Sustainable Vegetable Oil-Based Biomaterials: Synthesis and Biomedical Applications
Abstract
1. Introduction
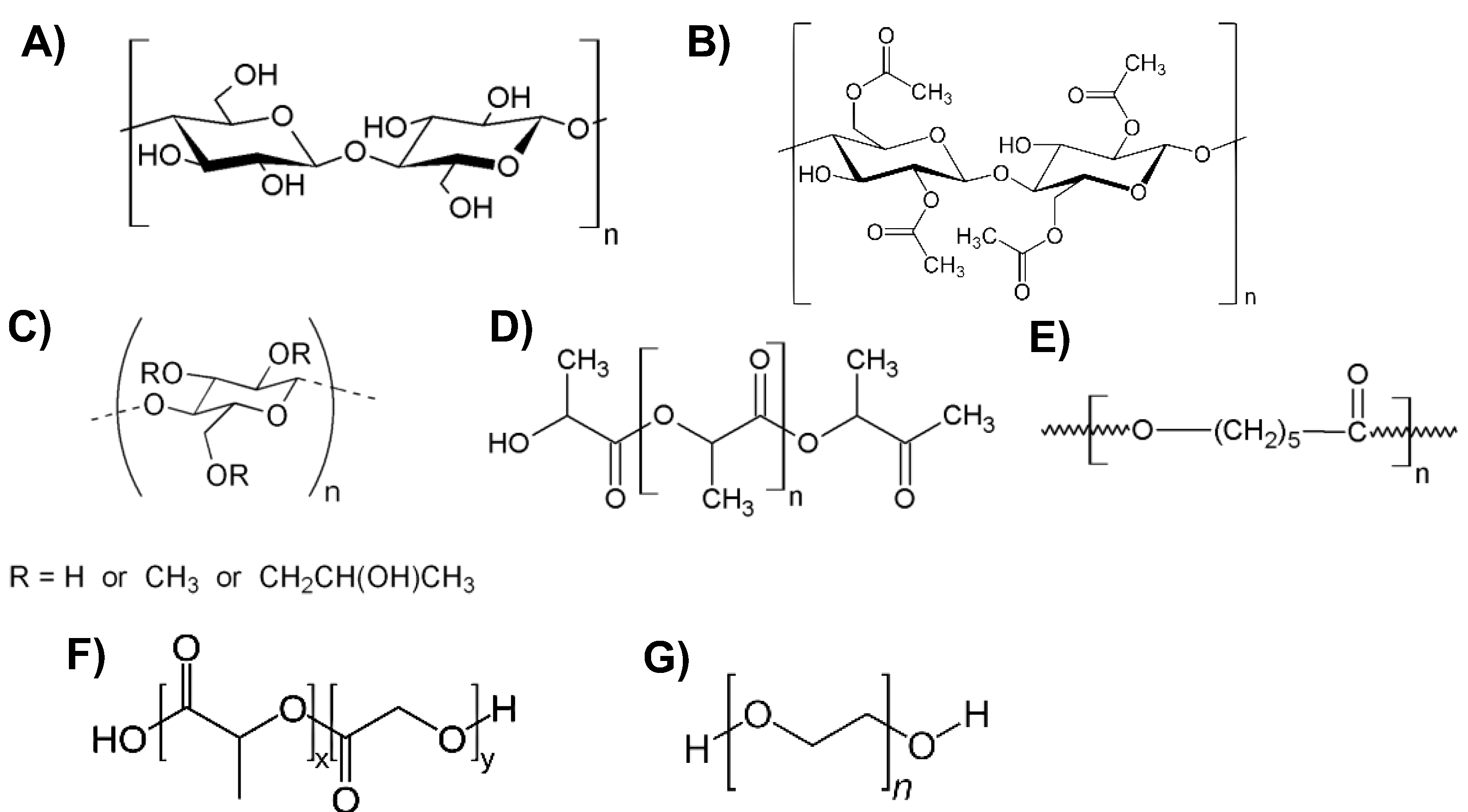
2. Biopolymers Based on Vegetable Oils
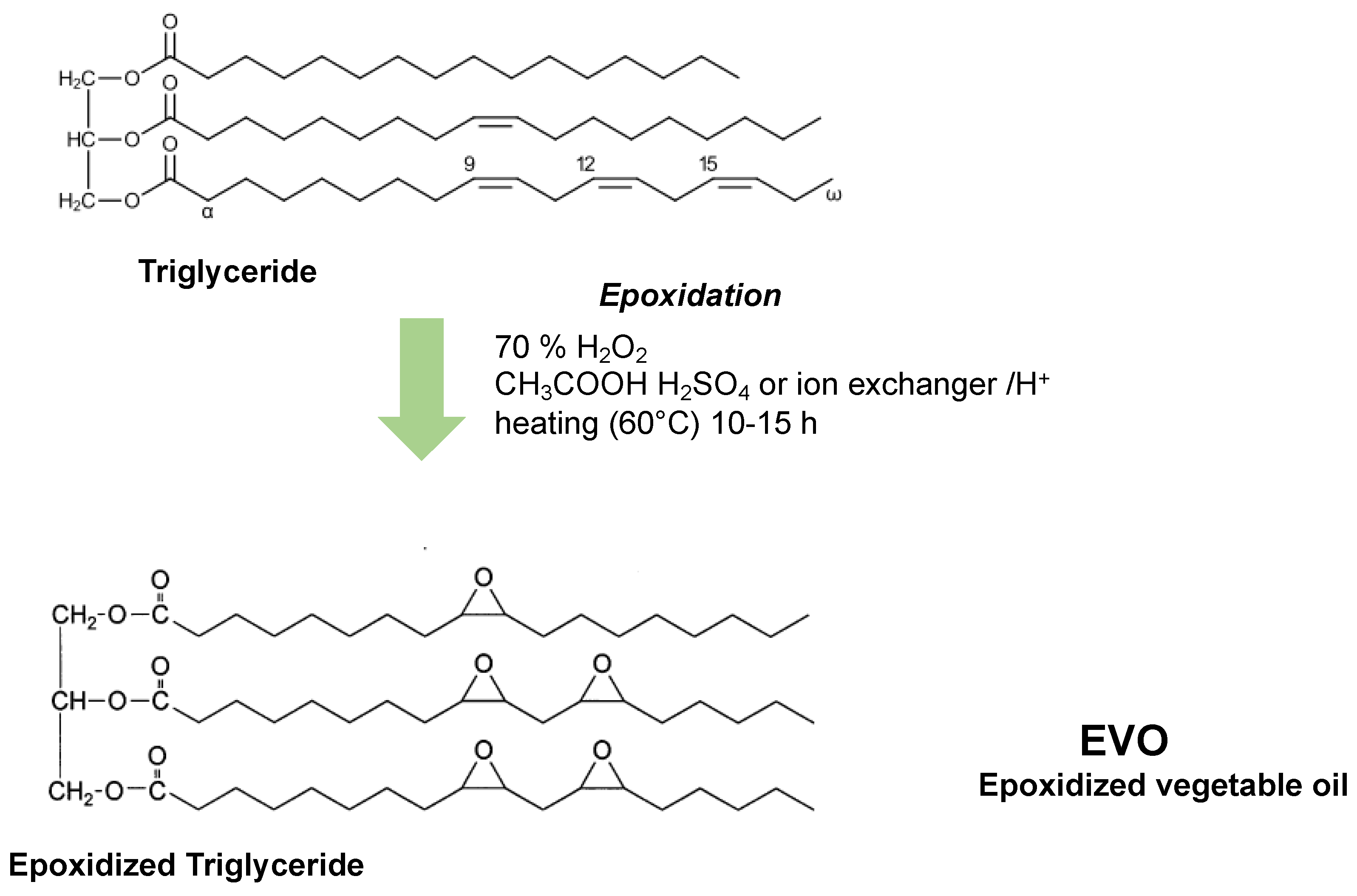
2.1. Synthesis of Vegetable Oil-Based Polymers
2.2. Synthesis Processes of Polymeric Biomaterials Based on Vegetable Oils
2.3. Cationic Photopolymerization
