Hybrid Molecules Consisting of Lysine Dendrons with Several Hydrophobic Tails: A SCF Study of Self-Assembling
Abstract
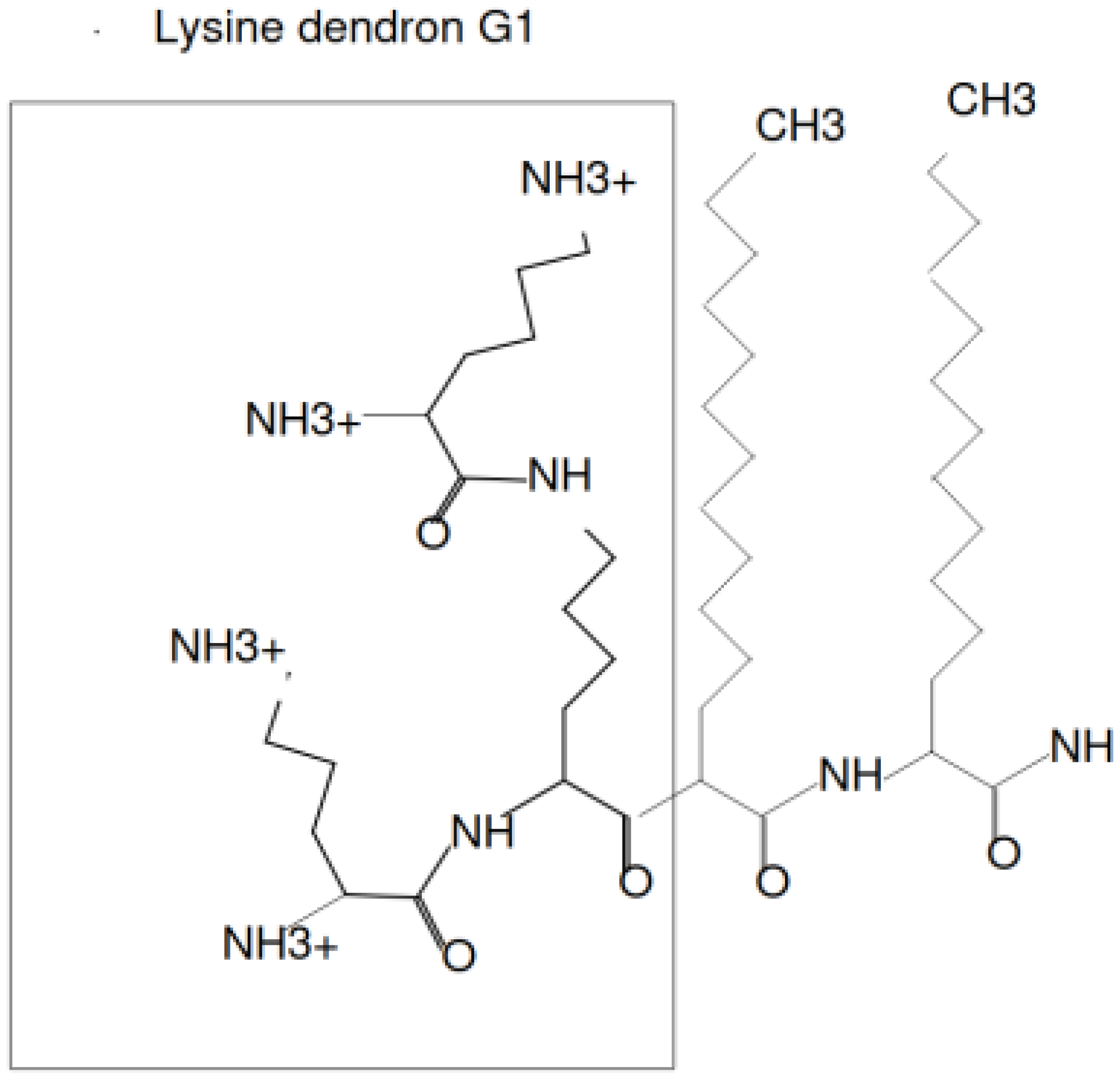
1. Introduction
2. Results and Discussion
2.1. Thermodynamic Characteristics
2.2. Structure
2.3. Electrostatic Properties
2.4. The Stratifications in Dendrons
3. Materials and Method
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nace, V. Nonionic Surfactants: Polyoxyalkylene Block Copolymers; CRC Press: Boca Raton, FL, USA, 1996; p. 284. [Google Scholar]
- Alexandridis, P.; Lindman, B. Amphiphilic Block Copolymers. Self-Assembly and Applications; Elsevier: Amsterdam, The Netherlands, 2000; p. 436. [Google Scholar]
- Hamley, I.W. Developments in Block Copolymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 367. [Google Scholar]
- Frechet, M.; Tomalia, D. Dendrimers and Other Dendritic Polymer; Wiley: Hoboken, NJ, USA, 2001; p. 647. [Google Scholar]
- Cheng, Y.; Tomalia, D. Dendrimer-Based Drug Delivery Systems: From Theory to Practice; Wiley: Hoboken, NJ, USA, 2012; p. 542. [Google Scholar]
- Klajnert, B.; Peng, L.; Cena, V. Dendrimers in Biomedical Applications; RSC Publishing: Cambridge, UK, 2013; p. 204. [Google Scholar]
- Narain, R. Polymers and Nanomaterials for Gene Therapy; Woodhead Publishing: Cambridge, UK, 2016; p. 279. [Google Scholar]
- Denkewalter, R.G.; Kolc, J.; Lukasavage, W.J. Macromolecular Highly Branched Homogeneous Compound Based on Lysine Units. U.S. Patent 4289872, 15 September 1981. [Google Scholar]
- Mirsharghi, S.; Knudsen, K.D.; Bagherifam, S.; Nystrom, B.; Boas, U. Preparation and self-assembly of amphiphilic polylysine dendrons. New J. Chem. 2016, 40, 3597–3611. [Google Scholar] [CrossRef]
- Bayele, H.K.; Ramaswamy, C.; Wilderspin, A.F.; Srai, K.S.; Toth, I.; Florence, A.T. Protein transduction by lipidic peptide dendrimers. J. Pharm. Sci. 2006, 95, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Rosati, M.; Acocella, A.; Pizzi, A.; Turtù, G.; Neri, G.; Demitri, N.; Nonappa; Raffaini, G.; Donnio, B.; Zerbetto, F.; et al. Azobenzene-containing linear-dendritic block copolymers prepared by sequential ATRP and click chemistry. Macromolecules 2022, 55, 2486–2496. [Google Scholar] [CrossRef]
- Liu, X.; Monzavi, T.; Gitsov, I. Controlled ATRP synthesis of novel linear- dendritic block copolymers and their directed selfassembly in breath figure arrays. Polymers 2019, 11, 539. [Google Scholar] [CrossRef]
- Kosakowska, K.; Casey, B.; Kurtz, S.; Lawson, L.; Grayson, S. Evaluation of Amphiphilic Star/Linear-Dendritic Polymer Reverse Micelles for Transdermal Drug Delivery: Directing Carrier Properties by Tailoring Core versus Peripheral Branching. Biomacromolecules 2018, 19, 3163–3176. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Peng, S.; Dong, C. Synthesis and characterization of linear-dendron-like poly(ϵ-caprolactone)-b-poly(ethyleneoxide) copolymers via the combination of ring-opening polymerization and click chemistry. Macromolecules 2008, 41, 6686–6695. [Google Scholar] [CrossRef]
