Yet Another Similarity between Mitochondrial and Bacterial Ribosomal Small Subunit Biogenesis Obtained by Structural Characterization of RbfA from S. aureus
Abstract
:1. Introduction
2. Results and Discussion
2.1. General Features of RbfA Homologs and Their Interaction with the Small Ribosomal Subunit
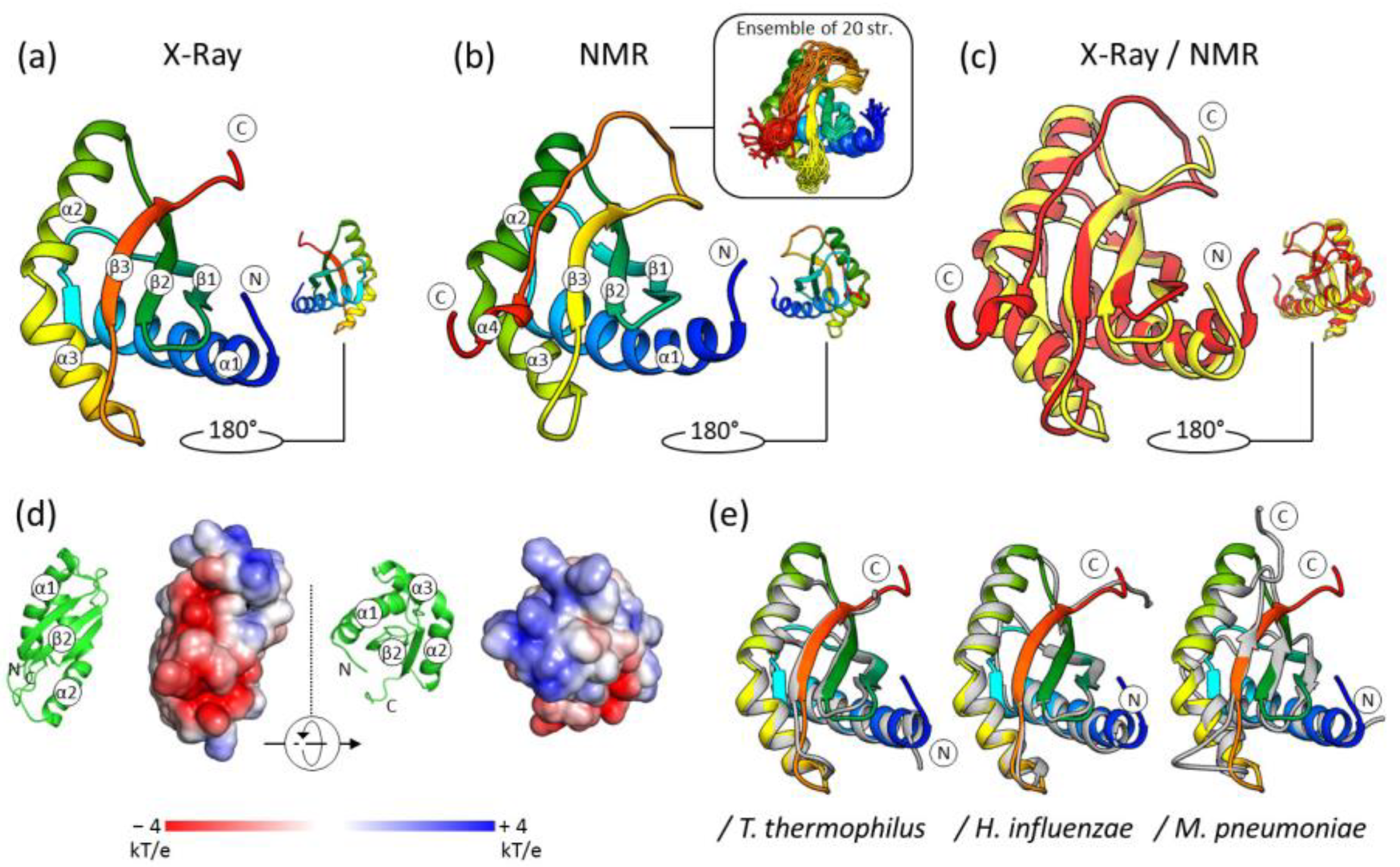
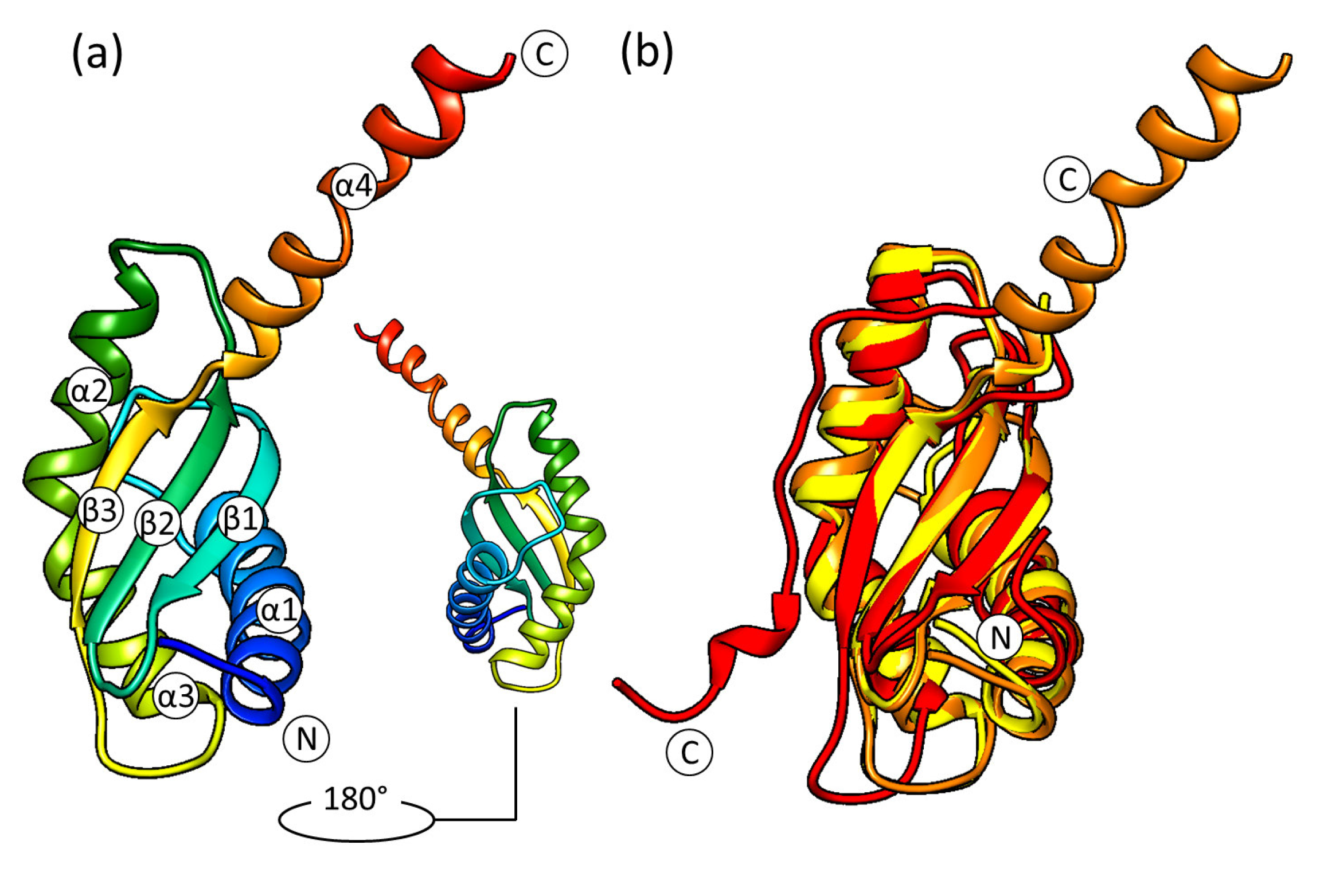
2.2. The Crystal Structure of S. aureus RbfA
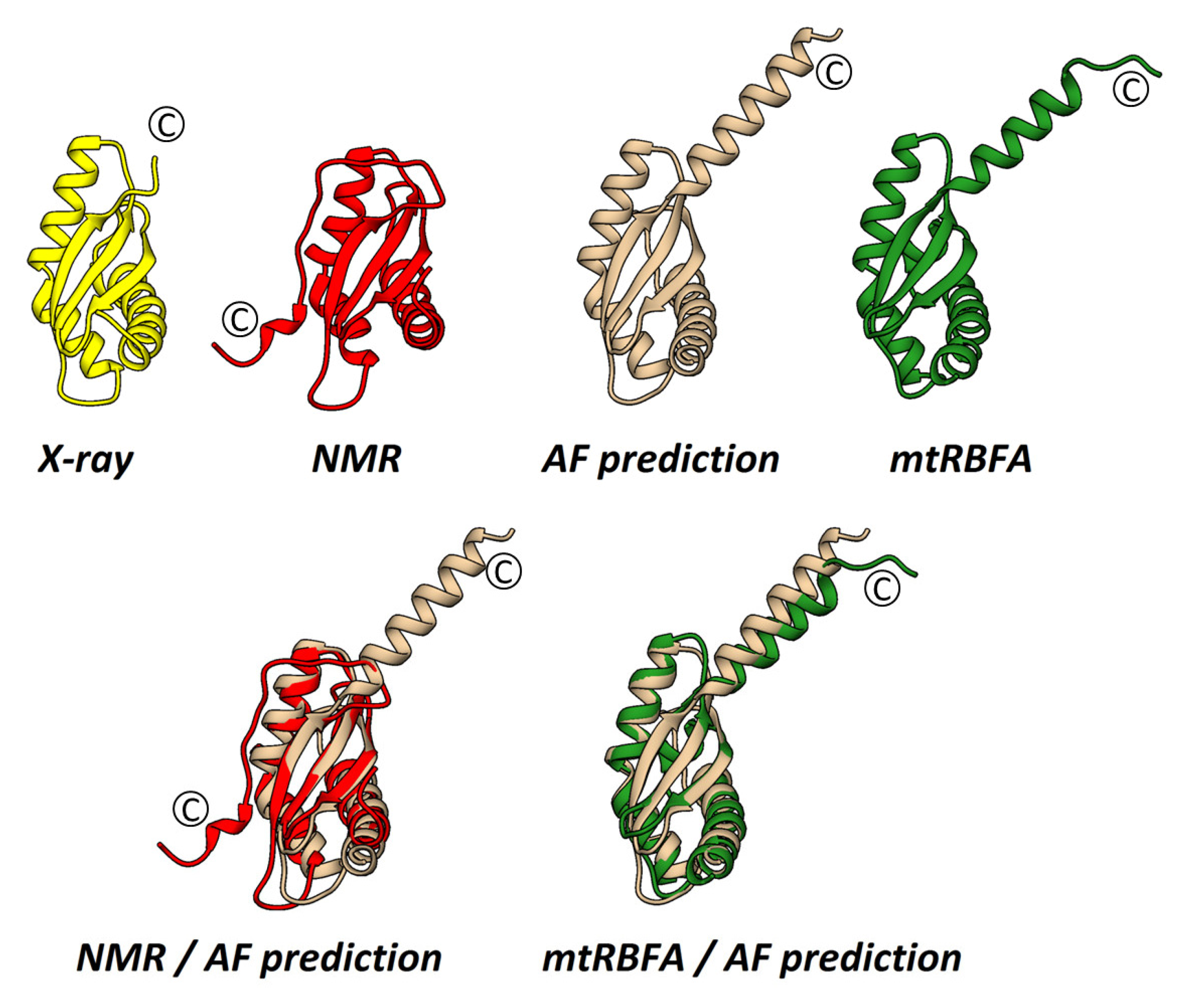
2.3. The Solution NMR Structure of S. aureus RbfA
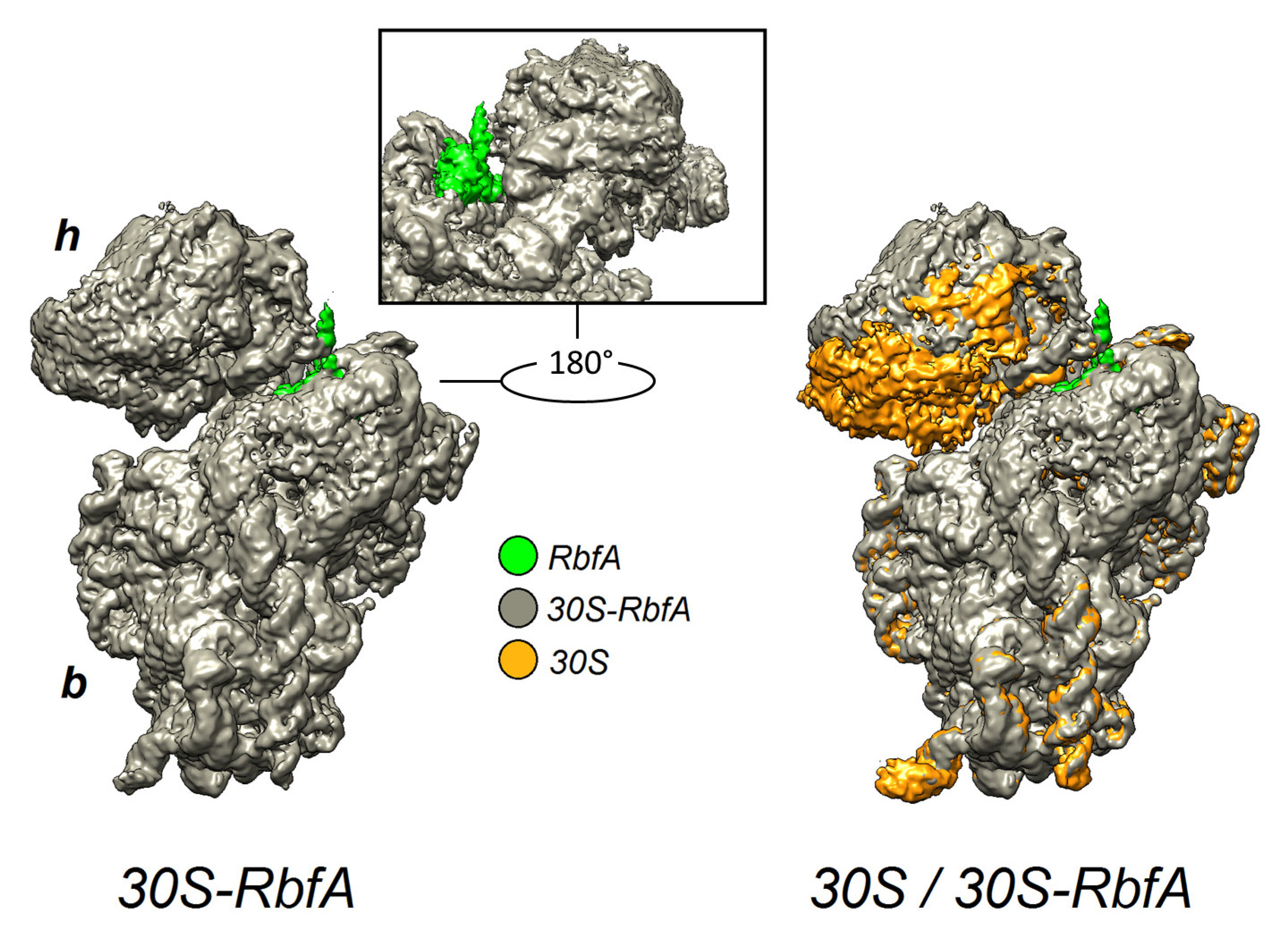
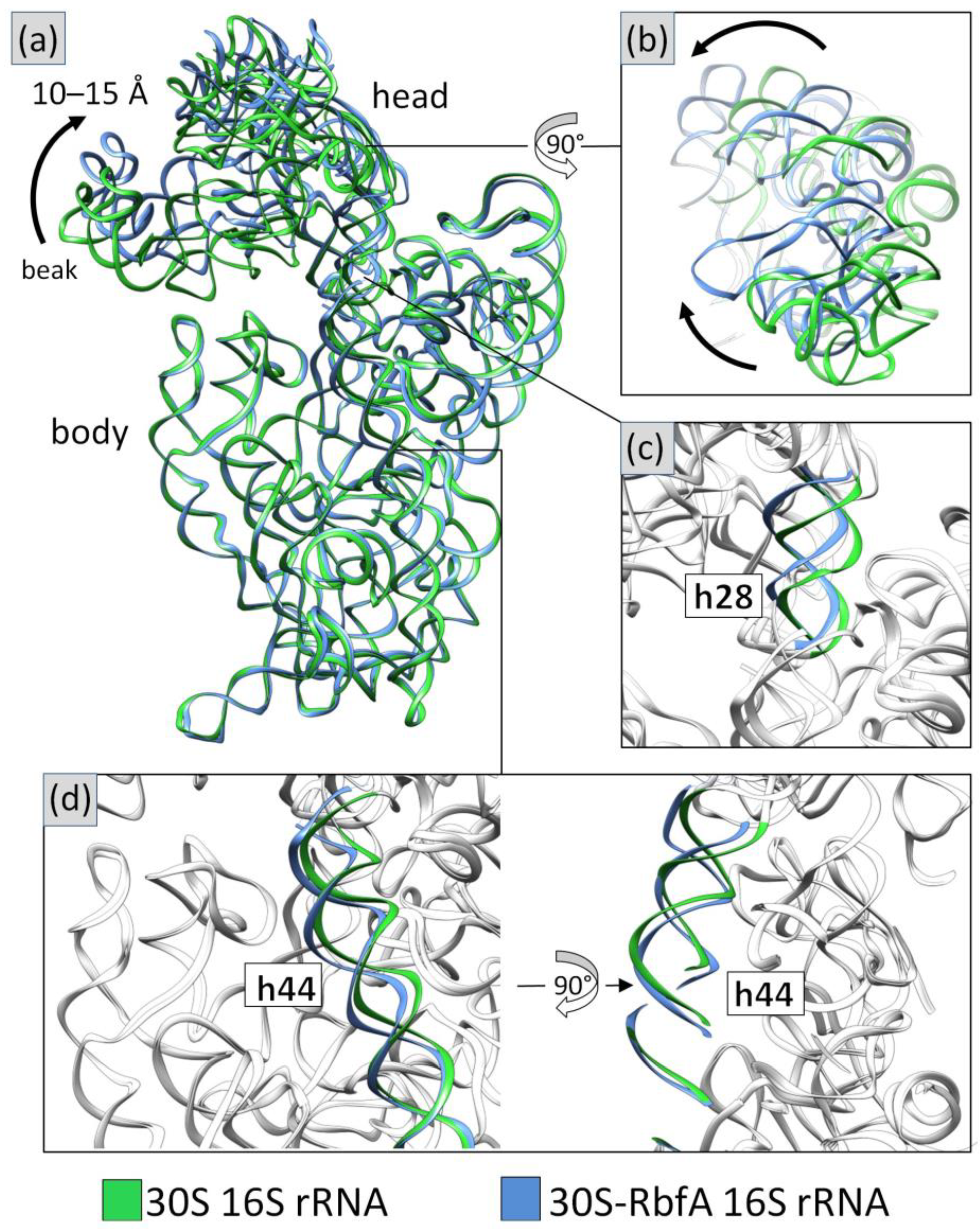
2.4. Cryo-EM Structure of the 30S–RbfA Complex from S. aureus
3. Materials and Methods
3.1. Expression and Purification of RbfA
3.2. Crystallization, Data Collection, and Structure Determination
3.3. NMR Characterization
3.4. Ribosome Purification and Dissociation
3.5. Cryo-EM Analysis
3.6. Structure Analysis and Visualization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crick, F. Central dogma of molecular biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Melnikov, S.; Ben-Shem, A.; Garreau de Loubresse, N.; Jenner, L.; Yusupova, G.; Yusupov, M. One core, two shells: Bacterial and eukaryotic ribosomes. Nat. Struct. Mol. Biol. 2012, 19, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Narla, A.; Ebert, B.L. Ribosomopathies: Human disorders of ribosome dysfunction. Blood 2010, 115, 3196–3205. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.N. Ribosome-targeting antibiotics and mechanisms of bacterial resistance. Nat. Rev. Microbiol. 2014, 12, 35–48. [Google Scholar] [CrossRef]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef]