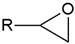
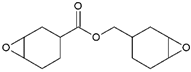
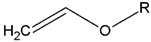
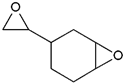
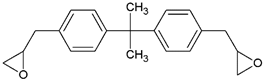
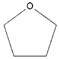
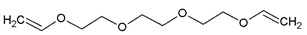
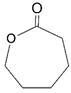
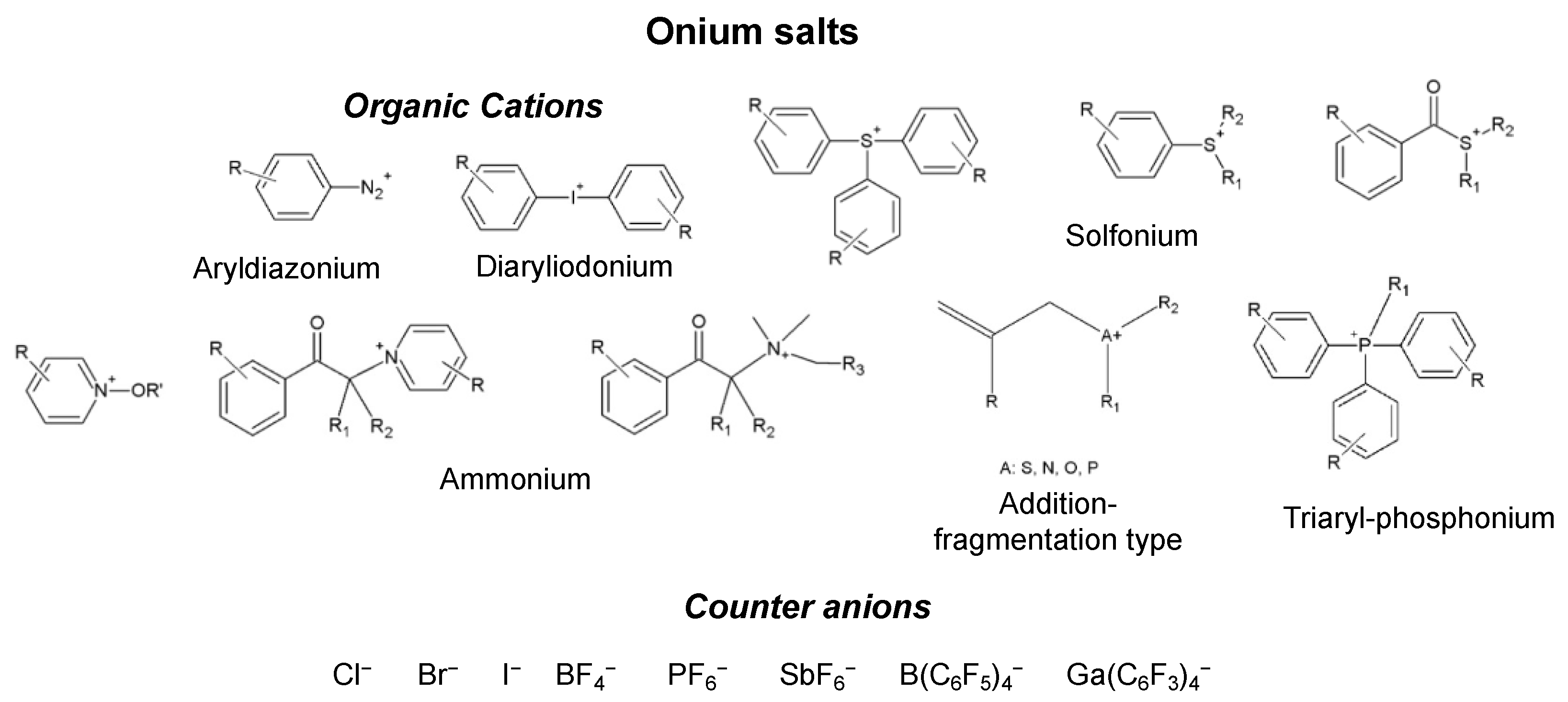
- Monomers or oligomers, the polymer precursors, which in the cationic photopolymerization are monomers that cannot be polymerized by radical mechanisms, such as epoxides, vinyl ethers and many heterocyclic compounds [66]. The monomer can contain one or more reactive groups, and this allows either linear polymers or a three-dimensional network to be obtained. Some of the most commonly used monomers in cationic polymerizations are summarized in Table 1.