- Del Barrio, J.; Oriol, L.; Alcalá, R.; Sánchez, C. Azobenzene-Containing linear-Dendritic diblock copolymers by click chemistry: Synthesis, characterization, morphological study, and photoinduction of optical anisotropy. Macromolecules 2009, 42, 5752–5760. [Google Scholar] [CrossRef]
- Blasco, E.; Del Barrio, J.; Piñol, M.; Oriol, L.; Berges, C.; Sánchez, C.; Alcalá, R. Azobenzene-containing linear-dendritic block copolymers prepared by sequential ATRP and click chemistry. Polymer 2012, 53, 4604–4613. [Google Scholar] [CrossRef]
- Qian, Y.; You, D.; Lin, F.; Wei, J.; Wang, Y.; Bi, Y. Enzyme triggered disassembly of amphiphilic linear-dendritic block copolymer micelles based on poly[N-(2-hydroxyethyl-L-glutamine)]. Polym. Chem. 2019, 10, 94–105. [Google Scholar] [CrossRef]
- Liu, X.; Gitsov, I. Nonionic amphiphilic linear dendritic block copolymers. solvent-induced self-assembly and morphology tuning. Macromolecules 2019, 52, 5563–5573. [Google Scholar] [CrossRef]
- Wei, J.; Lin, F.; You, D.; Qian, Y.; Wang, Y.; Bi, Y. Self-assembly and enzyme responsiveness of amphiphilic linear-dendritic block copolymers based on poly(N-vinylpyrrolidone) and dendritic phenylalanyl-lysine dipeptides. Polymers 2019, 11, 625. [Google Scholar] [CrossRef] [PubMed]
- Blasco, E.; Piñol, M.; Oriol, L. Responsive Linear-Dendritic Block Copolymers. Macromol. Rapid Commun. 2014, 35, 1090–1115. [Google Scholar] [CrossRef] [PubMed]
- Van Hest, J.; Delnoye, D.; Baars, M.; Van Genderen, M.; Meijer, E. Polystyrene-dendrimer amphiphilic block copolymers with a generation-dependent aggregation. Science 1995, 268, 1592–1595. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chang, H.; Sheng, Y.; Tsao, H. Photoresponsive polymersomes formed by amphiphilic linear-dendritic block copolymers: Generation-dependent aggregation behavior. Macromolecules 2012, 45, 7143–7156. [Google Scholar] [CrossRef]
- Blasco, E.; Del Barrio, J.; Sánchez-Somolinos, C.; Piñol, M.; Oriol, L. Light induced molecular release from vesicles based on amphiphilic linear-dendritic block copolymers. Polym. Chem. 2013, 4, 2246–2254. [Google Scholar] [CrossRef]
- Whitton, G.; Gillies, E. Functional aqueous assemblies of linear-dendron hybrids. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 148–172. [Google Scholar] [CrossRef]
- Mongkhontreerat, S.; Walter, M.; Cai, Y.; Brismar, H.; Hult, A.; Malkoch, M. Functional porous membranes from amorphous linear dendritic polyester hybrids. Polym. Chem. 2015, 6, 2390–2395. [Google Scholar] [CrossRef]
- Mongkhontreerat, S.; Walter, M.; Andrén, O.; Cai, Y.; Malkoch, M. Beyond State of the Art Honeycomb Membranes: High Performance Ordered Arrays from Multiprogrammable Linear-Dendritic Block Copolymers. Adv. Funct. Mater. 2015, 25, 4837–4843. [Google Scholar] [CrossRef]
- Del Barrio, J.; Oriol, L.; Sánchez, C.; Serrano, J.; Di Cicco, A.; Keller, P.; Li, M. Self-assembly of linear-dendritic diblock copolymers: From nanofibers to polymersomes. J. Am. Chem. Soc. 2010, 132, 3762–3769. [Google Scholar] [CrossRef]
