Influence of Light of Different Spectral Compositions on Growth Parameters, Photosynthetic Pigment Contents and Gene Expression in Scots Pine Plantlets
Abstract
:1. Introduction
2. Results
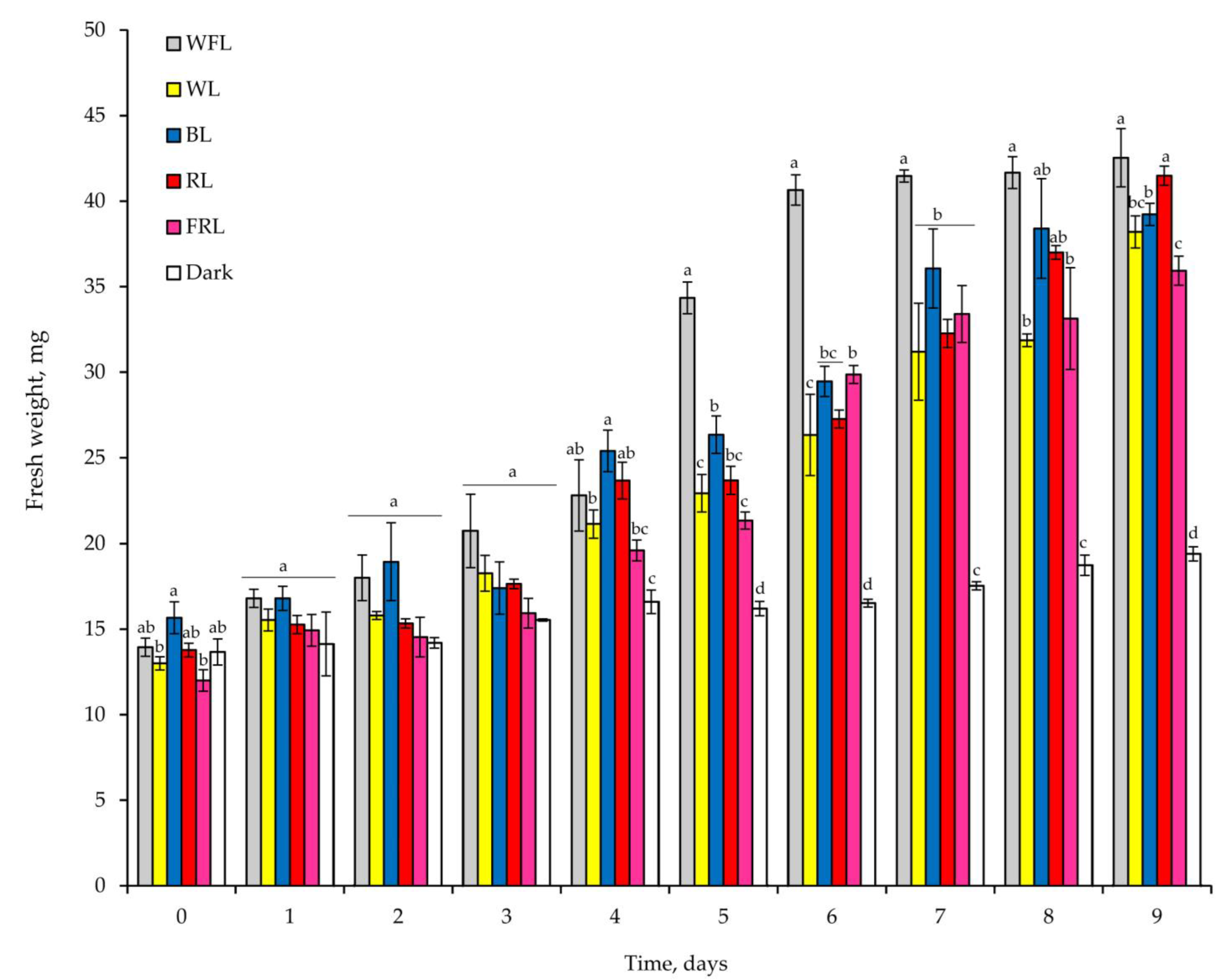
2.1. Seed Germination and Development of Plantlets
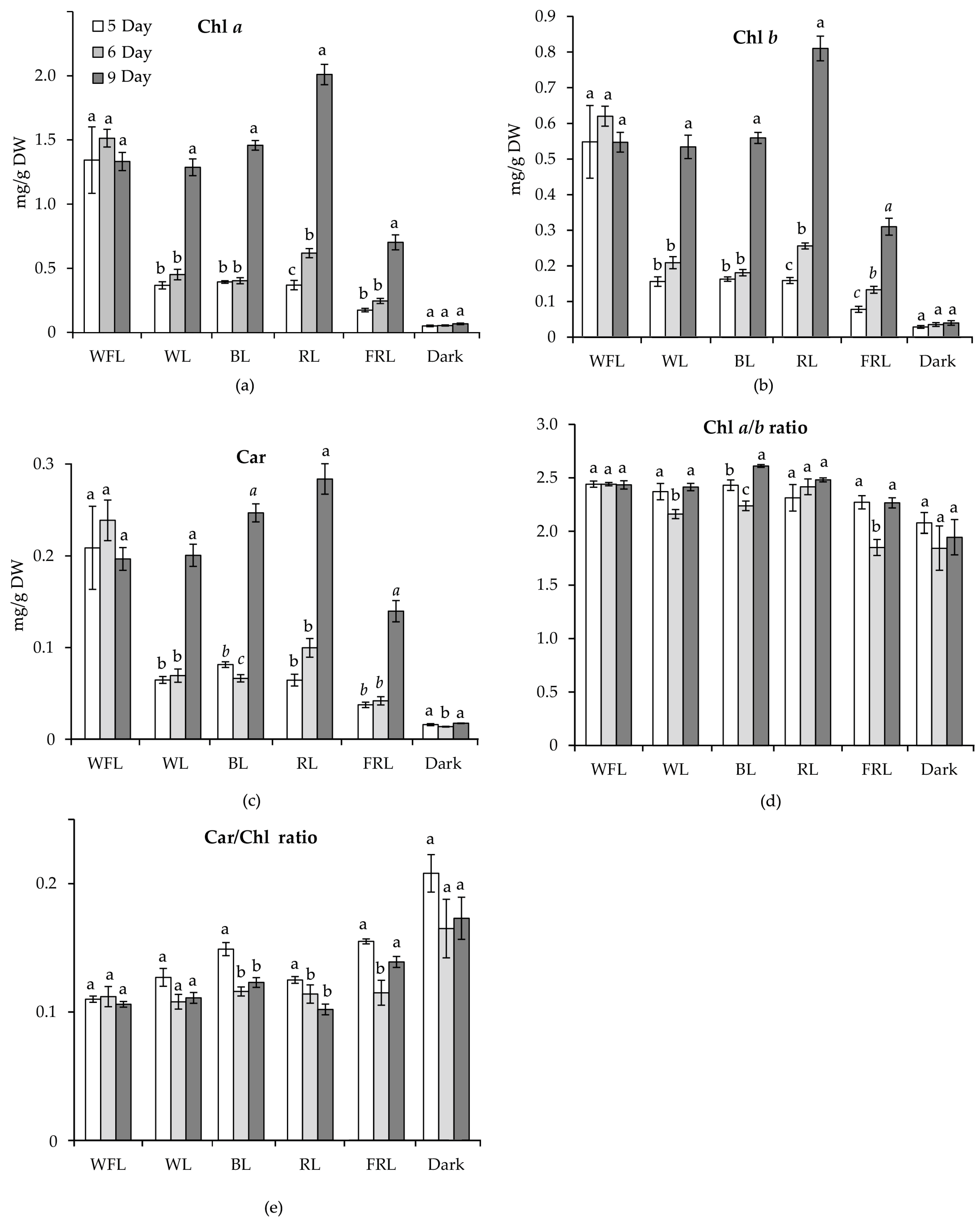
2.2. Content of Photosynthetic Pigments
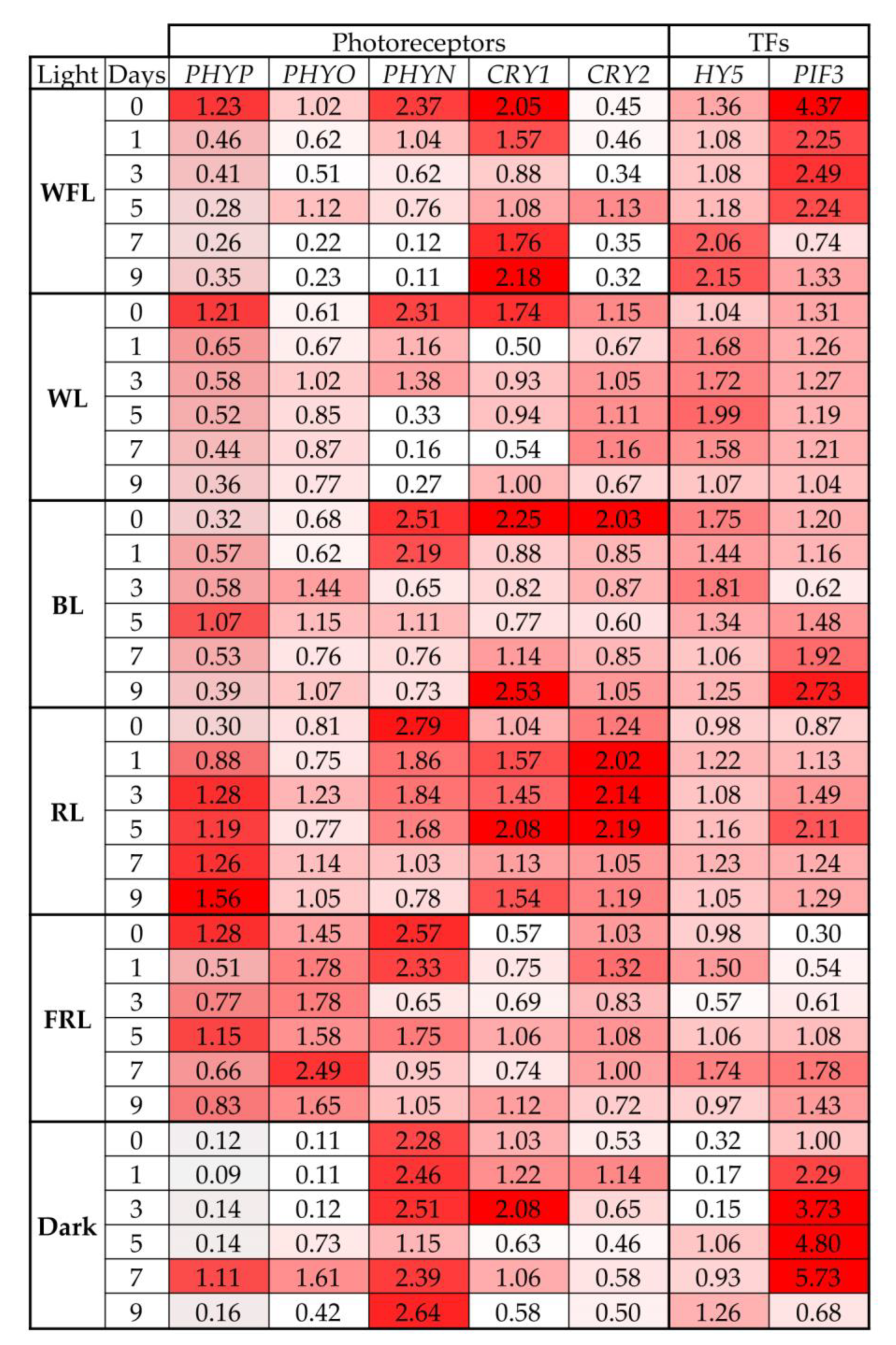
2.3. Transcript Levels of Photoreceptor Genes and TFs
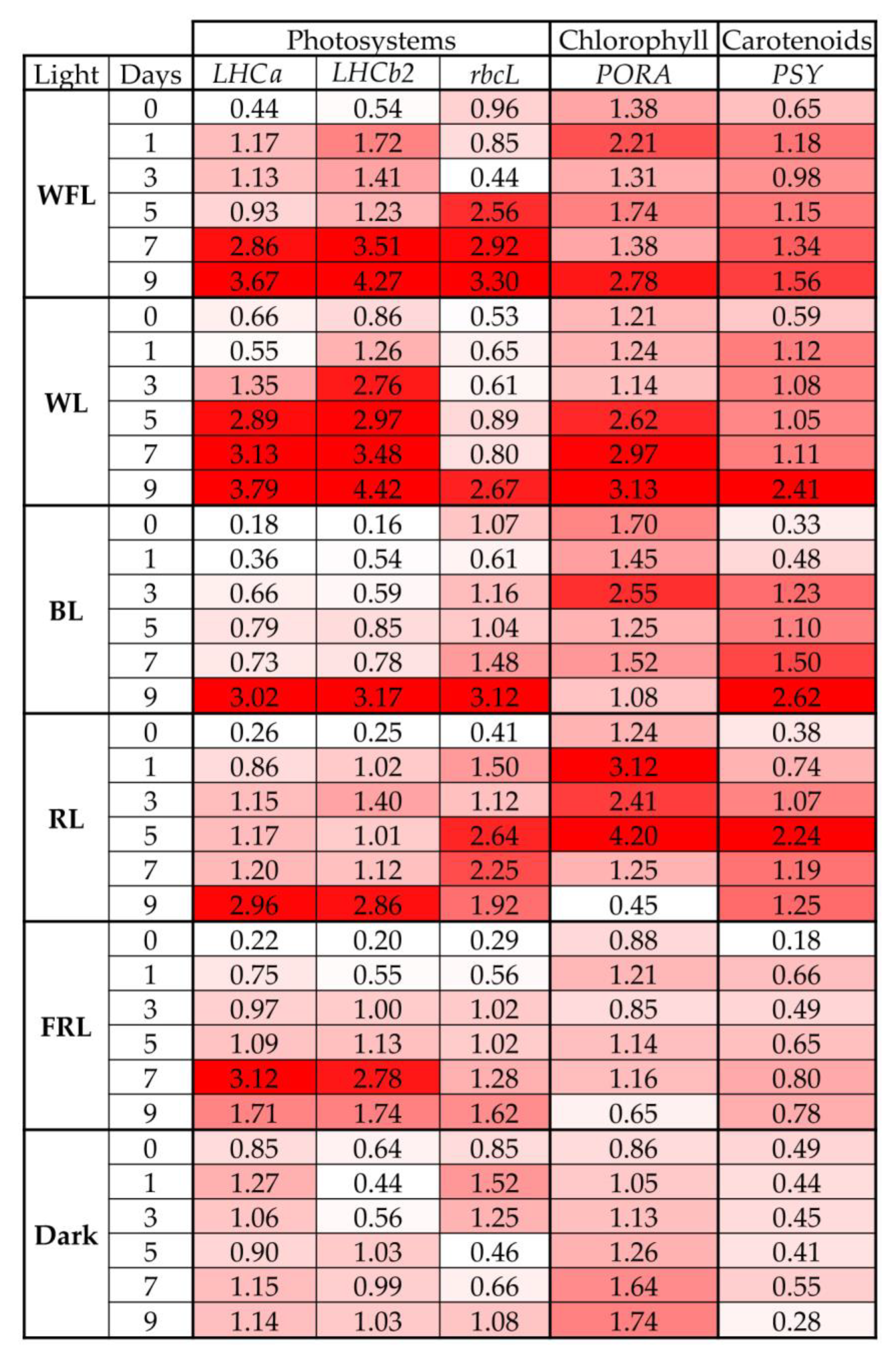
2.4. Transcript Levels of Genes for Photosystems and Pigment Biosynthesis
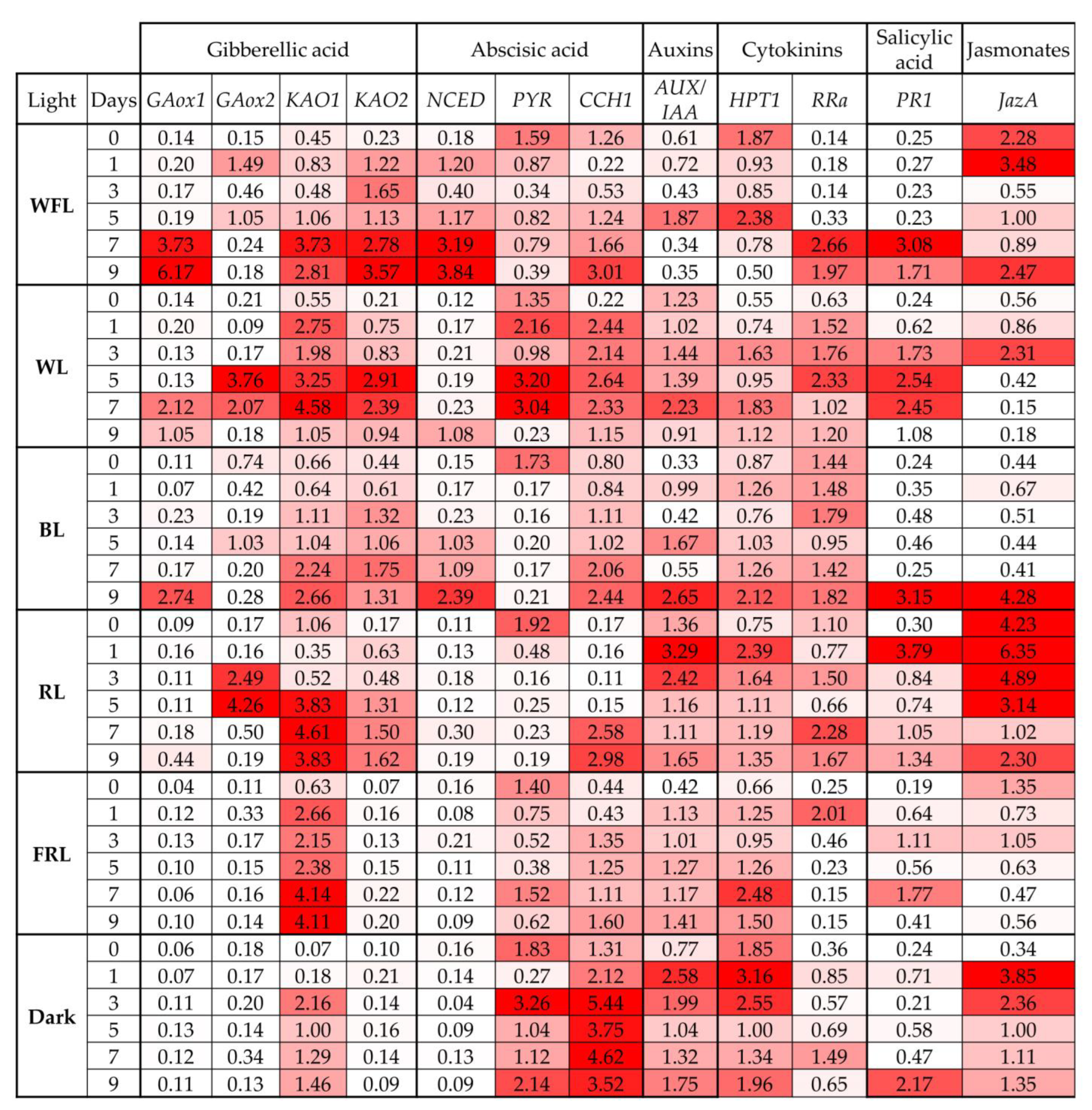
2.5. Expression of Phytohormone Biosynthesis and Signaling Genes
3. Discussion
4. Materials and Methods
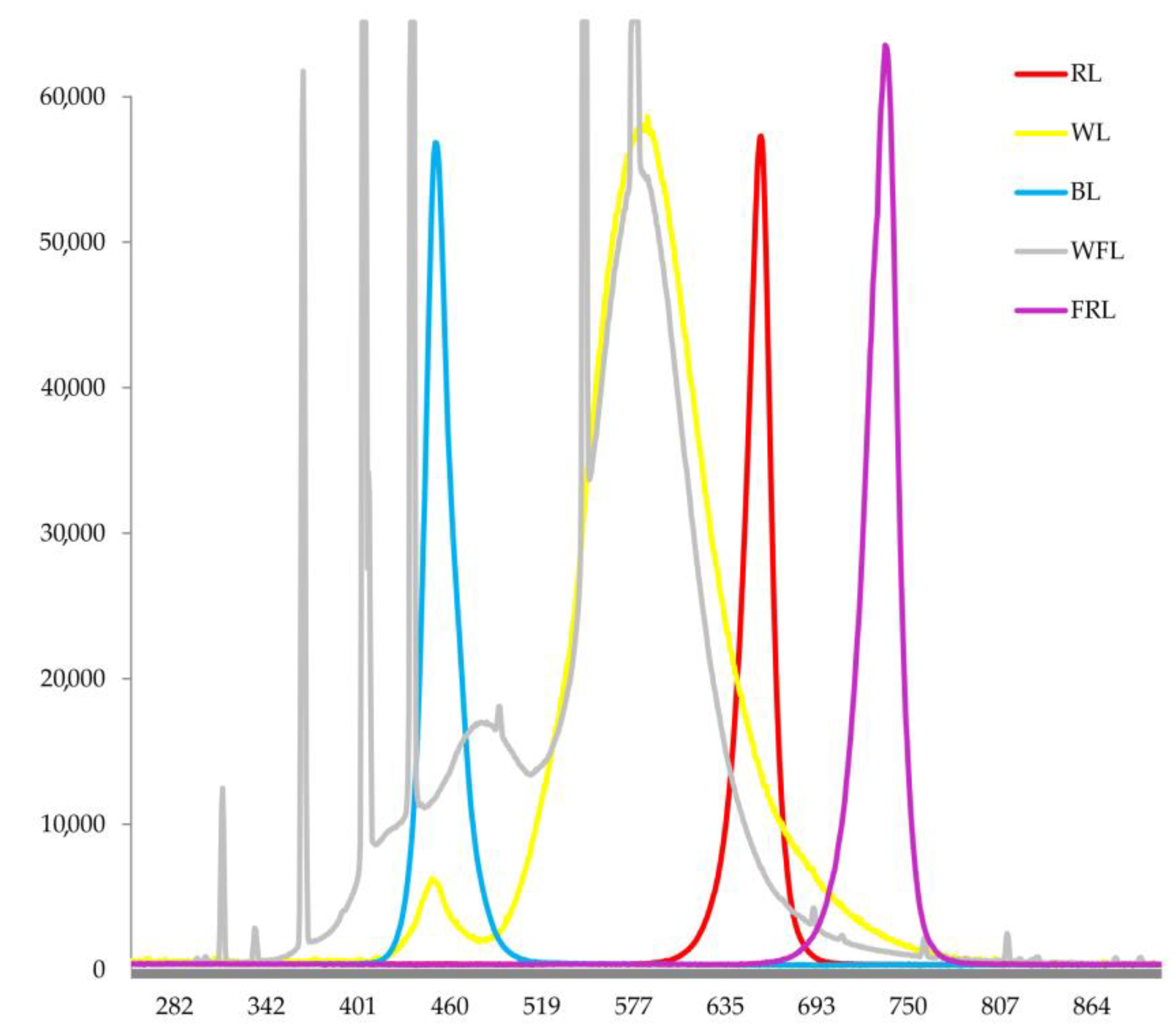
4.1. Experimental Design
4.2. Fresh Weight and Water Content
4.3. Morphometric Parameters
4.4. Determination of Photosynthetic Pigments
4.5. RNA Extraction and Quantitative RT–PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, H.; Wang, X.; Mo, X.; Tang, C.; Zhong, S.; Deng, X.W. Arabidopsis DET1 Degrades HFR1 but Stabilizes PIF1 to Precisely Regulate Seed Germination. Proc. Natl. Acad. Sci. USA 2015, 112, 3817–3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, J.; Clavijo Michelangeli, J.A.; Gezan, S.A.; Lee, H.; Vallejos, C.E. Maternal Effects on Seed and Seedling Phenotypes in Reciprocal F1 Hybrids of the Common Bean (Phaseolus Vulgaris L.). Front. Plant Sci. 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreslavski, V.D.; Los, D.A.; Schmitt, F.-J.; Zharmukhamedov, S.K.; Kuznetsov, V.V.; Allakhverdiev, S.I. The Impact of the Phytochromes on Photosynthetic Processes. Biochim. Biophys. Acta (BBA)-Bioenerg. 2018, 1859, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Lantzouni, O.; Bruggink, T.; Benjamins, R.; Lanfermeijer, F.; Denby, K.; Schwechheimer, C.; Bassel, G.W. A Molecular Signal Integration Network Underpinning Arabidopsis Seed Germination. Curr. Biol. 2020, 30, 3703–3712.e4. [Google Scholar] [CrossRef]