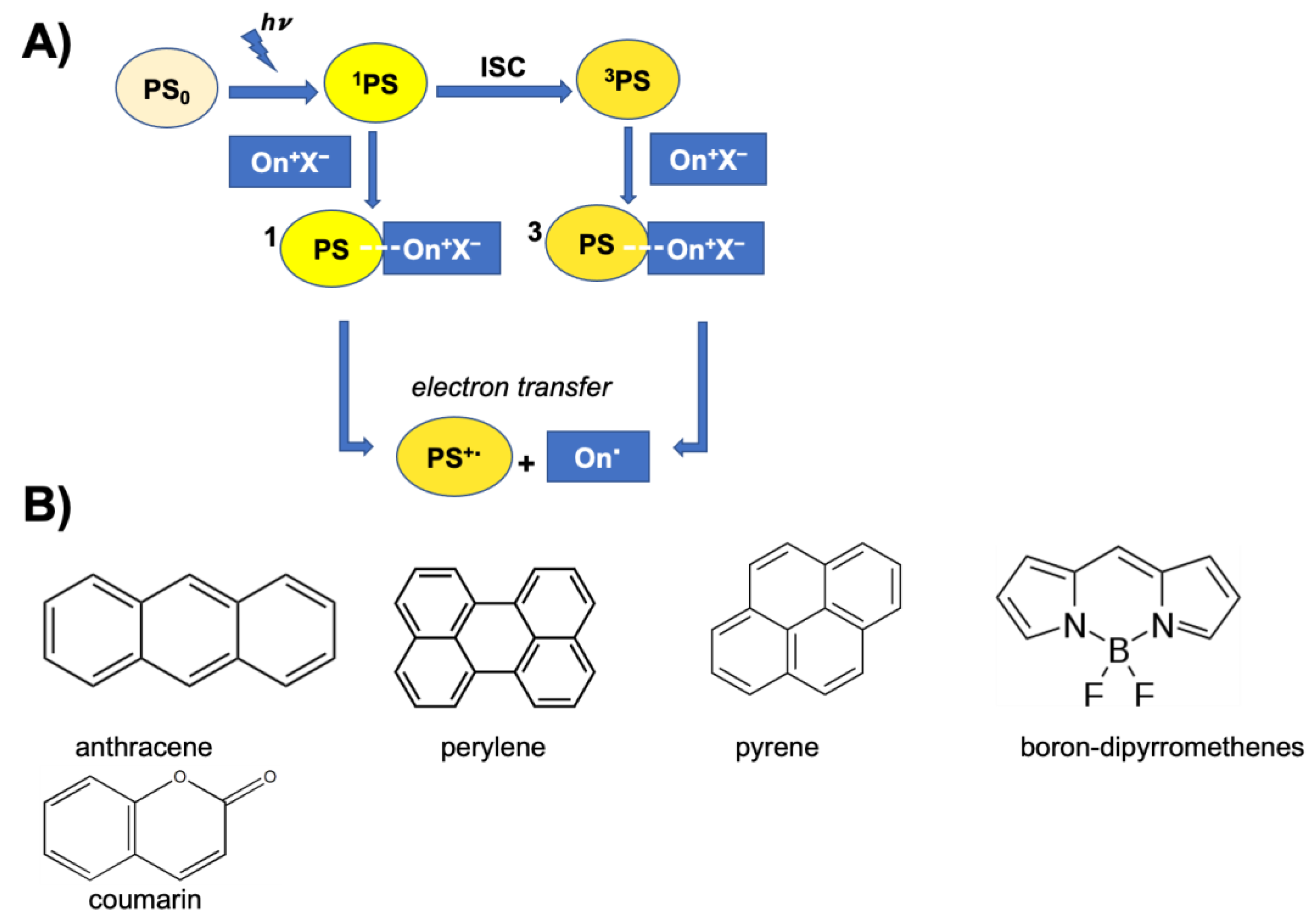
- Photoinitiator (PhI), which is a molecule able to form reactive species (radical or ionic), which initiate the polymerization reaction. These molecules are photosensitive molecules that absorb the light radiation and pass into an excited electronic state in which the reactive species is generated by homolytic or heterolytic cleavage of a bond. Its presence is essential when the monomers used are unable to absorb the radiation and to initiate the polymerization reaction autonomously. To properly choose the photoinitiator, it is important to consider that these molecules have a very selective light absorption and it is, therefore, necessary to use a molecule which shows high absorption capabilities in the emission range of the lamp chosen as the light source. In photochemical reactions, quantum yield is defined as the number of molecules reacted per quantum of the absorbed light. The quantum yield of the initiating species (Φi) correlates with photophysical deactivation of the excited states of PhI mainly by fluorescence and phosphorescence. Low quantum yields of fluorescence or phosphorescence of PhI lead to higher Φi, which is found to be proportional to the initiation rate of cationic photopolymerization [62]. In the cationic photopolymerization mechanism the photolysis step is dependent on the quantum yield of the PhI as well as on the intensity and wavelength of the light used [67].
