Triacylglycerol Composition and Chemical-Physical Properties of Cocoa Butter and Its Derivatives: NMR, DSC, X-ray, Rheological Investigation
Abstract
:1. Introduction
2. Results and Discussions
2.1. Differential Scanning Calorimetry
2.2. Rheological Measurements
2.3. Powder X-ray Diffraction
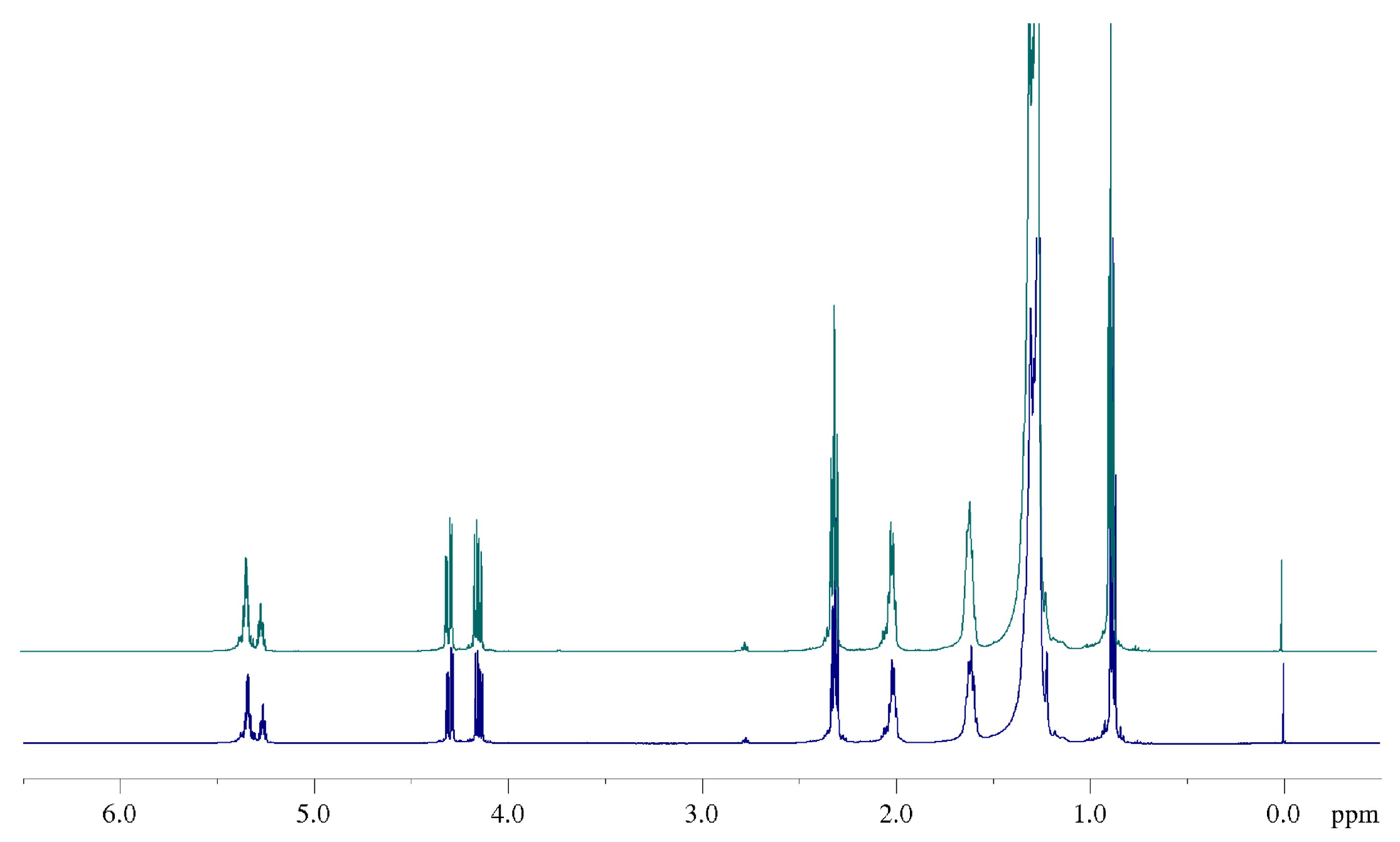
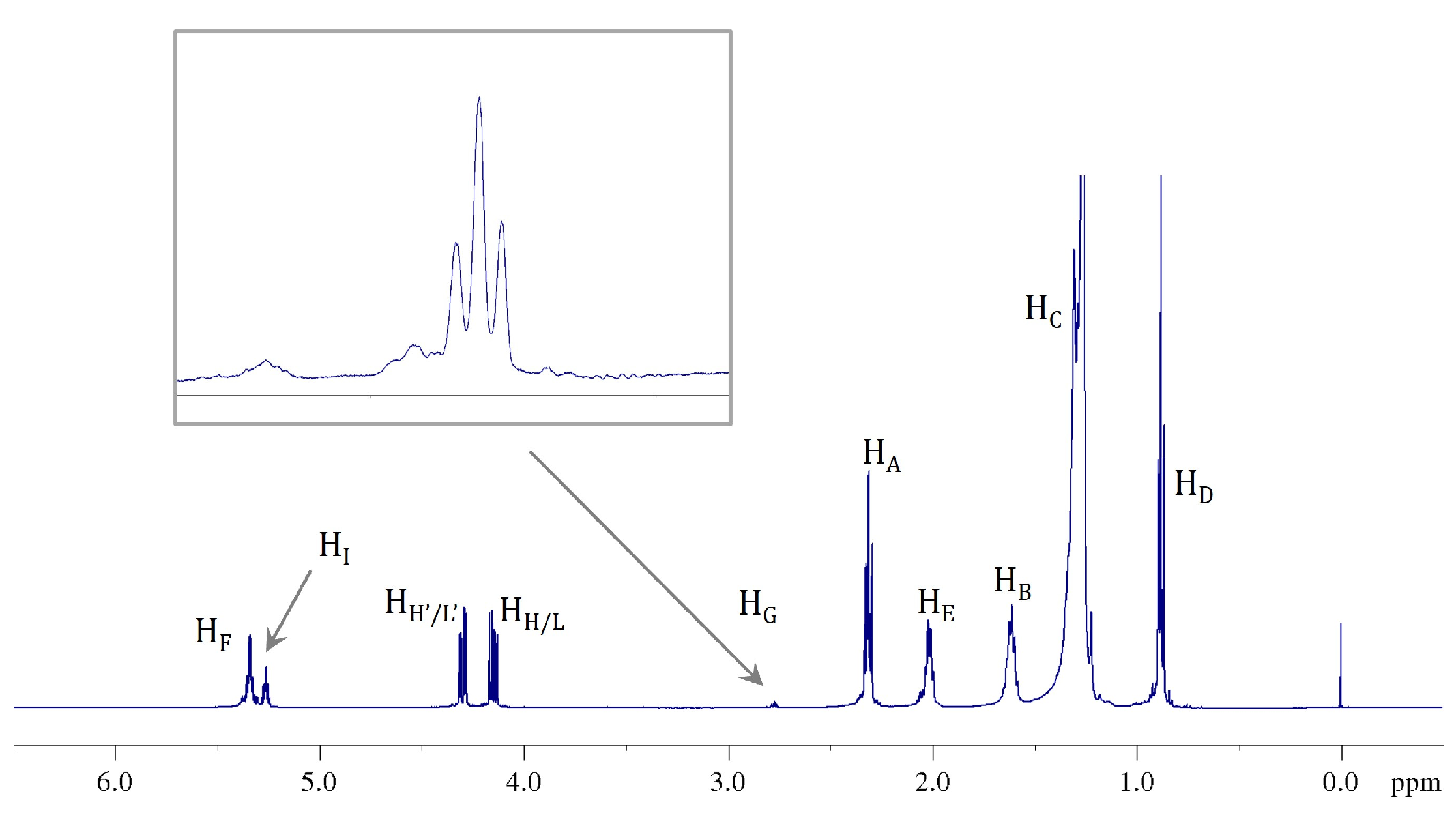
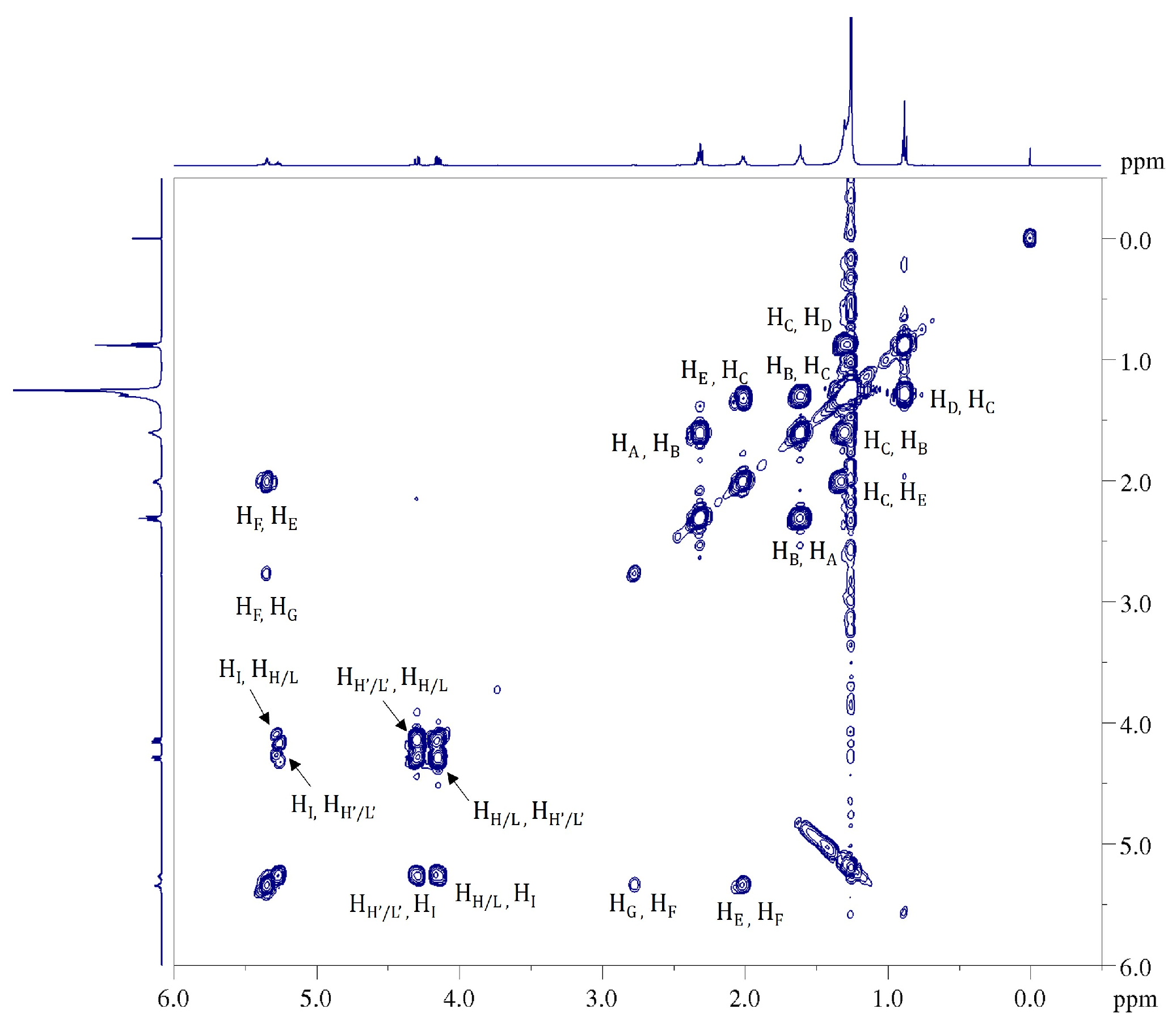
2.4. 1H NMR: Natural Cocoa Butter and Modified Samples
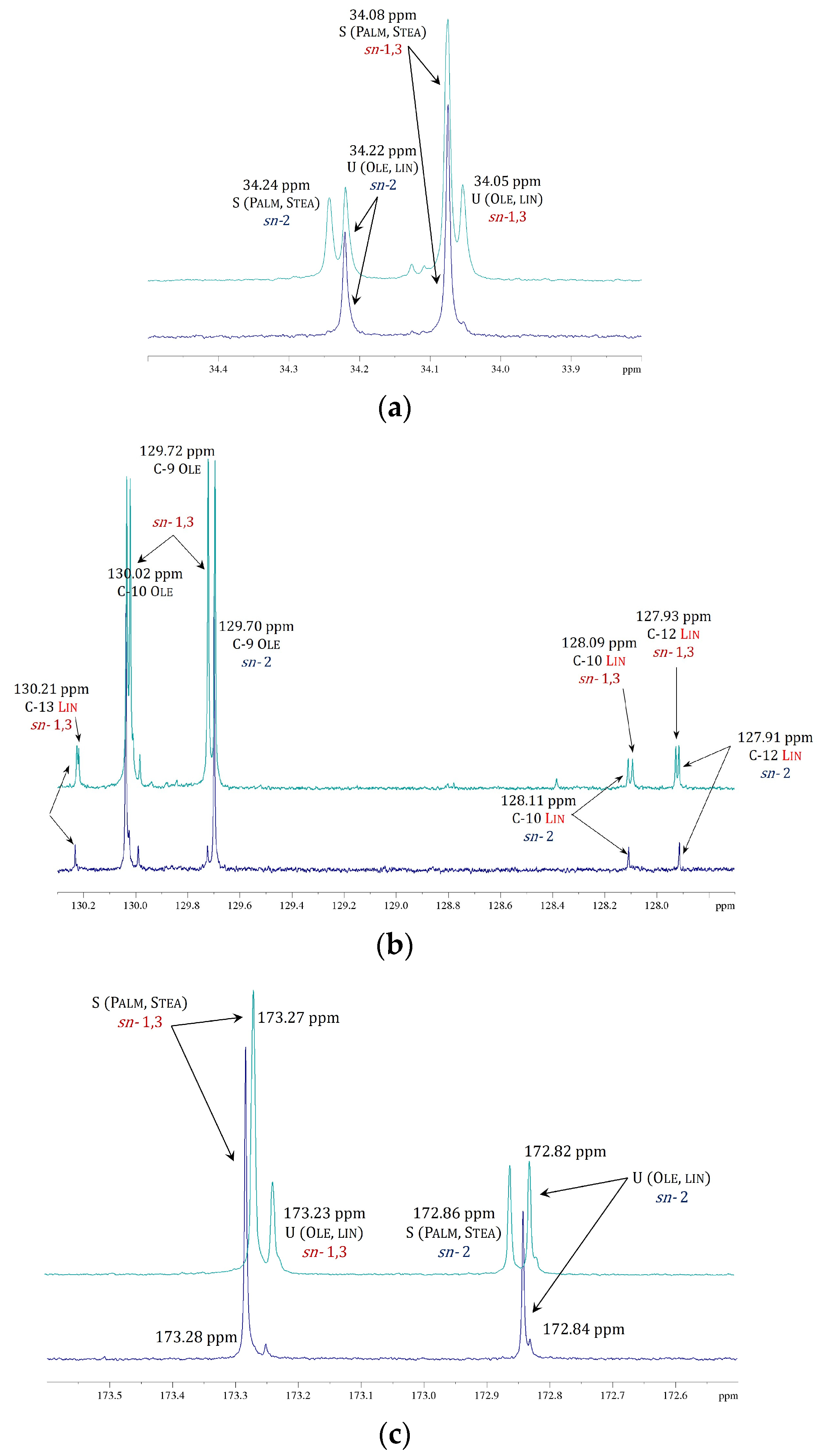
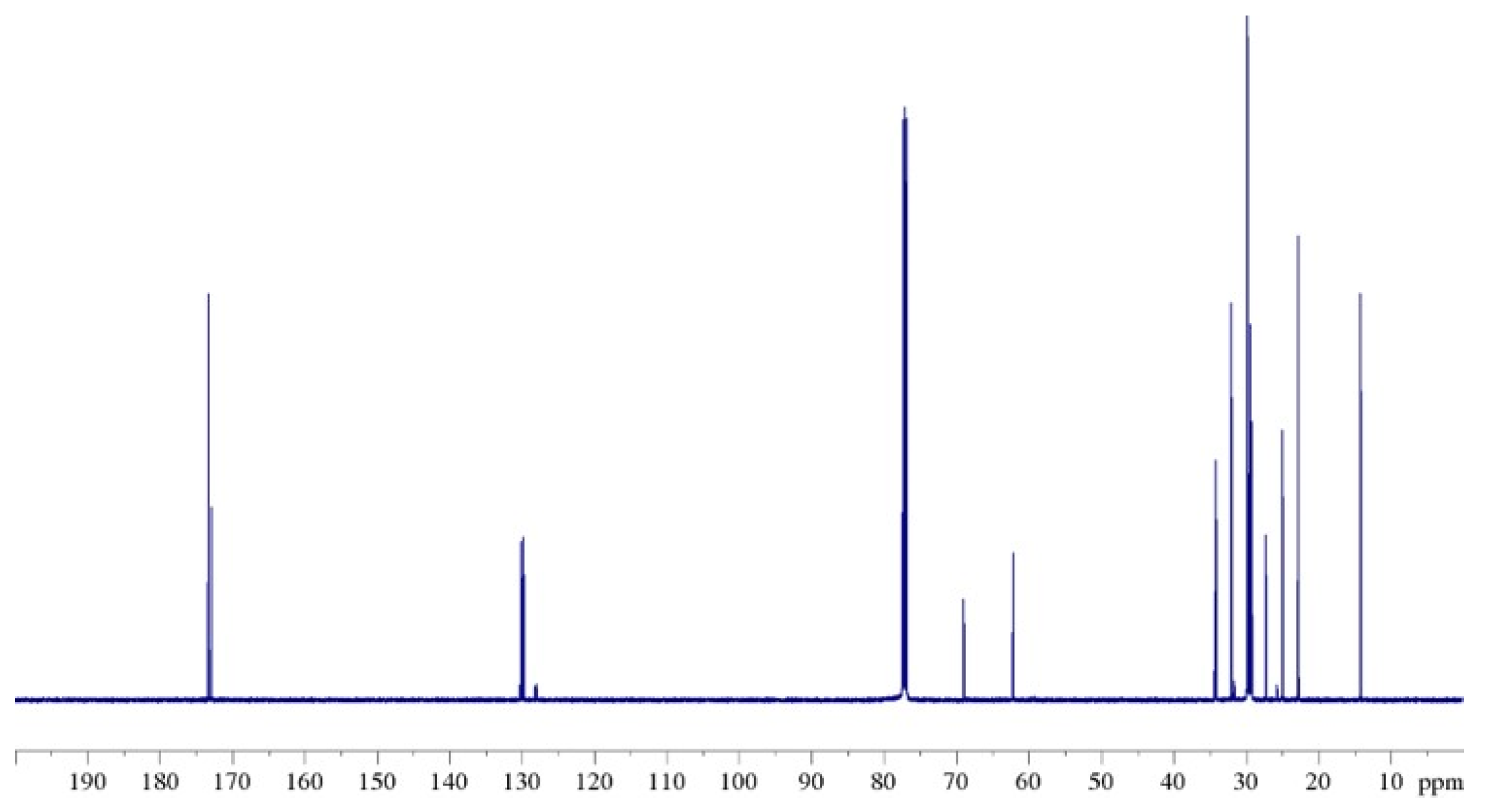
2.5. Parallel Study of 13C NMR Spectra of Natural and Modified Cocoa Butter
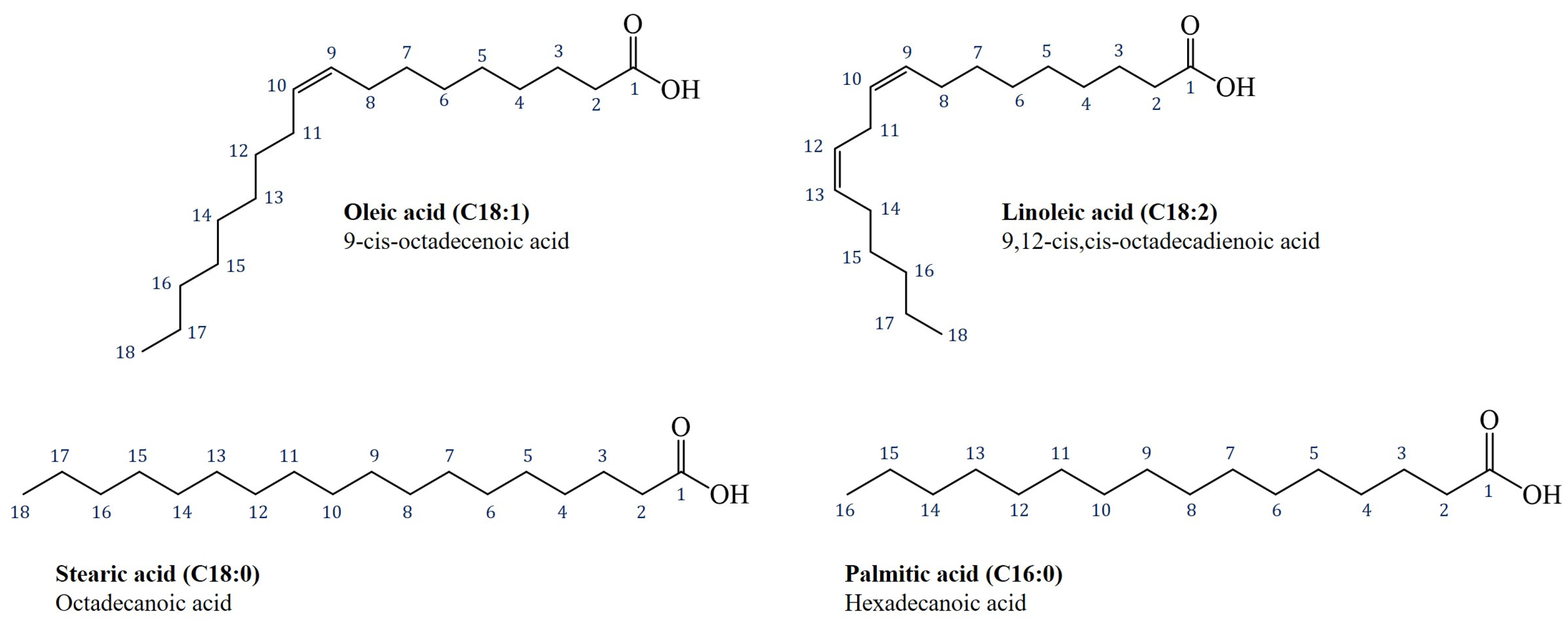
3. Materials and Methods
3.1. Starting Materials and Modified Fats
3.2. Differential Scanning Calorimetry
- Isotherm at 15 °C for 20 min
- Heating from 15 °C to 70 °C at 2 °C/min
- Cooling from 70 °C to 15 °C at 2 °C/min
- Isotherm at 15 °C for 10 min
- Heating from 15 °C to 70 °C at 2 °C/min
3.3. Rheological Measurement
3.4. Powder X-ray Diffraction
3.5. Nuclear Magnetic Resonance (NMR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marangoni, A.G.; Acevedo, N.; Maleky, F.; Co, E.; Peyronel, F.; Mazzanti, G.; Quinn, B.; Pink, D. Structure and functionality of edible fats. Soft Matter 2012, 8, 1275–1300. [Google Scholar] [CrossRef]
- Hidalgo, F.J.; Zamora, R. Triacylglycerols: Structures and Properties. In Encyclopedia of Food and Health, 1st ed.; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 351–356. [Google Scholar] [CrossRef]
- Pande, G.; Akoh, C.C.; Lai, O.M. Food Uses of Palm Oil and Its Components. In Palm Oil: Production, Processing, Characterization, and Uses, 1st ed.; Lai, O.-M., Tan, C.-P., Akoh, C.C., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 561–586. [Google Scholar]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry—A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Wijewardene, U.; Premlal Ranjith, H.M. Food safety and quality issues of dairy fats. In Fats in Food Technology, 2nd ed.; Kanes, K.R., Ed.; John Wiley & Sons: Chichester, UK, 2014; pp. 289–325. [Google Scholar] [CrossRef]
- Abd-Aziz, S.; Gozan, M.; Ibrahim, M.F.; Phang, L.-Y. Demand and Sustainability of Palm Oil Plantation. In Biorefinery of Oil Producing Plants for Value-Added Products, 1st ed.; Abd-Aziz, S., Gozan, M., Ibrahim, M.F., Phang, L.-Y., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2022. [Google Scholar] [CrossRef]
