, 136, 690–694. [Google Scholar] [CrossRef]
- Nimgulkar, C.; Ghosh, S.; Sankar, A.B.; Uday, K.P.; Surekha, M.V.; Madhusudhanachary, P.; Annapurna, B.R.; Raghu, P.; Bharatraj, D.K. Combination of spices and herbal extract restores macrophage foam cell migration and abrogates the athero-inflammatory signalling cascade of atherogenesis. Vasc. Pharmacol. 2015, 72, 53–63. [Google Scholar] [CrossRef]
- Chi, L.; Peng, L.; Pan, N.; Hu, X.; Zhang, Y. The anti-atherogenic effects of berberine on foam cell formation are mediated through the upregulation of sirtuin 1. Int. J. Mol. Med. 2014, 34, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Wang, X.; Bi, S.; Pan, Z.; Liu, S.; Yu, H.; Lu, H.; Lin, X.; Wang, X.; Ma, T.; et al. Inhibitory effects of resveratrol on foam cell formation are mediated through monocyte chemotactic protein-1 and lipid metabolism-related proteins. Int. J. Mol. Med. 2014, 33, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wang, C.J.; Wang, C.P.; Sheu, J.Y.; Lin, C.L.; Lin, H.H. Hibiscus sabdariffa leaf polyphenolic extract inhibits LDL oxidation and foam cell formation involving up-regulation of LXRα/ABCA1 pathway. Food Chem. 2013, 141, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.L.; Hu, H.J.; Liu, Y.B.; Hu, X.M.; Fan, X.J.; Zou, W.W.; Pan, Y.Q.; Zhou, W.Q.; Peng, M.W.; Gu, C.H. Allicin induces the upregulation of ABCA1 expression via PPARγ/LXRα signaling in THP-1 macrophage-derived foam cells. Int. J. Mol. Med. 2017, 39, 1452–1460. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Ren, K.; Chen, Q.; Li, H.; Yao, R.; Hu, H.; Lv, Y.C.; Zhao, G.J. Leonurine Prevents Atherosclerosis Via Promoting the Expression of ABCA1 and ABCG1 in a Pparγ/Lxrα Signaling Pathway-Dependent Manner. Cell. Physio. Biochem. 2017, 43, 1703–1717. [Google Scholar] [CrossRef] [PubMed]
- Bhansali, S.; Khatri, S.; Dhawan, V. Terminalia Arjuna bark extract impedes foam cell formation and promotes apoptosis in ox-LDL-stimulated macrophages by enhancing UPR-CHOP pathway. Lipids Health Dis. 2019, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Wing-Shing Cheung, D.; Koon, C.M.; Ng, C.F.; Leung, P.C.; Fung, K.P.; Kar-Sing Poon, S.; Bik-San Lau, C. The roots of Salvia miltiorrhiza (Danshen) and Pueraria lobata (Gegen) inhibit atherogenic events: A study of the combination effects of the 2-herb formula. J. Ethnopharmacol. 2012, 143, 859–866. [Google Scholar] [CrossRef]
- Liu, H.J.; Wang, X.L.; Zhang, L.; Qiu, Y.; Li, T.J.; Li, R.; Wu, M.C.; Wei, L.X.; Rui, Y.C. Inhibitions of vascular endothelial growth factor expression and foam cell formation by EGb 761, a special extract of Ginkgo biloba, in oxidatively modified low-density lipoprotein-induced human THP-1 monocytes cells. Phytomedicine 2009, 16, 138–145. [Google Scholar] [CrossRef]
- Zarrouk, A.; Martine, L.; Grégoire, S.; Nury, T.; Meddeb, W.; Camus, E.; Badreddine, A.; Durand, P.; Namsi, A.; Yammine, A.; et al. Profile of Fatty Acids, Tocopherols, Phytosterols and Polyphenols in Mediterranean Oils (Argan Oils, Olive Oils, Milk Thistle Seed Oils and Nigella Seed Oil) and Evaluation of their Antioxidant and Cytoprotective Activities. Curr. Pharm. Des. 2019, 25, 1791–1805. [Google Scholar] [CrossRef]