3. Functionalization and Biomedical Applications of Vegetable Oil-Based Biomaterials
3.1. Functionalization of Vegetable Oil-Based Biomaterials
3.2. Vegetable Oil-Based Biomaterials as Scaffolds for Stem Cell Growth
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. P T Peer-Rev. J. Formul. Manag. 2014, 39, 704–711. [Google Scholar]
- Yang, E.; Miao, S.; Zhong, J.; Zhang, Z.; Mills, D.K.; Zhang, L.G. Bio-Based Polymers for 3D Printing of Bioscaffolds. Polym. Rev. 2018, 58, 668–687. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Powell, L.C.; Chinga-Carrasco, G.; Gethin, D.T.; Syverud, K.; Hill, K.E.; Thomas, D.W. 3D Bioprinting of Carboxymethylated-Periodate Oxidized Nanocellulose Constructs for Wound Dressing Applications. BioMed Res. Int. 2015, 2015, 925757. [Google Scholar] [CrossRef] [PubMed]
- Okano, K.; Zhang, Q.; Shinkawa, S.; Yoshida, S.; Tanaka, T.; Fukuda, H.; Kondo, A. Efficient Production of Optically Pure d -Lactic Acid from Raw Corn Starch by Using a Genetically Modified l -Lactate Dehydrogenase Gene-Deficient and α-Amylase-Secreting Lactobacillus plantarum Strain. Appl. Environ. Microbiol. 2009, 75, 462–467. [Google Scholar] [CrossRef]
- Okano, K.; Uematsu, G.; Hama, S.; Tanaka, T.; Noda, H.; Kondo, A.; Honda, K. Metabolic Engineering of Lactobacillus plantarum for Direct L-Lactic Acid Production From Raw Corn Starch. Biotechnol. J. 2018, 13, e1700517. [Google Scholar] [CrossRef]
- Okano, K.; Zhang, Q.; Yoshida, S.; Tanaka, T.; Ogino, C.; Fukuda, H.; Kondo, A. d-lactic acid production from cellooligosaccharides and β-glucan using l-LDH gene-deficient and endoglucanase-secreting Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 2009, 85, 643–650. [Google Scholar] [CrossRef]
- Tateno, T.; Fukuda, H.; Kondo, A. Direct production of L-lysine from raw corn starch by Corynebacterium glutamicum secreting Streptococcus bovis α-amylase using cspB promoter and signal sequence. Appl. Microbiol. Biotechnol. 2007, 77, 533–541. [Google Scholar] [CrossRef]
- Aso, Y.; Hashimoto, A.; Ohara, H. Engineering Lactococcus lactis for D-Lactic Acid Production from Starch. Curr. Microbiol. 2019, 76, 1186–1192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Vadlani, P.V.; Kumar, A.; Hardwidge, P.R.; Govind, R.; Tanaka, T.; Kondo, A. Enhanced D-lactic acid production from renewable resources using engineered Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 2015, 100, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Vanaei, S.; Parizi, M.S.; Vanaei, S.; Salemizadehparizi, F.; Vanaei, H.R. An Overview on Materials and Techniques in 3D Bioprinting Toward Biomedical Application. Eng. Regen. 2021, 2, 1–18. [Google Scholar] [CrossRef]
- Lin, L.; Fang, Y.; Liao, Y.; Chen, G.; Gao, C.; Zhu, P. 3D Printing and Digital Processing Techniques in Dentistry: A Review of Literature. Adv. Eng. Mater. 2019, 21, 1801013. [Google Scholar] [CrossRef]
- Rosenzweig, D.H.; Carelli, E.; Steffen, T.; Jarzem, P.; Haglund, L. 3D-Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int. J. Mol. Sci. 2015, 16, 15118–15135. [Google Scholar] [CrossRef] [PubMed]