- Nomura, M.; Traub, P.; Guthrie, C.; Nashimoto, H. Assembly of ribosomes. Cell. Physiol. 1969, 74, 241–251. [Google Scholar] [CrossRef]
- Lahry, K.; Gopal, A.; Sahu, A.K.; Marbaniang, C.N.; Shah, R.A.; Mehta, A.; Varshney, U. Alternative Role of RluD in the Fidelity of Translation Initiation in Escherichia coli. J. Mol. Biol. 2022, 434, 501–526. [Google Scholar] [CrossRef]
- Shajani, Z.; Sykes, M.T.; Williamson, J.R. Assembly of bacterial ribosomes. Annu. Rev. Biochem. 2011, 80, 501–526. [Google Scholar] [CrossRef] [Green Version]
- Tamaru, D.; Amikura, K.; Shimizu, Y.; Nierhaus, K.H.; Ueda, T. Reconstitution of 30S ribosomal subunits in vitro using ribosome biogenesis factors. RNA. 2018, 24, 1512–1519. [Google Scholar] [CrossRef] [Green Version]
- Kaczanowska, M.; Ryden-Aulin, M. Ribosome biogenesis and the translation process in Escherichia coli. Microbiol. Mol. Biol. Rev. 2007, 71, 477–494. [Google Scholar] [CrossRef]
- Woodson, S.A. RNA folding and ribosome assembly. Curr. Opin. Chem. Biol. 2008, 12, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Woodson, S.A. RNA folding pathways and the self-assembly of ribosomes. Acc. Chem. Res. 2011, 44, 1312–1319. [Google Scholar] [CrossRef] [Green Version]
- Donhofer, A.; Sharma, M.; Datta, P.; Nierhaus, K.; Agrawal, R.; Wilson, D. Factor-mediated ribosome assembly in bacteria. In Encyclopedia of Life Sciences; American Cancer Society: Atlanta, GA, USA, 2009. [Google Scholar] [CrossRef]
- Sergeeva, O.V.; Sergiev, A.A.; Bogdanov, A.A.; Dontsova, O.A. Ribosome: Lessons of a molecular factory construction. Mol. Biol. 2014, 28, 468–484. [Google Scholar] [CrossRef]
- Britton, R.A. Role of GTPases in bacterial ribosome assembly. Annu. Rev. Microbiol. 2009, 63, 155–176. [Google Scholar] [CrossRef]