- Penson, P.E.; Banach, M. Natural compounds as anti-atherogenic agents: Clinical evidence for improved cardiovascular outcomes. Atherosclerosis 2021, 316, 58–65. [Google Scholar] [CrossRef]
- Jia, Q.; Cao, H.; Shen, D.; Li, S.; Yan, L.; Chen, C.; Xing, S.; Dou, F. Quercetin protects against atherosclerosis by regulating the expression of PCSK9, CD36, PPARγ, LXRα and ABCA1. Int. J. Mol. Med. 2019, 44, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-H.; Chen, J.-J.; Deng, W.-Y.; Xu, X.-D.; Liu, Q.-X.; Shi, M.-W.; Ren, K. Biochanin A Mitigates Atherosclerosis by Inhibiting Lipid Accumulation and Inflammatory Response. Oxidative Med. Cell. Longev. 2020, 2020, 8965047. [Google Scholar] [CrossRef] [PubMed]
- Fouache, A.; Zabaiou, N.; De Joussineau, C.; Morel, L.; Silvente-Poirot, S.; Namsi, A.; Lizard, G.; Poirot, M.; Makishima, M.; Baron, S.; et al. Flavonoids differentially modulate liver X receptors activity—Structure-function relationship analysis. J. Steroid Biochem. Mol. Biol. 2019, 190, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Namsi, A.; Nury, T.; Khan, A.S.; Leprince, J.; Vaudry, D.; Caccia, C.; Leoni, V.; Atanasov, A.G.; Tonon, M.-C.; Masmoudi-Kouki, O.; et al. Octadecaneuropeptide (ODN) Induces N2a Cells Differentiation through a PKA/PLC/PKC/MEK/ERK-Dependent Pathway: Incidence on Peroxisome, Mitochondria, and Lipid Profiles. Molecules 2019, 24, 3310. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide Biological Role of Hydroxytyrosol: Possible Therapeutic and Preventive Properties in Cardiovascular Diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rodriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M. Hydroxytyrosol: Bioavailability, toxicity, and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef]
- Liebgott, P.-P.; Amouric, A.; Comte, A.; Tholozan, J.-L.; Lorquin, J. Hydroxytyrosol from tyrosol using hydroxyphenylacetic acid-induced bacterial cultures and evidence of the role of 4-HPA 3-hydroxylase. Res. Microbiol. 2009, 160, 757–766. [Google Scholar] [CrossRef]
- Saija, A.; Trombetta, D.; Tomaino, A.; Lo Cascio, R.; Princi, P.; Uccella, N.; Bonina, F.; Castelli, F. ‘In vitro’ evaluation of the antioxidant activity and biomembrane interaction of the plant phenols oleuropein and hydroxytyrosol. Int. J. Pharm. 1998, 166, 123–133. [Google Scholar] [CrossRef]
- Calabriso, N.; Gnoni, A.; Stanca, E.; Cavallo, A.; Damiano, F.; Siculella, L.; Carluccio, M.A. Hydroxytyrosol Ameliorates Endothelial Function under Inflammatory Conditions by Preventing Mitochondrial Dysfunction. Oxidative Med. Cell. Longev. 2018, 2018, 9086947. [Google Scholar] [CrossRef]
- Carluccio, M.A.; Ancora, M.A.; Massaro, M.; Carluccio, M.; Scoditti, E.; Distante, A.; Storelli, C.; De Caterina, R. Homocysteine induces VCAM-1 gene expression through NF-κB and NAD(P)H oxidase activation: Protective role of Mediterranean diet polyphenolic antioxidants. Am. J. Physiol. Circ. Physiol. 2007, 293, H2344–H2354. [Google Scholar] [CrossRef]