- Chaves, I.; Pokorny, R.; Byrdin, M.; Hoang, N.; Ritz, T.; Brettel, K.; Essen, L.-O.; van der Horst, G.; Batschauer, A.; Ahmad, M. The Cryptochromes: Blue Light Photoreceptors in Plants and Animals. Annu. Rev. Plant Biol. 2011, 62, 335–364. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, N.C.; Su, Y.-S.; Lagarias, J.C. Phytochome Structure and Signaling Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 837–858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, E.; Kang, H.; Yamaguchi, S.; Park, J.; Lee, D.; Kamiya, Y.; Choi, G. Genome-Wide Analysis of Genes Targeted by PHYTOCHROME INTERACTING FACTOR 3-LIKE5 during Seed Germination in Arabidopsis. Plant Cell 2009, 21, 403–419. [Google Scholar] [CrossRef] [Green Version]
- Bae, G.; Choi, G. Decoding of Light Signals by Plant Phytochromes and Their Interacting Proteins. Annu. Rev. Plant Biol. 2008, 59, 281–311. [Google Scholar] [CrossRef] [Green Version]
- Pashkovskiy, P.; Kreslavski, V.; Khudyakova, A.; Kosobryukhov, A.; Kuznetsov, V.V.; Allakhverdiev, S. Influence of Phytochromes on MicroRNA Expression, Phenotype, and Photosynthetic Activity in A. Thaliana Phy Mutants under Light with Different Spectral Composition. Photosynthetica 2022, 61, 1–10. [Google Scholar] [CrossRef]
- Cheng, M.-C.; Kathare, P.K.; Paik, I.; Huq, E. Phytochrome Signaling Networks. Annu. Rev. Plant Biol. 2021, 72, 217–244. [Google Scholar] [CrossRef]
- Hornitschek, P.; Kohnen, M.V.; Lorrain, S.; Rougemont, J.; Ljung, K.; López-Vidriero, I.; Franco-Zorrilla, J.M.; Solano, R.; Trevisan, M.; Pradervand, S. Phytochrome Interacting Factors 4 and 5 Control Seedling Growth in Changing Light Conditions by Directly Controlling Auxin Signaling. Plant J. 2012, 71, 699–711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, M.; Nambara, E.; Choi, G.; Yamaguchi, S. Interaction of Light and Hormone Signals in Germinating Seeds. Plant Mol. Biol. 2008, 69, 463. [Google Scholar] [CrossRef] [PubMed]