- Dong, C.; Liu, G. Linear-dendritic biodegradable block copolymers: From synthesis to application in bionanotechnology. Polym. Chem. 2013, 4, 46–52. [Google Scholar] [CrossRef]
- Kalva, N.; Parekh, N.; Ambade, A. Controlled micellar disassembly of photo- and pH-cleavable linear-dendritic block copolymers. Polym. Chem. 2015, 6, 6826–6835. [Google Scholar] [CrossRef]
- Zhou, K.; Johnson, L.; Xiong, H.; Barrios, S.; Minnig, J.; Yan, Y.; Abram, B.; Yu, X.; Siegwart, D. Hydrophobic Domain Structure of Linear-Dendritic Poly(ethylene glycol) Lipids Affects RNA Delivery of Lipid Nanoparticles. Mol. Pharm. 2020, 17, 1575–1585. [Google Scholar] [CrossRef]
- Fedeli, E.; Lancelot, A.; Dominguez, J.; Serrano, J.; Calvo, P.; Sierra, T. Self-assembling hybrid linear-dendritic blockcopolymers:The design of nano-carriers for lipophilic antitumoral drugs. Nanomaterials 2019, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Kosakowska, K.; Casey, B.; Albert, J.; Wang, Y.; Ashbaugh, H.; Grayson, S. Synthesis and Self-Assembly of Amphiphilic Star/Linear-Dendritic Polymers: Effect of Core versus Peripheral Branching on Reverse Micelle Aggregation. Biomacromolecules 2018, 19, 3177–3189. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhao, Y.; Xu, W.; Li, L. Linear-dendritic block copolymer for drug and gene delivery. Mater. Sci. Eng. C 2016, 62, 943–959. [Google Scholar] [CrossRef]
- Andrén, O.; Zhang, Y.; Lundberg, P.; Hawker, C.; Nyström, A.; Malkoch, M. Therapeutic Nanocarriers via Cholesterol Directed Self-Assembly of Well-Defined Linear-Dendritic Polymeric Amphiphiles. Chem. Mater. 2017, 29, 3891–3898. [Google Scholar] [CrossRef]
- Najafi, F.; Salami-Kalajahi, M.; Roghani-Mamaqani, H. Janus-type dendrimers: Synthesis, properties, and applications. J. Mol. Liq. 2021, 347, 118396. [Google Scholar] [CrossRef]
- Lancelot, A.; Claveria-Gimeno, R.; Velazquez-Campoy, A.; Abian, O.; Serrano, J.; Sierra, T. Nanostructures based on ammonium-terminated amphiphilic Janus dendrimers as camptothecin carriers with antiviral activity. Eur. Polym. J. 2017, 90, 136–149. [Google Scholar] [CrossRef]
- Xiao, Q.; Rivera-Martinez, N.; Raab, C.; Bermudez, J.; Good, M.; Klein, M.; Percec, V. Co-assembly of liposomes, Dendrimersomes, and Polymersomes with amphiphilic Janus dendrimers conjugated to Mono- and Tris-Nitrilotriacetic Acid (NTA, TrisNTA) enhances protein recruitment. Giant 2021, 9, 100089. [Google Scholar] [CrossRef]
- Wagner, A.; Eto, H.; Joseph, A.; Kohyama, S.; Haraszti, T.; Zamora, R.A.; Vorobii, M.; Giannotti, M.; Schwille, P.; Rodriguez-Emmenegger, C. Dendrimersome Synthetic Cells Harbor Cell Division Machinery of Bacteria. Adv. Mater. 2022, 34, 703–709. [Google Scholar] [CrossRef]
- Potter, M.; Najer, A.; Klockner, A.; Zhang, S.; Holme, M.N.; Nele, V.; Che, J.; Massi, L.; Penders, J.; Saunders, C.; et al. Controlled Dendrimersome Nanoreactor System for Localized Hypochlorite-Induced Killing of Bacteria. ACS Nano 2020, 14, 17333–17353. [Google Scholar] [CrossRef]