- Usachev, K.S.; Yusupov, M.M.; Validov, S.Z. Hibernation as a stage of ribosome functioning. Biochemistry 2020, 85, 1434–1442. [Google Scholar] [CrossRef]
- Rubin, S.M.; Pelton, J.G.; Yokota, H.; Kim, R.; Wemmer, D.E. Solution structure of a putative ribosome binding protein from Mycoplasma pneumoniae and comparison to a distant homolog. J. Struct. Funct. Genom. 2003, 4, 235–243. [Google Scholar] [CrossRef]
- Rozanska, X.A.; Richter-Dennerlein, R.; Rorbach, J.; Gao, F.; Lewis, R.J.; Chrzanowska-Lightowlers, Z.M.; Lightowlers, R.N. The human rna-binding protein rbfa promotes the maturation of the mitochondrial ribosome. Biochem J. 2017, 474, 2145–2158. [Google Scholar] [CrossRef] [Green Version]
- Fristedt, R.; Scharff, L.B.; Clarke, C.A.; Wang, Q.; Lin, C.; Merchant, S.S.; Bock, R. RBF1, a plant homolog of the bacterial ribosome-binding factor RbfA, acts in processing of the chloroplast 16S ribosomal RNA. Plant Physiol. 2014, 164, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.Y.; Huang, Y.J.; Swapna, G.V.T.; Rajan, P.K.; Ke, H.; Xia, B.; Shukla, K.; Inouye, M.; Montelione, G.T. Solution NMR structure of ribosome-Binding factor A (RbfA), a cold-shock adaptation protein from Escherichia coli. J. Mol. Biol. 2003, 327, 521–536. [Google Scholar] [CrossRef]
- Dammel, C.S.; Noller, H.F. Suppression of a cold-sensitive mutation in 16S rRNA by overexpression of a novel ribosome-binding factor, RbfA. Genes Dev. 1995, 9, 626–637. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.G.; Inouye, M. RbfA, a 30S ribosomal binding factor, is a coldshock protein whose absence triggers the cold-shock response. Mol. Microbiol. 