- Fitzherbert, E.B.; Struebig, M.J.; Morel, A.; Danielsen, F.; Brühl, C.A.; Donald, P.F.; Phalan, B. How will oil palm expansion affect biodiversity? Trends Ecol. Evol. 2008, 10, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Meijaard, E.; Garcia-Ulloa, J.; Sheil, D.; Wich, S.A.; Carlson, K.M.; Juffe-Bignoli, D.; Brooks, T.M. Oil Palm and Biodiversity: A Situation Analysis by the IUCN Oil Palm Task Force; IUCN, Library System: Gland, Switzerland, 2018. [Google Scholar] [CrossRef] [Green Version]
- Gatti, R.C.; Liang, J.; Velichevskaya, A.; Zhou, M. Sustainable palm oil may not be so sustainable. Sci. Total Environ. 2019, 652, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Chew, C.L.; Ng, C.Y.; Hong, W.O.; Wu, T.Y.; Lee, Y.Y.; Low, L.E.; Low, L.E.; Kong, P.S.; Chan, E.S. Improving sustainability of palm oil production by increasing oil extraction rate: A review. Food Bioproc. Technol. 2021, 14, 573–586. [Google Scholar] [CrossRef]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Durazzo, A. Innovative and sustainable technologies to enhance the oxidative stability of vegetable oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- List, G.R.; Wang, T.; Shukla, V.K.S. Storage, Handling, and Transport of Oils and Fats. In Bailey’s Industrial Oil and Fat Products; Shahidi, F., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Nunes, A.; Marto, J.; Gonçalves, L.; Martins, A.M.; Fraga, C.; Ribeiro, H.M. Potential therapeutic of olive oil industry by-products in skin health: A review. Int. J. Food Sci. Technol. 2022, 57, 173–187. [Google Scholar] [CrossRef]
- Szabo, Z.; Marosvölgyi, T.; Szabo, E.; Koczka, V.; Verzar, Z.; Figler, M.; Decsi, T. Effects of Repeated Heating on Fatty Acid Composition of Plant-Based Cooking Oils. Foods 2022, 11, 192. [Google Scholar] [CrossRef]
- Nieto, G.; Lorenzo, J.M. Chapter 4: Plant source: Vegetable oils. In Food Lipids, 1st ed.; José, M.L., Sichetti Munekata, P.E., Pateiro, M., Barba, F.J., Domínguez, R., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 69–85. [Google Scholar] [CrossRef]
- Karupaiah, T.; Sundram, K. Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: A review of their nutritional implications. Nutr. Metab. 2007, 4, 16. [Google Scholar] [CrossRef] [Green Version]