- Torre, P.; Xiao, Q.; Buzzacchera, I.; Sherman, S.; Rahimi, K.; Kostina, N.; Rodriguez-Emmenegger, C.; Möller, M.; Wilson, C.; Klein, M.; et al. Encapsulation of hydrophobic components in dendrimersomes and decoration of their surface with proteins and nucleic acids. Proc. Natl. Acad. Sci. USA 2019, 116, 15378–15385. [Google Scholar] [CrossRef]
- Xiao, Q.; Rubien, J.; Wang, Z.; Reed, E.; Hammer, D.; Sahoo, D.; Heiney, P.; Yadavalli, S.; Goulian, M.; Wilner, S.; et al. Self-Sorting and Co-Assembly of Fluorinated, Hydrogenated, and Hybrid Janus Dendrimers into Dendrimersomes. J. Am. Chem. Soc. 2016, 138, 12655–12663. [Google Scholar] [CrossRef]
- Wang, L.; Shi, C.; Wang, X.; Guo, D.; Duncan, T.M.; Luo, J. Zwitterionic Janus Dendrimer with Distinct Functional Disparity for Enhanced Protein Delivery. Biomaterials 2019, 215, 119233. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of Self-Assembly of Hydrocarbon Amphiphiles into Micelles and Bilayers. Soc. Faraday Trans. 2 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Nagarajan, R.; Ganesh, K. Block copolymer selfassembly in selective solvents: Spherical micelles with segregated cores. J. Chem. Phys. 1989, 90, 5843–5856. [Google Scholar] [CrossRef]
- Nagarajan, R. Molecular Packing Parameter and Surfactant Self-Assembly: The Neglected Role of the Surfactant Tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Lebedeva, I.O.; Zhulina, E.B.; Borisov, O.V. Theory of Linear-Dendritic Block Copolymer Micelles. ACS Macro Lett. 2018, 7, 42–46. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, J.; Wang, L.; Xu, Z. Theoretical modeling and simulations of self-assembly of copolymers in solution. Prog. Polym. Sci. 2017, 75, 1–30. [Google Scholar] [CrossRef]
- Morris, K.F.; Billiot, E.J.; Billiot, F.H.; Lipkowitz, K.B.; Southerland, W.M.; Fan, Y. A Molecular Dynamics Simulation Study of Two Dipeptide Based Molecular Micelles: Effect of Amino Acid Order. Open J. Phys. Chem. 2013, 3, 20–29. [Google Scholar] [CrossRef]
- Colherinhas, G.; Fileti, E. Molecular Dynamics Study of Surfactant-Like Peptide Based Nanostructures. J. Phys. Chem. B 2014, 118, 12215–12222. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Wang, W.; Yu, C.; Zhou, Y.; Lu, Z.; Yan, D. Dissipative Particle Dynamics Simulation Study on Self-Assembly of Amphiphilic Hyperbranched Multiarm Copolymers with Different Degrees of Branching. Soft Matter 2015, 11, 8460–8470. [Google Scholar] [CrossRef]
- Yu, C.; Ma, L.; Li, K.; Li, S.; Liu, Y.; Liu, L.; Zhou, Y.; Yan, D. Computer Simulation Studies on the pH-Responsive Self-assembly of Amphiphilic Carboxy-Terminated Polyester Dendrimers in Aqueous Solution. Langmuir 2017, 33, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Yu, C.; Lu, Z.; Zhou, Y.; Yan, D. A dissipative particle dynamics simulation study on phase diagrams for the self-assembly of amphiphilic hyperbranched multiarm copolymers in various solvents. Soft Matter 2017, 13, 6178–6188. [Google Scholar] [CrossRef]