1996, 21, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Ke, H.; Shinde, U.; Inouye, M. The role of RbfA in 16S rRNA processing and cell growth at low temperature in Escherichia coli. J. Mol Biol. 2003, 332, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Datta, P.P.; Wilson, D.N.; Kawazoe, M.; Swami, N.K.; Kaminishi, T.; Sharma, M.R.; Booth, T.M.; Takemoto, C.; Fucini, P.; Yokoyama, S.; et al. Structural aspects of RbfA action during small ribosomal subunit assembly. Mol. Cell. 2007, 28, 434–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siomi, H.; Matunis, M.J.; Michael, W.M.; Dreyfuss, G. The pre-mRNA binding K protein contains a novel evolutionarily conserved motif. Nucleic Acids Res. 1993, 21, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Grishin, N.V. KH domain: One motif, two folds. Nucleic Acid Res. 2001, 29, 638–643. [Google Scholar] [CrossRef]
- Valverde, R.; Edwards, L.; Regan, L. Structure and function of KH domains. FEBS J. 2008, 275, 2712–2726. [Google Scholar] [CrossRef]
- Itoh, Y.; Khawaja, A.; Laptev, I.; Cipullo, M.; Atanassov, I.; Sergiev, P.; Rorbach, J.; Amunts, A. Mechanism of mitoribosomal small subunit biogenesis and preinitiation. Nature 2022, 606, 603–608. [Google Scholar] [CrossRef]
- Schedlbauer, A.; Iturrioz, I.; Ochoa-Lizarralde, B.; Diercks, T.; Lopez-Alonso, J.P.; Lavin, J.L.; Kaminishia, T.; Capunia, R.; Dhimole, N.; de Astigarraga, E.; et al. A conserved rRNA switch is central to decoding site maturation on the small ribosomal subunit. Sci. Adv. 2021, 7, eabf7547. [Google Scholar] [CrossRef]