- Norazlina, M.R.; Jahurul, M.H.A.; Hasmadi, M.; Mansoor, A.H.; Norliza, J.; Patricia, M.; Fan, H.Y. Trends in blending vegetable fats and oils for cocoa butter alternative application: A review. Trends Food Sci. Technol. 2021, 116, 102–114. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, T.; Liang, Y.; Jiang, L.; Sui, X. Dietary bioactive lipids: A review on absorption, metabolism, and health properties. J. Agric. Food Chem. 2021, 69, 8929–8943. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; Liu, R.; Chang, M.; Wei, W.; Jin, Q.; Wang, X. Reviews of medium-and long-chain triglyceride with respect to nutritional benefits and digestion and absorption behavior. Int. Food Res. J. 2022, 115, 111058. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.E. Triacylglycerol structure and interesterification of palmitic and stearic acid-rich fats: An overview and implications for cardiovascular disease. Nutr. Res. Rev. 2009, 22, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afoakwa, E.O.; Paterson, A.; Fowler, M. Factors influencing rheological and textural qualities in chocolate—A review. Trends Food Sci. Technol. 2007, 18, 290–298. [Google Scholar] [CrossRef]
- Smith, K.W. Cocoa butter and cocoa butter equivalents. In Structured and Modified Lipids, 1st ed.; Gunstone, F.D., Ed.; Unilever Research Colworth: Bedfordshire, UK, 2001; pp. 401–422. [Google Scholar]
- Campos, R.; Marangoni, A.G. Molecular composition dynamics and structure of cocoa butter. In Cocoa Butter and Related Compounds, 1st ed.; Garti, N., Widlak, N.R., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 103–150. [Google Scholar]
- Liu, W.; Yao, Y.; Li, C. Effect of tempered procedures on the crystallization behavior of different positions of cocoa butter products. Food Chem. 2022, 370, 131002. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, Z.; Xie, D.; Cao, Z.; Chen, L.; Wang, H.; Jiang, L.; Shen, Q. Rhizomucor miehei lipase-catalyzed synthesis of cocoa butter equivalent from palm mid-fraction and stearic acid: Characteristics and feasibility as cocoa butter alternative. Food Chem. 2021, 343, 128407. [Google Scholar] [CrossRef]
- Ribeiro, A.P.B.; Basso, R.C.; dos Santos, A.O.; Andrade, G.C.; Cardoso, L.P.; Kieckbusch, T.G. Hardfats as crystallization modifiers of cocoa butter. Eur. J. Lipid Sci. Technol. 2013, 115, 1462–1473. [Google Scholar] [CrossRef]
- Sivakanthan, S.; Madhujith, T. Current trends in applications of enzymatic interesterification of fats and oils: A review. LWT—Food Sci. Technol. 2020, 132, 109880. [Google Scholar] [CrossRef]
- Ornla-ied, P.; Podchong, P.; Sonwai, S. Synthesis of cocoa butter alternatives from palm kernel stearin, coconut oil and fully hydrogenated palm stearin blends by chemical interesterification. J. Sci. Food Agric. 2022, 102, 1619–1627. [Google Scholar] [CrossRef]