- Gruen, D.W. A statistical mechanical model of the lipid bilayer above its phase transition. Biochim. Et Biophys. Acta 1980, 595, 161–183. [Google Scholar] [CrossRef]
- Gruen, D.W. The packing of amphiphile chains in a small spherical micelle. J. Colloid Inferface Sci. 1981, 84, 281–283. [Google Scholar] [CrossRef]
- Ben-Shaul, A.; Szleifer, I.; Gelbart, W.M. Chain organization and thermodynamics in micelles and bilayers. I. Theory. J. Chem. Phys. 1985, 83, 3597–3611. [Google Scholar] [CrossRef]
- Szleifer, I.; Ben-Shaul, A.; Gelbart, W.M. Chain organization and thermodynamics in micelles and bilayers. II. Model calculations. J. Chem. Phys. 1985, 83, 3612–3620. [Google Scholar] [CrossRef]
- Szleifer, I.; Ben-Shaul, A.; Gelbart, W.M. Statistical thermodynamics of molecular organization in mixed micelles and bilayers. J. Chem. Phys. 1987, 86, 7094–7109. [Google Scholar] [CrossRef]
- Leermakers, F.; Lyklema, J. On the self-consistent field theory of surfactant micelles. Colloids Surfaces 1992, 67, 239–255. [Google Scholar] [CrossRef]
- Meijer, L.; Leermakers, F.; Nelson, A. Modelling of the electrolyte ion-phospholipid layer interaction. Langmuir 1994, 10, 1199–1206. [Google Scholar] [CrossRef]
- Shusharina, N.P.; Linse, P.; Khokhlov, A.R. Lattice Mean-Field Modeling of Charged Polymeric Micelles. Macromolecules 2000, 33, 8488–8496. [Google Scholar] [CrossRef]
- Al-Anber, Z.A.; Avalos, J.B.; Mackie, A.D. Prediction of the critical micelle concentration in a lattice model for amphiphiles using a single-chain mean-field theory. J. Chem. Phys. 2005, 122, 104910. [Google Scholar] [CrossRef]
- Daful, A.G.; Baulin, V.A.; Avalos, J.B.; Mackie, A.D. Accurate Critical Micelle Concentrations from a Microscopic Surfactant Model. J. Phys. Chem. B 2011, 115, 3434–3443. [Google Scholar] [CrossRef]
- Muller, M.; Schmid, F. Incorporating Fluctuations and Dynamics in Self-consistent Field Theories for Polymer Blends. Adv. Polym. Sci. 2005, 185, 1–58. [Google Scholar] [CrossRef]
- Zhang, L.; Sevink, A.; Schmid, F. Hybrid Lattice Boltzmann/Dynamic Self-Consistent Field Simulations of Microphase Separation and Vesicle Formation in Block Copolymer Systems. Macromolecules 2011, 44, 9434–9447. [Google Scholar] [CrossRef]
- Shavykin, O.V.; Leermakers, F.A.; Neelov, I.M.; Darinskii, A.A. Self-Assembly of Lysine-Based Dendritic Surfactants Modeled by the Self-Consistent Field Approach. Langmuir 2018, 34, 1613–1626. [Google Scholar] [CrossRef]
- Shavykin, O.V.; Neelov, I.M.; Borisov, O.V.; Darinskii, A.A.; Leermakers, F.A.M. SCF Theory of Uniformly Charged Dendrimers: Impact of Asymmetry of Branching, Generation Number, and Salt Concentration. Macromolecules 2020, 53, 7298–7311. [Google Scholar] [CrossRef]
- Okrugin, B.M.; Richter, R.P.; Leermakers, F.A.M.; Neelov, I.M.; Borisov, O.V.; Zhulina, E.B. Structure and properties of polydisperse polyelectrolyte brushes studied by self-consistent field theory. Soft Matter. 2018, 14, 6230–6242. [Google Scholar] [CrossRef]
- Lebedeva, I.O.; Shavykin, O.V.; Neelov, I.M.; Zhulina, E.B.; Leermakers, F.A.M.; Borisov, O.V. Non-linear elasticity effects and stratification in brushes of branched polyelectrolytes. J. Chem. Phys. 2019, 151, 214902. [Google Scholar] [CrossRef]