- Maksimova, E.M.; Korepanov, A.P.; Kravchenko, O.V.; Baymukhametov, T.N.; Myasnikov, A.G.; Vassilenko, K.S.; Afonina, Z.A.; Stolboushkina, E.A. RbfA Is Involved in Two Important Stages of 30S Subunit Assembly: Formation of the Central Pseudoknot and Docking of Helix 44 to the Decoding Center. Int. J. Mol. Sci. 2021, 22, 6140. [Google Scholar] [CrossRef]
- Sharma, I.M.; Woodson, S.A. RbfA and IF3 couple ribosome biogenesis and translation initiation to increase stress tolerance. Nucleic Acids Res. 2020, 48, 359–372. [Google Scholar] [CrossRef]
- Blokhin, D.S.; Bikmullin, A.G.; Nurullina, L.I.; Garaeva, N.S.; Validov, S.Z.; Klochkov, V.V.; Aganov, A.V.; Khusainov, I.S.; Yusupov, M.M.; Usachev, K.S. Backbone and side chain NMR assignments for the ribosome binding factor A (RbfA) from Staphylococcus aureus. Biomol. NMR Assign. 2019, 13, 27–30. [Google Scholar] [CrossRef]
- Lovell, S.C.; Davis, I.W.; Arendall, W.B., III; de Bakker, P.I.W.; Word, Y.M.; Prisant, V.G.; Richardson, J.S.; Richardson, D.C. Structure validation by Cα geometry: ϕ, ψ and Cβ deviation. Proteins 2003, 50, 437–450. [Google Scholar] [CrossRef]
- Kabsch, W.; Sander, C. Dictionary of protein secondary structure: Pattern recognition of hydrogen-bonded and geometrical features. Biopolymers 1983, 22, 2577–2637. [Google Scholar] [CrossRef]
- Belinite, M.; Khusainov, I.; Soufari, H.; Marzi, S.; Romby, R.; Yusupov, M.; Hashem, Y. Stabilization of Ribosomal RNA of the Small Subunit by Spermidine in Staphylococcus aureus. Front. Mol. Biosci. 2021, 8, 738752. [Google Scholar] [CrossRef]
- Khusainov, I.; Vicens, Q.; Ayupov, R.; Usachev, K.; Myasnikov, A.; Simonetti, A.; Validov, S.; Kieffer, B.; Yusupova, G.; Yusupov, M.; et al. Structures and dynamics of hibernating ribosomes from Staphylococcus aureus mediated by intermolecular interactions of HPF. EMBO J. 2017, 36, 2073–2087. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Afonine, P.V.; Poon, B.K.; Read, R.J.; Sobolev, O.V.; Terwilliger, T.C.; Urzhumtsev, A.; Adams, P.D. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. Sect. D Struct. Biol. 2018, 74, 531–544. [Google Scholar] [CrossRef] [Green Version]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Culver, G.M. Assembly of the 30S ribosomal subunit. Biopolymers 2003, 68, 234–249. [Google Scholar] [CrossRef]
- Delvillani, F.; Papiani, G.; Deho, G.; Briani, F. S1 ribosomal protein and the interplay between translation and mRNA decay. Nucleic Acids Res. 2011, 39, 7702–7715. [Google Scholar] [CrossRef] [Green Version]
- Saguy, M.; Gilet, R.; Skorski, P.; Hermann-Le Denmat, S.; Felden, B. Ribosomal protein S1 influences trans-translation in vitro and in vivo. Nucleic Acids Res. 2007, 35, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2021, 50, D439–D444. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. XDS program package. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Schrodinger, L.L.C. The PyMOL Molecular Graphics System. Version 1.8; PyMOL: Mannheim, Germany, 2015. [Google Scholar]
- Golovanov, A.P.; Hautbergue, G.M.; Wilson, S.A.; Lian, L.Y. A simple method for improving protein solubility and long-term stability. J. Am. Chem. Soc. 2004, 126, 8933–8939. [Google Scholar] [CrossRef]
- Sattler, M.; Schleucher, J.; Griesinger, C. Heteronuclear multidimensional NMR experiments for the structure determination of proteins in solution employing pulsed field gradients. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 34, 93–158. [Google Scholar] [CrossRef]