- Aktas, A.B.; Ozen, B. Chemical and physical properties of fats produced by chemical interesterification of tallow with vegetable oils. Grasas Aceites 2021, 72, e418. [Google Scholar] [CrossRef]
- Loisel, C.; Keller, G.; Lecq, G.; Bourgaux, C.; Ollivon, M. Phase transitions and polymorphism of cocoa butter. J. Am. Oil. Chem. Soc. 1998, 75, 425–439. [Google Scholar] [CrossRef]
- Declerck, A.; Nelis, V.; Danthine, S.; Dewettinck, K.; Van der Meeren, P. Characterisation of Fat Crystal Polymorphism in Cocoa Butter by Time-Domain NMR and DSC Deconvolution. Foods 2021, 10, 520. [Google Scholar] [CrossRef]
- Bresson, S.; Lecuelle, A.; Bougrioua, F.; El Hadri, M.; Baeten, V.; Courty, M.; Faivre, V. Comparative structural and vibrational investigations between cocoa butter (CB) and cocoa butter equivalent (CBE) by ESI/MALDI-HRMS, XRD, DSC, MIR and Raman spectroscopy. Food Chem. 2021, 363, 130319. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Careglio, E. Influence of free fatty acids as additives on the crystallization kinetics of cocoa butter. J. Food Res. 2018, 7, 86–97. [Google Scholar] [CrossRef]
- Ioannidi, E.; Risbo, J.; Aarøe, E.; van den Berg, F.W. Thermal Analysis of Dark Chocolate with Differential Scanning Calorimetry—Limitations in the Quantitative Evaluation of the Crystalline State. Food Anal. Methods 2021, 14, 2556–2568. [Google Scholar] [CrossRef]
- Ray, J.; Smith, K.W.; Bhaggan, K.; Stapley, A.G. Crystallization and Polymorphism of Cocoa Butter Equivalents from blends of Palm Mid Fraction and Hard Stearins Produced by Enzymatic Acidolysis of High Oleic Sunflower Oil. Eur. J. Lipid Sci. Technol. 2022, 124, 2100228. [Google Scholar] [CrossRef]
- Castro-Alayo, E.M.; Torrejón-Valqui, L.; Medina-Mendoza, M.; Cayo-Colca, I.S.; Cárdenas-Toro, F.P. Kinetics Crystallization and Polymorphism of Cocoa Butter throughout the Spontaneous Fermentation Process. Foods 2022, 11, 1769. [Google Scholar] [CrossRef]
- De Clercq, N.; Danthine, S.; Nguyen, M.T.; Gibon, V.; Dewettinck, K. Enzymatic interesterification of palm oil and fractions: Monitoring the degree of interesterification using different methods. J. Am. Oil. Chem. Soc. 2012, 89, 219–229. [Google Scholar] [CrossRef]