- Shavykin, O.; Mikhailov, I.; Darinskii, A.; Neelov, I.; Leermakers, F. Effect of an asymmetry of branching on structural characteristics of dendrimers revealed by Brownian dynamics simulations. Polymer 2018, 146, 256–266. [Google Scholar] [CrossRef]
- Moorefield, C.N.; Newkome, G.R. Unimolecular micelles: Supramolecular use of dendritic constructs to create versatile molecular containers. C. R. Chim. 2003, 6, 715–724. [Google Scholar] [CrossRef]
- Cao, W.; Zhu, L. Synthesis and Unimolecular Micelles of Amphiphilic Dendrimer-like Star Polymer with Various Functional Surface Groups. Macromolecules 2011, 44, 1500–1512. [Google Scholar] [CrossRef]
- De Gennes, P.; Hervet, H. Statistics of starburst polymers. J. Phys. Lett. 1983, 44, 351–360. [Google Scholar] [CrossRef]
- Lescanec, R.; Muthukumar, M. Density profiles of simulated comburst molecules. Macromolecules 1991, 24, 4892–4897. [Google Scholar] [CrossRef]
- Boris, D.; Rubinstein, M. A Self-Consistent Mean Field Model of a Starburst Dendrimer: Dense Core vs Dense Shell. Macromolecules 1996, 29, 7251–7260. [Google Scholar] [CrossRef]
- Murat, M.; Grest, G. Molecular Dynamics Study of Dendrimer Molecules in Solvents of Varying Quality. Macromolecules 1996, 29, 1278–1285. [Google Scholar] [CrossRef]
- Neelov, I.; Falkovich, S.; Markelov, D.; Paci, E.; Darinskii, A.; Tenhu, H. Molecular Dynamics of Lysine Dendrimers. Computer Simulation and NMR. In Dendrimers in Biomedical Applications; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 99–114. [Google Scholar] [CrossRef]
- Okrugin, B.; Neelov, I.; Borisov, O.; Leermakers, F. Structure of asymmetrical peptide dendrimers: Insights given by self-consistent field theory. Polymer 2017, 125, 292–302. [Google Scholar] [CrossRef]
- Sommerfeld, N.; Hejl, M.; Klose, M.; Schreiber-Brynzak, E.; Bileck, A.; Meier, S.; Gerner, C.; Jakupec, M.; Galanski, M.; Keppler, B. Low-Generation Polyamidoamine Dendrimers as Drug Carriers for Platinum(IV) Complexes. Eur. J. Inorg. Chem. 2017, 2017, 1713–1720. [Google Scholar] [CrossRef]
- Shah, N.; Steptoe, R.; Parekh, H. Low-generation asymmetric dendrimers exhibit minimal toxicity and effectively complex DNA. J. Pept. Sci. 2011, 17, 470–478. [Google Scholar] [CrossRef]
- Gorzkiewicz, M.; Konopka, M.; Janaszewska, A.; Tarasenko, I.; Sheveleva, N.; Gajek, A.; Neelov, I.; Klajnert-Maculewicz, B. Application of new lysine-based peptide dendrimers D3K2 and D3G2 for gene delivery: Specific cytotoxicity to cancer cells and transfection in vitro. Bioorg. Chem. 2020, 95, 103504. [Google Scholar] [CrossRef] [PubMed]