- Manna, C.; Napoli, D.; Cacciapuoti, G.; Porcelli, M.; Zappia, V. Olive Oil Phenolic Compounds Inhibit Homocysteine-Induced Endothelial Cell Adhesion Regardless of Their Different Antioxidant Activity. J. Agric. Food Chem. 2009, 57, 3478–3482. [Google Scholar] [CrossRef] [PubMed]
- González-Santiago, M.; Martín-Bautista, E.; Carrero, J.; Fonollá, J.; Baró, L.; Bartolomé, M.; Gil-Loyzaga, P.; López-Huertas, E. One-month administration of hydroxytyrosol, a phenolic antioxidant present in olive oil, to hyperlipemic rabbits improves blood lipid profile, antioxidant status and reduces atherosclerosis development. Atherosclerosis 2006, 188, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Warleta, F.; Quesada, C.S.; Campos, M.; Allouche, Y.; Beltran, G.; Gaforio, J.J. Hydroxytyrosol Protects against Oxidative DNA Damage in Human Breast Cells. Nutrients 2011, 3, 839–857. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; Bouaziz, M.; Fki, I.; El Feki, A.; Sayadi, S. Hypolipidimic and antioxidant activities of oleuropein and its hydrolysis derivative-rich extracts from Chemlali olive leaves. Chem. Interactions 2008, 176, 88–98. [Google Scholar] [CrossRef]
- Romero, M.; Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016, 7, 584–593. [Google Scholar] [CrossRef]
- Yammine, A.; Namsi, A.; Vervandier-Fasseur, D.; Mackrill, J.; Lizard, G.; Latruffe, N. Polyphenols of the Mediterranean Diet and Their Metabolites in the Prevention of Colorectal Cancer. Molecules 2021, 26, 3483. [Google Scholar] [CrossRef]
- de Freitas, F.A.; Levy, D.; Reichert, C.O.; Cunha-Neto, E.; Kalil, J.; Bydlowski, S.P. Effects of Oxysterols on Immune Cells and Related Diseases. Cells 2022, 11, 1251. [Google Scholar] [CrossRef]
- Li, L.; Du, Z.; Rong, B.; Zhao, D.; Wang, A.; Xu, Y.; Zhang, H.; Bai, X.; Zhong, J. Foam cells promote atherosclerosis progression by releasing CXCL12. Biosci. Rep. 2020, 40, BSR20193267. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef]
- Gui, Y.; Zheng, H.; Cao, R.Y. Foam Cells in Atherosclerosis: Novel Insights Into Its Origins, Consequences, and Molecular Mechanisms. Front. Cardiovasc. Med. 2022, 9, 845942. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Zhang, M.; Chen, L.-Y.; Gong, D.; Xia, X.-D.; Yu, X.-H.; Wang, S.-Q.; Ou, X.; Dai, X.-Y.; Zheng, X.-L.; et al. Heat shock protein 70 accelerates atherosclerosis by downregulating the expression of ABCA1 and ABCG1 through the JNK/Elk-1 pathway. Biochim. et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2018, 1863, 806–822. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yan, G.; Wang, H.; Lou, D. Septin 4 activates PPARγ/LXRα signaling by upregulating ABCA1 and ABCG1 expression to inhibit the formation of THP-1 macrophage-derived foam cells. Exp. Ther. Med. 2021, 22, 763. [Google Scholar] [CrossRef] [PubMed]