- Ghazani, S.M.; Marangoni, A.G. Molecular origins of polymorphism in cocoa butter. Annu. Rev. Food Sci. Technol. 2021, 12, 567–590. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. 1H nuclear magnetic resonance as a fast tool for determining the composition of acyl chains in acylglycerol mixtures. Eur. J. Lipid Sci. Technol. 2003, 105, 502–507. [Google Scholar] [CrossRef]
- Siudem, P.; Zielińska, A.; Paradowska, K. Application of 1H NMR in the study of fatty acids composition of vegetable oils. J. Pharm. Biomed. Anal. 2022, 212, 114658. [Google Scholar] [CrossRef] [PubMed]
- McNaught, A.D.; Wilkinson, A. IUPAC. Compendium of Chemical Terminology (the “Gold Book”), 2nd ed.; Online Version (2019-) Created by S. J. Chalk; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar] [CrossRef]
- Mannina, L.; Luchinat, C.; Emanuele, M.C.; Segre, A. Acyl positional distribution of glycerol tri-esters in vegetable oils: A 13C NMR study. Chem. Phys. Lipids 1999, 103, 47–55. [Google Scholar] [CrossRef]
- Alemany, L.B. Using simple 13C NMR linewidth and relaxation measurements to make detailed chemical shift assignments in triacylglycerols and related compounds. Chem. Phys. Lipids 2022, 120, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Simova, S.; Ivanova, G.; Spassov, S.L. Alternative NMR method for quantitative determination of acyl positional distribution in triacylglycerols and related compounds. Chem. Phys. Lipids 2003, 126, 167–176. [Google Scholar] [CrossRef]
- Hama, J.R.; Fitzsimmons-Thoss, V. Determination of Unsaturated Fatty Acids Composition in Walnut (Juglans regia L.) Oil Using NMR Spectroscopy. Food Anal. Methods 2022, 15, 1226–1236. [Google Scholar] [CrossRef]
- Kildahl-Andersen, G.; Gjerlaug-Enger, E.; Rise, F.; Haug, A.; Egelandsdal, B. Quantification of Fatty Acids and their Regioisomeric Distribution in Triacylglycerols from Porcine and Bovine Sources Using 13C NMR Spectroscopy. Lipids 2021, 56, 111–122. [Google Scholar] [CrossRef]
- Tarvainen, M.; Kallio, H.; Yang, B. Regiospecific analysis of triacylglycerols by ultrahigh-performance-liquid chromatography–electrospray ionization–tandem mass spectrometry. Anal. Chem. 2019, 91, 13695–13702. [Google Scholar] [CrossRef]
- Kalpio, M.; Linderborg, K.M.; Fabritius, M.; Kallio, H.; Yang, B. Strategy for stereospecific characterization of natural triacylglycerols using multidimensional chromatography and mass spectrometry. J. Chromatogr. A 2021, 1641, 461992. [Google Scholar] [CrossRef]