- Gavassi, M.A.; Fernandes, G.C.; Monteiro, C.C.; Peres, L.E.P.; Carvalho, R.F. Seed Germination in Tomato: A Focus on Interaction between Phytochromes and Gibberellins or Abscisic Acid. Am. J. Plant Sci. 2014, 2014, 2163–2169. [Google Scholar] [CrossRef] [Green Version]
- Zdarska, M.; Dobisová, T.; Gelová, Z.; Pernisová, M.; Dabravolski, S.; Hejátko, J. Illuminating Light, Cytokinin, and Ethylene Signalling Crosstalk in Plant Development. J. Exp. Bot. 2015, 66, 4913–4931. [Google Scholar] [CrossRef] [Green Version]
- Itoh, H.; Ueguchi-Tanaka, M.; Matsuoka, M. Chapter 6 Molecular Biology of Gibberellins Signaling in Higher Plants. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2008; Volume 268, pp. 191–221. [Google Scholar]
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.; Koshiba, T.; et al. Regulation of Hormone Metabolism in Arabidopsis Seeds: Phytochrome Regulation of Abscisic Acid Metabolism and Abscisic Acid Regulation of Gibberellin Metabolism. Plant J. 2006, 48, 354–366. [Google Scholar] [CrossRef]
- Oh, E.; Yamaguchi, S.; Hu, J.; Yusuke, J.; Jung, B.; Paik, I.; Lee, H.-S.; Sun, T.; Kamiya, Y.; Choi, G. PIL5, a Phytochrome-Interacting BHLH Protein, Regulates Gibberellin Responsiveness by Binding Directly to the GAI and RGA Promoters in Arabidopsis Seeds. Plant Cell 2007, 19, 1192–1208. [Google Scholar] [CrossRef] [Green Version]
- Nakabayashi, K.; Okamoto, M.; Koshiba, T.; Kamiya, Y.; Nambara, E. Genome-Wide Profiling of Stored MRNA in Arabidopsis Thaliana Seed Germination: Epigenetic and Genetic Regulation of Transcription in Seed. Plant J. 2005, 41, 697–709. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Huq, E.; Rodríguez-Concepción, M. Direct Regulation of Phytoene Synthase Gene Expression and Carotenoid Biosynthesis by Phytochrome-Interacting Factors. Proc. Natl. Acad. Sci. USA 2010, 107, 11626–11631. [Google Scholar] [CrossRef] [Green Version]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed Dormancy and the Control of Germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Kucera, B.; Cohn, M.A.; Leubner-Metzger, G. Plant Hormone Interactions during Seed Dormancy Release and Germination. Seed Sci. Res. 2005, 15, 281–307. [Google Scholar] [CrossRef]
- Voitsekhovskaja, O.V. Phytochromes and Other (Photo)Receptors of Information in Plants. Russ. J. Plant Physiol. 2019, 66, 351–364. [Google Scholar] [CrossRef]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain Dormancy and Light Quality Effects on Germination in the Model Grass Brachypodium Distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Barrero, J.M.; Hughes, T.; Julkowska, M.; Taylor, J.M.; Xu, Q.; Gubler, F. Roles for Blue Light, Jasmonate and Nitric Oxide in the Regulation of Dormancy and Germination in Wheat Grain (Triticum Aestivum L.). Planta 2013, 238, 121–138. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xiang, Y.; Zhu, H.; Xu, H.; Zhang, Z.; Zhang, C.; Zhang, L.; Ma, Z. Wheat Cryptochromes: Subcellular Localization and Involvement in Photomorphogenesis and Osmotic Stress Responses. Plant Physiol. 2009, 149, 760–774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrero, J.M.; Downie, A.B.; Xu, Q.; Gubler, F. A Role for Barley CRYPTOCHROME1 in Light Regulation of Grain Dormancy and Germination. Plant Cell 2014, 26, 1094–1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoang, H.H.; Sechet, J.; Bailly, C.; Leymarie, J.; Corbineau, F. Inhibition of Germination of Dormant Barley (Hordeum Vulgare L.) Grains by Blue Light as Related to Oxygen and Hormonal Regulation. Plant Cell Environ. 