- Shen, Y.; Delaglio, F.; Cornilescu, G.; Bax, A. TALOS+: A hybrid method for predicting protein backbone torsion angles from NMR chemical shifts. J. Biomol. NMR. 2009, 44, 213–223. [Google Scholar] [CrossRef]
- Bardiaux, B.; Malliavin, T.; Nilges, M. ARIA for solution and solid-state NMR. Methods Mol. Biol. 2012, 831, 453–483. [Google Scholar] [CrossRef]
- Laskowski, R.A.; Rullmannn, J.A.; MacArthur, M.W.; Kaptein, R.; Thornton, J.M. AQUA and PROCHECK-NMR: Programs for checking the quality of protein structures solved by NMR. J. Biomol. NMR 1996, 8, 477–486. [Google Scholar] [CrossRef]
- Vranken, W.F.; Boucher, W.; Stevens, T.J.; Fogh, R.H.; Pajon, A.; Llinas, M.; Ulrich, E.L.; Markley, J.L.; Ionides, J.; Laue, E.D. The CCPN data model for NMR spectroscopy: Development of a software pipeline. Proteins 2005, 59, 687–696. [Google Scholar] [CrossRef]
- Belinite, M.; Khusainov, I.; Marzi, S. Staphylococcus aureus 30S ribosomal subunit purification and its biochemical and cryo-EM analysis. Bio-Protoc. J. 2022, 12, 20. [Google Scholar] [CrossRef]
- Khusainov, I.; Vicens, Q.; Bochler, A.; Grosse, F.; Myasnikov, A.; Ménétret, J.F.; Chicher, J.; Marzi, S.; Romby, P.; Yusupova, G.; et al. Structure of the 70S ribosome from human pathogen Staphylococcus aureus. Nucleic Acids Res. 2016, 44, 10491–10504. [Google Scholar] [CrossRef] [Green Version]
- Bikmullin, A.G.; Nurullina, L.I.; Garaeva, N.S.; Klochkova, E.A.; Blokhin, D.S.; Golubev, A.A.; Validov, S.Z.; Khusainov, I.S.; Usachev, K.S.; Yusupov, M.M. In vitro reconstitution of the S. aureus 30S ribosomal subunit and RbfA factor complex for structural studies. Biochemistry 2020, 85, 545–552. [Google Scholar] [CrossRef]