- Heming, M.; Gran, S.; Jauch, S.-L.; Fischer-Riepe, L.; Russo, A.; Klotz, L.; Hermann, S.; Schäfers, M.; Roth, J.; Barczyk-Kahlert, K. Peroxisome Proliferator-Activated Receptor-γ Modulates the Response of Macrophages to Lipopolysaccharide and Glucocorticoids. Front. Immunol. 2018, 9, 893. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A natural compound with promising pharmacological activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Hazas, M.D.C.L.D.L.; Rubió, L.; Macia, A.; Motilva, M.J. Hydroxytyrosol: Emerging Trends in Potential Therapeutic Applications. Curr. Pharm. Des. 2018, 24, 2157–2179. [Google Scholar] [CrossRef]
- Pastor, R.; Bouzas, C.; Tur, J.A. Beneficial effects of dietary supplementation with olive oil, oleic acid, or hydroxytyrosol in metabolic syndrome: Systematic review and meta-analysis. Free. Radic. Biol. Med. 2021, 172, 372–385. [Google Scholar] [CrossRef]
- Javadifar, A.; Rastgoo, S.; Banach, M.; Jamialahmadi, T.; Johnston, T.; Sahebkar, A. Foam Cells as Therapeutic Targets in Atherosclerosis with a Focus on the Regulatory Roles of Non-Coding RNAs. Int. J. Mol. Sci. 2021, 22, 2529. [Google Scholar] [CrossRef]
- Liao, Y.; Zhu, E.; Zhou, W. Ox-LDL Aggravates the Oxidative Stress and Inflammatory Responses of THP-1 Macrophages by Reducing the Inhibition Effect of miR-491-5p on MMP-9. Front. Cardiovasc. Med. 2021, 8, 697236. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, Y.; Zhang, X.; Aa, J.; Wang, G.; Xie, Y. Curcumin regulates endogenous and exogenous metabolism via Nrf2-FXR-LXR pathway in NAFLD mice. Biomed. Pharmacother. 2018, 105, 274–281. [Google Scholar] [CrossRef]
- Napolitano, G.; Fasciolo, G.; Venditti, P. Mitochondrial Management of Reactive Oxygen Species. Antioxidants 2021, 10, 1824. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Kurihara, Y.; Takeya, M.; Kamada, N.; Kataoka, M.; Jishage, K.; Ueda, O.; Sakaguchi, H.; Higashi, T.; Suzuki, T.; et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 1997, 386, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Kuchibhotla, S.; Vanegas, D.; Kennedy, D.J.; Guy, E.; Nimako, G.; Morton, R.E.; Febbraio, M. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 2008, 78, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef]
- Kim, K.-W.; Ivanov, S.; Williams, J.W. Monocyte Recruitment, Specification, and Function in Atherosclerosis. Cells 2020, 10, 15. [Google Scholar] [CrossRef]
- Sprague, A.H.; Khalil, R.A. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem. Pharmacol. 2009, 78, 539–552. [Google Scholar] [CrossRef]
- Franceschelli, S.; Gatta, D.M.P.; Pesce, M.; Ferrone, A.; Quiles, J.L.; Genovese, S.; Epifano, F.; Fiorito, S.; Taddeo, V.A.; Patruno, A.; et al. Modulation of CAT-2B-Mediated l-Arginine Uptake and Nitric Oxide Biosynthesis in HCT116 Cell Line Through Biological Activity of 4′-Geranyloxyferulic Acid Extract from Quinoa Seeds. Int. J. Mol. Sci. 2019, 20, 3262. [Google Scholar] [CrossRef]
- Franceschelli, S.; Gatta, D.M.P.; Pesce, M.; Ferrone, A.; Patruno, A.; De Lutiis, M.A.; Grilli, A.; Felaco, M.; Croce, F.; Speranza, L. New Approach in Translational Medicine: Effects of Electrolyzed Reduced Water (ERW) on NF-κB/iNOS Pathway in U937 Cell Line under Altered Redox State. Int. J. Mol. Sci. 2016, 17, 1461. [Google Scholar] [CrossRef]