2014, 37, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Qamaruddin, M.; Tillberg, E. Rapid Effects of Red Light on the Isopentenyladenosine Content in Scots Pine Seeds 1. Plant Physiol. 1989, 91, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Tillberg, E. Effect of Light on Abscisic Acid Content in Photosensitive Scots Pine (Pinus Sylvestris L.) Seed. Plant Growth Regul. 1992, 11, 147–152. [Google Scholar] [CrossRef]
- Razzak, A.; Ranade, S.S.; Strand, Å.; García-Gil, M.R. Differential Response of Scots Pine Seedlings to Variable Intensity and Ratio of Red and Far-Red Light. Plant Cell Environ. 2017, 40, 1332–1340. [Google Scholar] [CrossRef]
- Skordilis, A.; Thanos, C.A. Seed Stratification and Germination Strategy in the Mediterranean Pines Pinus Brutia and P. Halepensis. Seed Sci. Res. 1995, 5, 151–160. [Google Scholar] [CrossRef]
- Sano, N.; Marion-Poll, A. ABA Metabolism and Homeostasis in Seed Dormancy and Germination. Int. J. Mol. Sci. 2021, 22, 5069. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Delhomme, N.; García-Gil, M.R. Transcriptome Analysis of Shade Avoidance and Shade Tolerance in Conifers. Planta 2019, 250, 299–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernbach, E.; Mohr, H. Coaction of Blue/Ultraviolet-A Light and Light Absorbed by Phytochrome in Controlling Growth of Pine (Pinus Sylestris L.) Seedlings. Planta 1990, 180, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Humbert, L.; Gagnon, D.; Kneeshaw, D.; Messier, C. A Shade Tolerance Index for Common Understory Species of Northeastern North America. Ecol. Indic. 2007, 7, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Giertych, M.J.; Karolewski, P.; Oleksyn, J. Carbon Allocation in Seedlings of Deciduous Tree Species Depends on Their Shade Tolerance. Acta Physiol. Plant 2015, 37, 216. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Li, L. Hormonal Regulation in Shade Avoidance. Front. Plant Sci. 2017, 8, 1527. [Google Scholar] [CrossRef] [Green Version]