- Punjani, A.; Rubinstein, J.L.; Fleet, D.J.; Brubaker, M.A. cryoSPARC: Algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 2017, 14, 290–296. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Coudert, E.; Gehant, S.; de Castro, E.; Pozzato, M.; Baratin, D.; Neto, T.; Sigrist, C.J.A.; Redaschi, N.; Bridge, A. UniProt Consortium. Annotation of biologically relevant ligands in UniProtKB using ChEBI. Bioinformatics 2023, 39, btac793. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikmullin, A.G.; Fatkhullin, B.; Stetsenko, A.; Gabdulkhakov, A.; Garaeva, N.; Nurullina, L.; Klochkova, E.; Golubev, A.; Khusainov, I.; Trachtmann, N.; et al. Yet Another Similarity between Mitochondrial and Bacterial Ribosomal Small Subunit Biogenesis Obtained by Structural Characterization of RbfA from S. aureus. Int. J. Mol. Sci. 2023, 24, 2118. https://doi.org/10.3390/ijms24032118
Bikmullin AG, Fatkhullin B, Stetsenko A, Gabdulkhakov A, Garaeva N, Nurullina L, Klochkova E, Golubev A, Khusainov I, Trachtmann N, et al. Yet Another Similarity between Mitochondrial and Bacterial Ribosomal Small Subunit Biogenesis Obtained by Structural Characterization of RbfA from S. aureus. International Journal of Molecular Sciences. 2023; 24(3):2118. https://doi.org/10.3390/ijms24032118
Chicago/Turabian StyleBikmullin, Aydar G., Bulat Fatkhullin, Artem Stetsenko, Azat Gabdulkhakov, Natalia Garaeva, Liliia Nurullina, Evelina Klochkova, Alexander Golubev, Iskander Khusainov, Natalie Trachtmann, and et al. 2023. "Yet Another Similarity between Mitochondrial and Bacterial Ribosomal Small Subunit Biogenesis Obtained by Structural Characterization of RbfA from S. aureus" International Journal of Molecular Sciences 24, no. 3: 2118. https://doi.org/10.3390/ijms24032118