- Alexandri, E.; Ahmed, R.; Siddiqui, H.; Choudhary, M.I.; Tsiafoulis, C.G.; Gerothanassis, I.P. High resolution NMR spectroscopy as a structural and analytical tool for unsaturated lipids in solution. Molecules 2017, 22, 1663. [Google Scholar] [CrossRef] [Green Version]
- Gouk, S.W.; Cheng, S.F.; Malon, M.; Ong, A.S.H.; Chuah, C.H. Critical considerations for fast and accurate regiospecific analysis of triacylglycerols using quantitative 13C NMR. Anal. Methods 2013, 5, 2064–2073. [Google Scholar] [CrossRef]
- Hadri, M.E.; Bresson, S.; Lecuelle, A.; Bougrioua, F.; Baeten, V.; Nguyen, V.H.; Courty, M. Structural and Vibrational Investigations of Mixtures of Cocoa Butter (CB), Cocoa Butter Equivalent (CBE) and Anhydrous Milk Fat (AMF) to Understand Fat Bloom Process. Appl. Sci. 2022, 12, 6594. [Google Scholar] [CrossRef]
- Caputo, P.; Oliviero Rossi, C. Differential Scanning Calorimetry as a New Method to Evaluate the Effectiveness of Rejuvenating Agents in Bitumens. Appl. Sci. 2021, 11, 6528. [Google Scholar] [CrossRef]
- Malkin, A.Y.; IIsayev, A. Rheometry Experimental Methods. In Rheology Concepts, Methods, and Applications, 3rd ed.; Malkin, A.Y., IIsayev, A., Eds.; ChemTec Publishing: Toronto, ON, Canada, 2017; pp. 265–375. [Google Scholar] [CrossRef]
- Joyner (Melito), H.S.; Daubert, C.R. Rheological Principles for Food Analysis. In Food Analysis, 1st ed.; Nielsen, S.S., Ed.; Food Science Text Series; Springer: Cham, Germany, 2017; pp. 511–527. [Google Scholar] [CrossRef]
- Caputo, P.; Porto, M.; Calandra, P.; De Santo, M.P.; Oliviero Rossi, C. Effect of epoxidized soybean oil on mechanical properties of bitumen and aged bitumen. Mol. Cryst. Liq. 2018, 675, 68–74. [Google Scholar] [CrossRef]
- Reuhs, B.L.; Simsek, S. Nuclear Magnetic Resonance. In Food Analysis, 1st ed.; Nielsen, S.S., Ed.; Food Science Text Series; Springer: Cham, Germany, 2017; pp. 151–163. [Google Scholar] [CrossRef]
- Truzzi, E.; Marchetti, L.; Benvenuti, S.; Ferroni, A.; Rossi, M.C.; Bertelli, D. Novel strategy for the recognition of adulterant vegetable oils in essential oils commonly used in food industries by applying 13C NMR spectroscopy. J. Agric. Food Chem. 2021, 69, 8276–8286. [Google Scholar] [CrossRef]
- Eisenmann, P.; Ehlers, M.; Weinert, C.H.; Tzvetkova, P.; Silber, M.; Rist, M.J.; Luy, B.; Muhle-Goll, C. Untargeted NMR Spectroscopic Analysis of the Metabolic Variety of New Apple Cultivars. Metabolites 2016, 6, 29. [Google Scholar] [CrossRef]
- TopSpin. Available online: https://www.bruker.com/en/products-and-solutions/mr/nmr-software/topspin.html (accessed on 10 October 2022).