- Lorrain, S.; Allen, T.; Duek, P.D.; Whitelam, G.C.; Fankhauser, C. Phytochrome-Mediated Inhibition of Shade Avoidance Involves Degradation of Growth-Promoting BHLH Transcription Factors. Plant J. 2008, 53, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Carlos, L. Ballaré,Ronald Pierik The Shade-Avoidance Syndrome: Multiple Signals and Ecological Consequences. Plant Cell Environ. 2017, 40, 2530–2543. [Google Scholar] [CrossRef]
- Li, J.; Li, G.; Wang, H.; Wang Deng, X. Phytochrome Signaling Mechanisms. Arab. Book 2011, 9, e0148. [Google Scholar] [CrossRef] [Green Version]
- Franklin, K.A.; Whitelam, G.C. Red: Far-Red Ratio Perception and Shade Avoidance. Light Plant Dev. 2007, 30, 211–234. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Kreslavski, V.D.; Ivanov, Y.; Ivanova, A.; Kartashov, A.; Shmarev, A.; Strokina, V.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of Light of Different Spectral Compositions on the Growth, Photosynthesis, and Expression of Light-Dependent Genes of Scots Pine Seedlings. Cells 2021, 10, 3284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Peng, T.; Chen, C.-Y.; Ji, R.; Gu, D.; Li, T.; Zhang, D.; Tu, Y.-T.; Wu, K.; Liu, X. HY5 Interacts with the Histone Deacetylase HDA15 to Repress Hypocotyl Cell Elongation in Photomorphogenesis. Plant Physiol. 2019, 180, 1450–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, S.; Zhang, M.; Ye, J.; Du, J.; Jiang, Y.; Hu, Y. Auxin Contributes to Jasmonate-Mediated Regulation of Abscisic Acid Signaling during Seed Germination in Arabidopsis. Plant Cell 2022, koac362, online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.V.; Savochkin, Y.V.; Kuznetsov, V.V. Scots Pine as a Model Plant for Studying the Mechanisms of Conifers Adaptation to Heavy Metal Action: 1. Effects of Continuous Zinc Presence on Morphometric and Physiological Characteristics of Developing Pine Seedlings. Russ. J. Plant Physiol. 2011, 58, 871–878. [Google Scholar] [CrossRef]