- Gorzkiewicz, M.; Kopeć, O.; Janaszewska, A.; Konopka, M.; Pedziwiatr-Werbicka, E.; Tarasenko, I.; Bezrodnyi, V.; Neelov, I.; Klajnert-Maculewicz, B. Poly(lysine) Dendrimers Form Complexes with siRNA and Provide Its Efficient Uptake by Myeloid Cells: Model Studies for Therapeutic Nucleic Acid Delivery. Int. J. Mol. Sci. 2020, 21, 3138. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Lesniak, W.; Islam, M.; MuÑiz, M.; Balogh, L.; Baker, J. Comprehensive characterization of surface-functionalized poly(amidoamine) dendrimers with acetamide, hydroxyl, and carboxyl groups. Colloids Surfaces A Physicochem. Eng. Asp. 2006, 272, 139–150. [Google Scholar] [CrossRef]
- Trinchi, A.; Muster, T. A Review of Surface Functionalized Amine Terminated Dendrimers for Application in Biological and Molecular Sensing. Supramol. Chem. 2007, 19, 431–445. [Google Scholar] [CrossRef]
- Caminade, A.; Turrin, C. Dendrimers for drug delivery. J. Mater. Chem. B 2014, 2, 4055–4066. [Google Scholar] [CrossRef]
- Sheveleva, N.; Markelov, D.; Vovk, M.; Mikhailova, M.; Tarasenko, I.; Neelov, I.; Lähderanta, E. NMR studies of excluded volume interactions in peptide dendrimers. Sci. Rep. 2018, 8, 8916. [Google Scholar] [CrossRef]
- Sheveleva, N.; Markelov, D.; Vovk, M.; Tarasenko, I.; Mikhailova, M.; Ilyash, M.; Neelov, I.; Lahderanta, E. Stable Deuterium Labeling of Histidine-Rich Lysine-Based Dendrimers. Molecules 2019, 24, 2481. [Google Scholar] [CrossRef]
- Sheveleva, N.; Markelov, D.; Vovk, M.; Mikhailova, M.; Tarasenko, I.; Tolstoy, P.; Neelov, I.; Lähderanta, E. Lysine-based dendrimer with double arginine residues. RSC Adv. 2019, 9, 18018–18026. [Google Scholar] [CrossRef]
- Yang, H.; Lopina, S. Penicillin V-conjugated PEG-PAMAM star polymers. J. Biomater. Sci. Polym. Ed. 2003, 14, 1043–1056. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.; Gupta, U.; Greish, K.; Iyer, A. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef]
- Suek, N.; Lamm, M. Computer Simulation of Architectural and Molecular Weight Effects on the Assembly of Amphiphilic Linear-Dendritic Block Copolymers in Solution. Langmuir 2008, 24, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Miranda, V.; Araya-Durán, I.; Camarada, M.; Comer, J.; Valencia-Gallegos, J.; González-Nilo, F. Self-Assembly of Amphiphilic Dendrimers: The Role of Generation and Alkyl Chain Length in siRNA Interaction. Sci. Rep. 2016, 6, 29436. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.; Frey, H. Linear–dendritic block copolymers: The state of the art and exciting perspectives. Prog. Polym. Sci. 2011, 36, 1–52. [Google Scholar] [CrossRef]
- Pera, H.; Kleijn, J.; Leermakers, F. Linking lipid architecture to bilayer structure and mechanics using self-consistent field modelling. J. Chem. Phys. 2014, 140, 065102. [Google Scholar] [CrossRef] [PubMed]
- Tanford, C. The Hydrophobic Effect: Formation of Micelles and Biological Membranes, 2nd ed.; Wiley: New York, NY, USA, 1980. [Google Scholar]
- Gowdy, J.; Batchelor, M.; Neelov, I.; Paci, E. Non-Exponential Kinetics of Loop Formation in Proteins and Peptides: A Signature of Rugged Free Energy Landscapes? J. Phys. Chem. B 2017, 121, 9518–9525. [Google Scholar] [CrossRef]
- Darinskii, A.; Gotlib, Y.; Lyulin, A.; Neelov, I. Computer simulation of local dynamics of polymer chain in the orienting field of the LC type. Vysok. Soedin. Seriya A 1991, 33, 1211–1220. [Google Scholar]