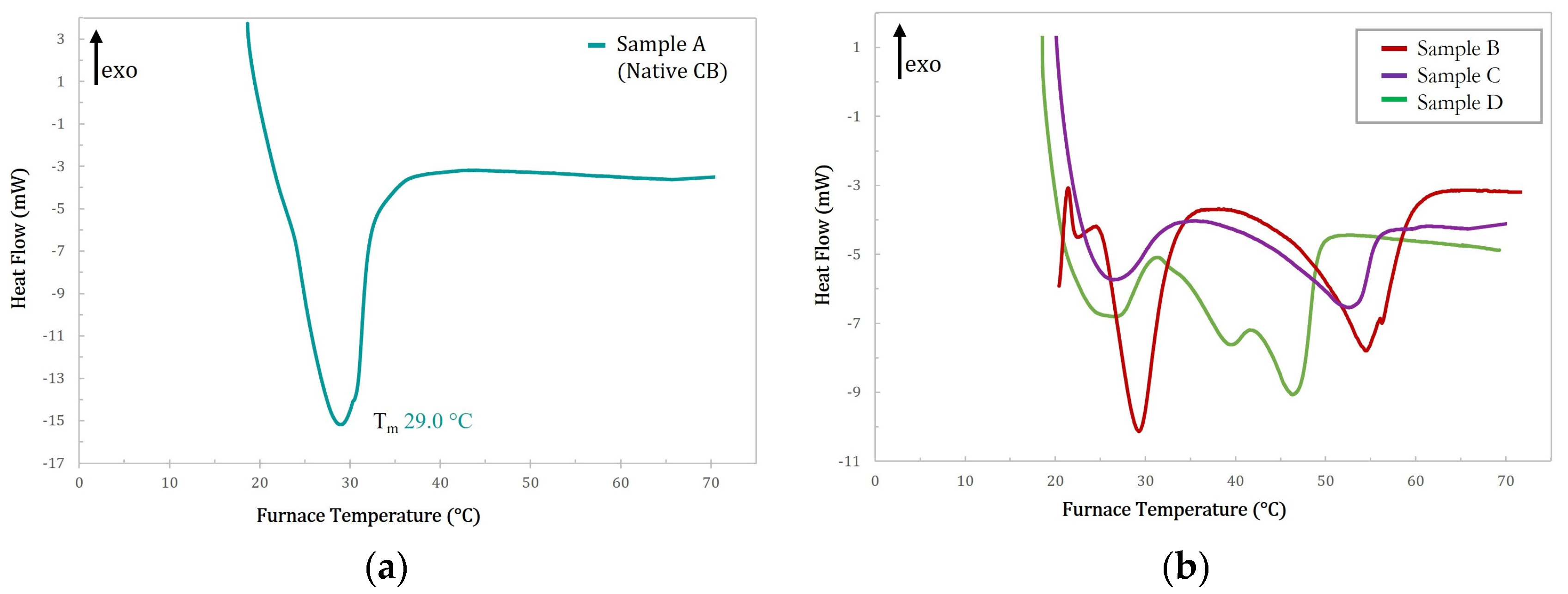



| A-Native CB | 29.0 | 53.5 |
| B | 28.6 | 20.8 |
| 53.3 | 29.2 | |
| C | 26.1 | 7.7 |
| 52.6 | 11.0 | |
| D | 24.6 | 10.7 |
| 39.1 | 2.9 | |
| 46.8 | 9.6 | |
| PO | 26.6 | 5.6 |
| 42.7 | 5.1 | |
| 52.5 | 12.5 |
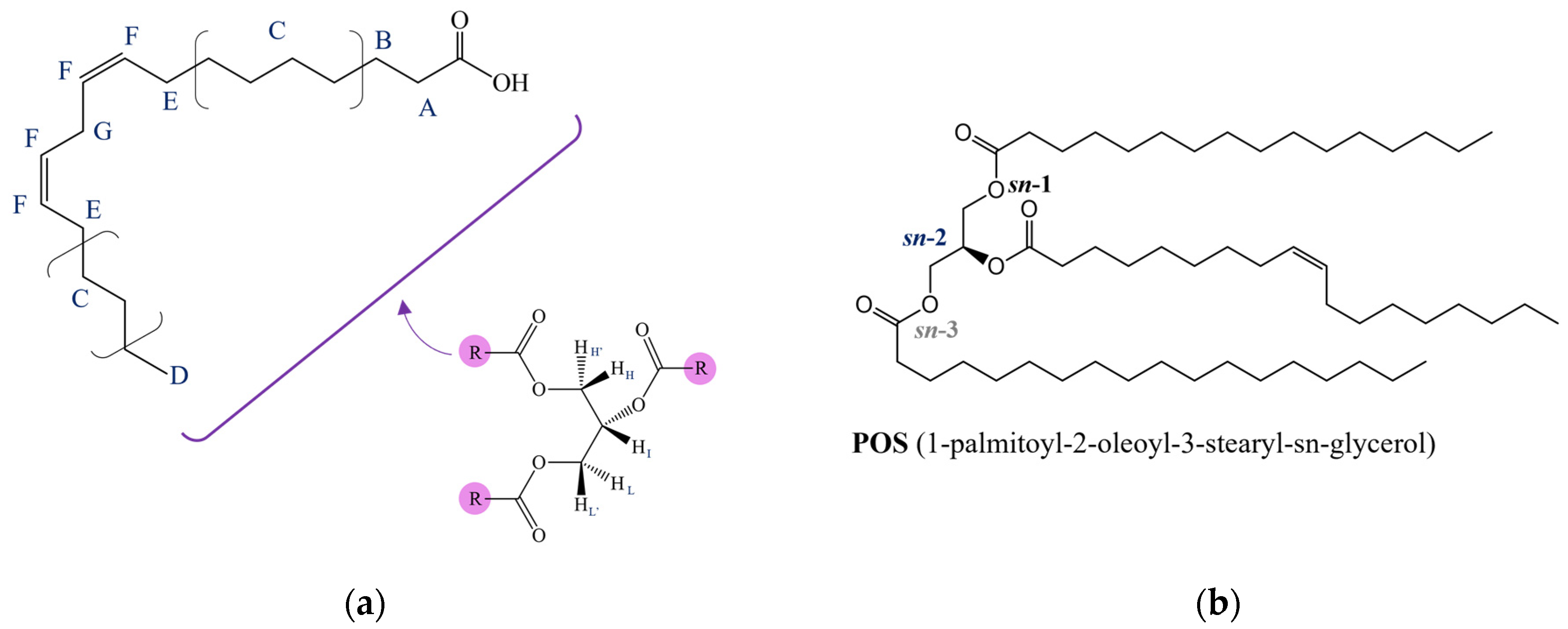
| Position | δH, Multiplicity 1 (J in Hz) | COSY |
|---|---|---|
| A | 2.20–2.43, m | B |
| B | 1.50–1.73, m | A, C |
| C | 1.15–1.45, m | B, D, E |
| D | 0.88, m | C |
| E | 1.93–2.15, m | C, F |
| F | 5.28–5.46, m (11.00) | E, G |
| G | 2.73–2.81, (t, 6.5) | F |
| H, L (Gly 1) | 4.14, dd (11.88, 5.93) | H′, L′, I |
| I (Gly 1) | 5.26, m | H, H′, L, L′ |
| H′, L′ (Gly 1) | 4.29, dd (11.88, 4.31) | H, L, I |
| Triglycerides | δ 13C ppm | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Palmitic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | ||||||
| A | B | A | B | A | B | A | B | ||
| C-1 | sn-1,3 | 173.28 | 173.27 | 173.28 | 173.27 | - | 173.23 | - | 173.23 |
| sn-2 | - | 172.86 | - | 172.86 | 172.84 | 172.82 | 172.84 | 172.82 | |
| C-2 | sn-1,3 | 34.08 | 34.08 | 34.08 | 34.08 | - | 34.05 | - | 34.05 |
| sn-2 | - | 34.24 | - | 34.24 | 34.22 | 34.22 | 34.22 | 34.22 | |
| C-3 | sn-1,3 | 24.89 | 24.89 | 24.89 | 24.89 | - | 24.88 | - | 24.88 |
| sn-2 | - | 24.94 | - | 24.94 | 24.91 | 24.91 | 24.87 | 24.87 | |
| C-4 | sn-1,3 | 29.16 | 29.16 | 29.16 | 29.16 | - | 28.12 | - | |
| sn-2 | - | 29.12 | - | 29.12 | 28.08 | 28.08 | - | ||
| C-5 | sn-1,3 | 29.30 | 29.36 | 29.36 | - | 29.22 | - | ||
| sn-2 | - | 29.33 | - | 29.36 (b 1) | 29.23 | 29.23 | - | ||
| C-6 | sn-1,3 | 29.51 | 29.51 | 29.51 | 29.51 | - | 29.14 | - | 29.14 |
| sn-2 | - | 29.53 | - | 29.53 | 29.15 | 29.15 | 29.15 | 29.15 | |
| C-7 | sn-1,3 | 29.65 | 29.65 | 29.65 | 29.65 | - | 29.75 (b 1) | - | 29.64 |
| sn-2 | - | 29.67 | - | 29.67 | 29.75 | 29.75 | - | ||
| C-8 | sn-1,3 | 29.68–29.73 | - | 27.20 | - | 27.22 | |||
| sn-2 | 27.20 | 27.22 | 27.22 | ||||||
| C-9 | sn-1,3 | 29.68–29.73 | - | 128.72 | - | 129.98 | |||
| sn-2 | 128.72 | 128.72 | - | ||||||
| C-10 | sn-1,3 | 29.68–29.73 | - | 130.02 | - | 128.08 | |||
| sn-2 | 130.03 | 130.03 | 128.11 | 128.11 | |||||
| C-11 | sn-1,3 | 29.68–29.73 | - | 27.25 | 22.66 | ||||
| sn-2 | 27.25 | 27.25 | - | ||||||