- Ivanov, Y.V.; Kartashov, A.V.; Ivanova, A.I.; Ivanov, V.P.; Marchenko, S.I.; Nartov, D.I.; Kuznetsov, V.V. Long-Term Impact of Cement Plant Emissions on the Elemental Composition of Both Soils and Pine Stands and on the Formation of Scots Pine Seeds. Environ. Pollut. 2018, 243, 1383–1393. [Google Scholar] [CrossRef]
- Ivanov, Y.V.; Ivanova, A.I.; Kartashov, A.V.; Kuznetsov, V.V. Phytotoxicity of Short-Term Exposure to Excess Zinc or Copper in Scots Pine Seedlings in Relation to Growth, Water Status, Nutrient Balance, and Antioxidative Activity. Environ. Sci. Pollut. Res. 2021, 28, 14828–14843. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Plant Cell Membranes; Academic Press: Cambridge, MA, USA, 1987; Volume 148, pp. 350–382. [Google Scholar] [CrossRef]
- Kolosova, N.; Miller, B.; Ralph, S.; Ellis, B.E.; Douglas, C.; Ritland, K.; Bohlmann, J. Isolation of High-Quality RNA from Gymnosperm and Angiosperm Trees. BioTechniques 2004, 36, 821–824. [Google Scholar] [CrossRef]
- Pashkovskiy, P.P.; Vankova, R.; Zlobin, I.E.; Dobrev, P.; Ivanov, Y.V.; Kartashov, A.V.; Kuznetsov, V.V. Comparative Analysis of Abscisic Acid Levels and Expression of Abscisic Acid-Related Genes in Scots Pine and Norway Spruce Seedlings under Water Deficit. Plant Physiol. Biochem. 2019, 140, 105–112. [Google Scholar] [CrossRef]

| Light | Seed Vigor, % | Seed Germination, % |
|---|---|---|
| WFL | 73.2 ± 3.3 a | 80.6 ± 4.9 a |
| WL | 65.6 ± 4.7 ab | 78.4 ± 5.8 a |
| BL | 55.7 ± 2.8 b | 80.2 ± 4.7 a |
| RL | 58.9 ± 5.0 ab | 80.8 ± 3.3 a |
| FRL | 48.1 ± 7.4 b | 81.6 ± 6.6 a |
| Dark | 11.4 ± 4.3 c | 56.6 ± 3.9 b |
| Light | Plantlet Length cm | Root Length cm | Hypocotyl Length cm | Cotyledons Length, cm | Plantlet Dry Weight (without Seed Coat), mg | Water Content in Plantlet, % |