- Shavykin, O.V.; Neelov, I.M.; Darinskii, A.A. Is the manifestation of the local dynamics in the spin-lattice NMR relaxation in dendrimers sensitive to excluded volume interactions? Phys. Chem. Chem. Phys. 2016, 18, 24307–24317. [Google Scholar] [CrossRef]
- Neelov, I.; Adolf, D.; McLeish, T.; Paci, E. Molecular dynamics simulation of dextran extension by constant force in single molecule AFM. Biophys. J. 2006, 91, 3579–3588. [Google Scholar] [CrossRef]
- Ennari, J.; Neelov, I.; Sundholm, F. Simulation of a PEO based solid polyelectrolyte, comparison of the CMM and the Ewald summation method. Polymer 2000, 41, 2149–2155. [Google Scholar] [CrossRef]
- Okrugin, B.; Ilyash, M.; Markelov, D.; Neelov, I. Lysine dendrigraft nanocontainers. Influence of topology 5on their size and internal structure. Pharmaceutics 2018, 10, 129. [Google Scholar] [CrossRef]
- Sadovnichy, V.; Tikhonravov, A.; Voevodin, V.; Opanasenko, V. Contemporary High Performance Computing: From Petascale toward Exascale; Chapman and Hall/CRC: Boca Raton, FL, USA, 2013; pp. 283–307. [Google Scholar]













| (a) | |||||||
| W | C | NH | NH | O | Na | Cl | |
| W | 0 | 0 | 0 | 0 | |||
| C | 1.2 | 0 | 3 | 2 | 2 | 2 | 2 |
| NH | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
| NH | 2 | 0 | 0 | 0 | 0 | 0 | |
| O | 2 | 0 | 0 | 0 | 0 | 0 | |
| Na | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| Cl | 0 | 2 | 0 | 0 | 0 | 0 | 0 |
| (b) | |||||||
| v | 0 | 0 | 1 | 0 | 0 | 1 | |
| 80 | 2 | 5 | 5 | 5 | 10 | 10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shavykin, O.V.; Mikhtaniuk, S.E.; Fatullaev, E.I.; Neelov, I.M.; Leermakers, F.A.M.; Brito, M.E.; Holm, C.; Borisov, O.V.; Darinskii, A.A. Hybrid Molecules Consisting of Lysine Dendrons with Several Hydrophobic Tails: A SCF Study of Self-Assembling. Int. J. Mol. Sci. 2023, 24, 2078. https://doi.org/10.3390/ijms24032078
Shavykin OV, Mikhtaniuk SE, Fatullaev EI, Neelov IM, Leermakers FAM, Brito ME, Holm C, Borisov OV, Darinskii AA. Hybrid Molecules Consisting of Lysine Dendrons with Several Hydrophobic Tails: A SCF Study of Self-Assembling. International Journal of Molecular Sciences. 2023; 24(3):2078. https://doi.org/10.3390/ijms24032078
Chicago/Turabian StyleShavykin, Oleg V., Sofia E. Mikhtaniuk, Emil I. Fatullaev, Igor M. Neelov, Frans A. M. Leermakers, Mariano E. Brito, Christian Holm, Oleg V. Borisov, and Anatoly A. Darinskii. 2023. "Hybrid Molecules Consisting of Lysine Dendrons with Several Hydrophobic Tails: A SCF Study of Self-Assembling" International Journal of Molecular Sciences 24, no. 3: 2078. https://doi.org/10.3390/ijms24032078
APA StyleShavykin, O. V., Mikhtaniuk, S. E., Fatullaev, E. I., Neelov, I. M., Leermakers, F. A. M., Brito, M. E., Holm, C., Borisov, O. V., & Darinskii, A. A. (2023). Hybrid Molecules Consisting of Lysine Dendrons with Several Hydrophobic Tails: A SCF Study of Self-Assembling. International Journal of Molecular Sciences, 24(3), 2078. https://doi.org/10.3390/ijms24032078