| C-12 | sn-1,3 | 29.68–29.73 | - | 29.80 | - | 127.93 | |||
| sn-2 | 29.80 | 29.80 | 127.91 | 127.91 | |||||
| C-13 | sn-1,3 | 29.40 | 29.40 | 29.68–29.73 | - | 29.35 | - | 130.21 | |
| sn-2 | 29.68–29.73 | 29.35 | 29.35 | 130.23 | 130.23 | ||||
| C-14 | sn-1,3 | 31.96 | 31.96 | 29.68–29.73 | - | 29.56 (b 1) | - | 27.23 | |
| sn-2 | - | 31.96 | - | 29.68–29.73 | 29.56 | 27.23 | |||
| C-15 | sn-1,3 | 22.72 | 22.72 | 29.40 | 29.40 | - | 29.35 | - | 29.37 |
| sn-2 | - | 22.72 | - | 29.40 | 29.35 | 29.35 | 29.37 | 29.37 | |
| C-16 | sn-1,3 | 14.13 | 14.13 | 31.96 | 31.96 | - | 31.94 | - | 31.55 |
| sn-2 | - | 14.13 | - | 31.96 | 31.94 | 31.55 | |||
| C-17 | sn-1,3 | 22.71 | 22.72 | - | 22.71 | - | 22.60 | ||
| sn-2 | - | 22.72 | 22.60 | 22.60 | - | ||||
| C-18 | sn-1,3 | 14.13 | 14.13 | - | 14.12 | - | 14.08 | ||
| sn-2 | - | 14.12 | 14.08 | 14.08 | - | - | |||
| -CHO (Gly 1) | sn-2 | - | 68.91 | - | 68.91 | 68.92 | |||
| -CH2O (Gly 1) | sn-1,3 | 62.11 | - | 62.12 | - | 62.12 | |||
| Unsaturated | Saturated | |||
|---|---|---|---|---|
| sn-1,3 | sn-2 | sn-1,3 | sn-2 | |
| Sample A | 32.7 0.3 | 67.3 0.2 | - | |
| Sample B | 15.2 0.4 | 17.7 0.4 | 30.2 0.2 | 0.2 |
| Sample C | 0.6 | 0.3 | 40.5 0.3 | 25.7 0.5 |
| Sample D | 21.4 0.3 | 10.8 0.5 | 20.2 0.6 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colella, M.F.; Marino, N.; Oliviero Rossi, C.; Seta, L.; Caputo, P.; De Luca, G. Triacylglycerol Composition and Chemical-Physical Properties of Cocoa Butter and Its Derivatives: NMR, DSC, X-ray, Rheological Investigation. Int. J. Mol. Sci. 2023, 24, 2090. https://doi.org/10.3390/ijms24032090
Colella MF, Marino N, Oliviero Rossi C, Seta L, Caputo P, De Luca G. Triacylglycerol Composition and Chemical-Physical Properties of Cocoa Butter and Its Derivatives: NMR, DSC, X-ray, Rheological Investigation. International Journal of Molecular Sciences. 2023; 24(3):2090. https://doi.org/10.3390/ijms24032090
Chicago/Turabian StyleColella, Maria Francesca, Nadia Marino, Cesare Oliviero Rossi, Lucia Seta, Paolino Caputo, and Giuseppina De Luca. 2023. "Triacylglycerol Composition and Chemical-Physical Properties of Cocoa Butter and Its Derivatives: NMR, DSC, X-ray, Rheological Investigation" International Journal of Molecular Sciences 24, no. 3: 2090. https://doi.org/10.3390/ijms24032090