|---|---|---|---|---|---|---|
| WFL | 5.62 ± 0.11 c | 1.37 ± 0.06 c | 2.98 ± 0.09 c | 1.24 ± 0.04 c | 3.78 ± 0.08 ab | 86.40 ± 0.21 b |
| WL | 6.58 ± 0.10 b | 1.93 ± 0.08 b | 3.47 ± 0.09 b | 1.14 ± 0.03 c | 3.86 ± 0.10 ab | 87.10 ± 0.24 b |
| BL | 7.27 ± 0.12 a | 1.84 ± 0.08 b | 4.03 ± 0.07 a | 1.37 ± 0.04 b | 4.43 ± 0.19 a | 86.33 ± 0.10 b |
| RL | 7.42 ± 0.19 a | 2.29 ± 0.14 a | 3.49 ± 0.11 b | 1.59± 0.03 a | 4.64 ± 0.16 a | 86.60 ± 0.23 b |
| FRL | 6.60 ± 0.19 b | 1.75 ± 0.08 b | 3.79± 0.13 a | 1.02± 0.04 d | 3.19 ± 0.09 b | 88.68 ± 0.13 a |
| Dark | 1.89 ± 0.09 d | 1.42 ± 0.09 c | ND | ND | 3.57 ± 0.37 ab | 65.73 ± 2.08 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashkovskiy, P.; Ivanov, Y.; Ivanova, A.; Kreslavski, V.D.; Vereshchagin, M.; Tatarkina, P.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of Light of Different Spectral Compositions on Growth Parameters, Photosynthetic Pigment Contents and Gene Expression in Scots Pine Plantlets. Int. J. Mol. Sci. 2023, 24, 2063. https://doi.org/10.3390/ijms24032063
Pashkovskiy P, Ivanov Y, Ivanova A, Kreslavski VD, Vereshchagin M, Tatarkina P, Kuznetsov VV, Allakhverdiev SI. Influence of Light of Different Spectral Compositions on Growth Parameters, Photosynthetic Pigment Contents and Gene Expression in Scots Pine Plantlets. International Journal of Molecular Sciences. 2023; 24(3):2063. https://doi.org/10.3390/ijms24032063
Chicago/Turabian StylePashkovskiy, Pavel, Yury Ivanov, Alexandra Ivanova, Vladimir D. Kreslavski, Mikhail Vereshchagin, Polina Tatarkina, Vladimir V. Kuznetsov, and Suleyman I. Allakhverdiev. 2023. "Influence of Light of Different Spectral Compositions on Growth Parameters, Photosynthetic Pigment Contents and Gene Expression in Scots Pine Plantlets" International Journal of Molecular Sciences 24, no. 3: 2063. https://doi.org/10.3390/ijms24032063