- Pesce, M.; Ferrone, A.; Rizzuto, A.; Tatangelo, R.; Iezzi, I.; Ladu, S.; Franceschelli, S.; Speranza, L.; Patruno, A.; Felaco, M.; et al. The SHP-1 expression is associated with cytokines and psychopathological status in unmedicated first episode Schizophrenia patients. Brain Behav. Immun. 2014, 41, 251–260. [Google Scholar] [CrossRef]
- Sancilio, S.; Nobilio, S.; Ruggiero, A.G.; Di Filippo, E.S.; Stati, G.; Fulle, S.; Bellomo, R.G.; Saggini, R.; Di Pietro, R. Effects of Focused Vibrations on Human Satellite Cells. Int. J. Mol. Sci. 2022, 23, 6026. [Google Scholar] [CrossRef]
- Speranza, L.; Franceschelli, S.; Pesce, M.; Menghini, L.; Patruno, A.; Vinciguerra, I.; A De Lutiis, M.; Felaco, M.; Felaco, P.; Grilli, A. Anti-inflammatory properties of the plant Verbascum mallophorum. J. Biol. Regul. Homeost. Agents 2009, 23, 189–195. [Google Scholar] [PubMed]

| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| IL-1β | TGAGGATGACTTGTTCTTTGAAG | GTGGTGGTCGGAGATTCG |
| TNFα | CCTTCCTGATCGTGGCAG | GCTTGAGGGTTTGCTACAAC |
| VCAM-1 | GTGGACATAAGAAACTGGAAAAGGG | CATTCACGAGGCCACCACTC |
| ICAM-1 | TGATGGGCAGTCAACAGCTA | GGGTAAGGTTCTTGCCCACT |
| SR-A1 | CTCGTGTTTGCAGTTCTCA | CCATGTTGCTCATGTGTTCC |
| CD36 | CAAGCTCCTTGGCATGGTAGA | TGGATTTGCACAATATGAA |
| ABCG1 | GAAGGTTGCCACAGCTTCTC | CATGGTCTTGGCCAGGTAGT |
| LOX-1 | TTACTCTCCATGGTGGTGCC | AGCTTCTTCTGCTTGTTGCC |
| PPARγ | GCAGTGGGGATGTCTCATAATGC | CAGGGGGGTGATGTGTTTGAA |
| LXRα | AAGCCCTGCATGCCTACGT | TGCAGACGCAGTGCAAACA |
| ABCA1 | CCCTGTGGAATGTACCTATGTG | GAGGTGTCCCAAAGATGCAA |
| 18s | CTTTGCCATCACTGCCATTAAG | TCCATCCTTTACATCCTTCTGTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceschelli, S.; De Cecco, F.; Pesce, M.; Ripari, P.; Guagnano, M.T.; Nuevo, A.B.; Grilli, A.; Sancilio, S.; Speranza, L. Hydroxytyrosol Reduces Foam Cell Formation and Endothelial Inflammation Regulating the PPARγ/LXRα/ABCA1 Pathway. Int. J. Mol. Sci. 2023, 24, 2057. https://doi.org/10.3390/ijms24032057
Franceschelli S, De Cecco F, Pesce M, Ripari P, Guagnano MT, Nuevo AB, Grilli A, Sancilio S, Speranza L. Hydroxytyrosol Reduces Foam Cell Formation and Endothelial Inflammation Regulating the PPARγ/LXRα/ABCA1 Pathway. International Journal of Molecular Sciences. 2023; 24(3):2057. https://doi.org/10.3390/ijms24032057
Chicago/Turabian StyleFranceschelli, Sara, Federica De Cecco, Mirko Pesce, Patrizio Ripari, Maria Teresa Guagnano, Arturo Bravo Nuevo, Alfredo Grilli, Silvia Sancilio, and Lorenza Speranza. 2023. "Hydroxytyrosol Reduces Foam Cell Formation and Endothelial Inflammation Regulating the PPARγ/LXRα/ABCA1 Pathway" International Journal of Molecular Sciences 24, no. 3: 2057. https://doi.org/10.3390/ijms24032057
APA StyleFranceschelli, S., De Cecco, F., Pesce, M., Ripari, P., Guagnano, M. T., Nuevo, A. B., Grilli, A., Sancilio, S., & Speranza, L. (2023). Hydroxytyrosol Reduces Foam Cell Formation and Endothelial Inflammation Regulating the PPARγ/LXRα/ABCA1 Pathway. International Journal of Molecular Sciences, 24(3), 2057. https://doi.org/10.3390/ijms24032057