- Serra, T.; Planell, J.A.; Navarro, M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013, 9, 5521–5530. [Google Scholar] [CrossRef] [PubMed]
- Gregor, A.; Filová, E.; Novák, M.; Kronek, J.; Chlup, H.; Buzgo, M.; Blahnová, V.; Lukášová, V.; Bartoš, M.; Nečas, A.; et al. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J. Biol. Eng. 2017, 11, 31. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Almeida, C.R.; Serra, T.; Oliveira, M.I.; Planell, J.A.; Barbosa, M.A.; Navarro, M. Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-D structures on inflammation. Acta Biomater. 2014, 10, 613–622. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2019, 10, 381–388. [Google Scholar] [CrossRef]
- Gonçalves, E.M.; Oliveira, F.J.; Silva, R.F.; Neto, M.A.; Fernandes, M.H.; Amaral, M.; Vallet-Regí, M.; Vila, M. Three-dimensional printed PCL -hydroxyapatite scaffolds filled with CNT s for bone cell growth stimulation. J. Biomed. Mater. Res. B Appl. Biomater. 2015, 104, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, P.P.; Flôres, S.H.; Rios, A.D.O.; Chisté, R.C. Biodegradable polymers as wall materials to the synthesis of bioactive compound nanocapsules. Trends Food Sci. Technol. 2016, 53, 23–33. [Google Scholar] [CrossRef]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef]
- Engebretson, B.; Sikavitsas, V.I. Long-Term In Vivo Effect of Peg Bone Tissue Engineering Scaffolds. J. Autom. Inf. Sci. 2012, 22, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.W.; Singh, N.; Burns, K.L.; Babensee, J.E.; Lyon, L.A.; García, A.J. Reduced acute inflammatory responses to microgel conformal coatings. Biomaterials 2008, 29, 4605–4615. [Google Scholar] [CrossRef]
- Hutanu, D.; Frishberg, M.D.; Guo, L.; Darie, C.C. Recent Applications of Polyethylene Glycols (PEGs) and PEG Derivatives. Mod. Chem. Appl. 2014, 2, 1–6. [Google Scholar] [CrossRef]
- Rafat, M.; Li, F.; Fagerholm, P.; Lagali, N.S.; Watsky, M.A.; Munger, R.; Matsuura, T.; Griffith, M. PEG-stabilized carbodiimide crosslinked collagen–chitosan hydrogels for corneal tissue engineering. Biomaterials 2008, 29, 3960–3972. [Google Scholar] [CrossRef] [PubMed]
- Kraehenbuehl, T.P.; Zammaretti, P.; Van der Vlies, A.J.; Schoenmakers, R.G.; Lutolf, M.P.; Jaconi, M.E.; Hubbell, J.A. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: Systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials 2008, 29, 2757–2766. [Google Scholar] [CrossRef]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef]
- Mauretti, A.; Neri, A.; Kossover, O.; Seliktar, D.; Di Nardo, P.; Melino, S. Design of a Novel Composite H2 S-Releasing Hydrogel for Cardiac Tissue Repair. Macromol Biosci 2016, 16, 847–858. [Google Scholar] [CrossRef]
- Ciocci, M.; Cacciotti, I.; Seliktar, D.; Melino, S. Injectable silk fibroin hydrogels functionalized with microspheres as adult stem cells-carrier systems. Int. J. Biol. Macromol. 2018, 108, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Buonvino, S.; Ciocci, M.; Seliktar, D.; Melino, S. Photo-Polymerization Damage Protection by Hydrogen Sulfide Donors for 3D-Cell Culture Systems Optimization. Int. J. Mol. Sci. 2021, 22, 6095. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Schuman, T.P. Vegetable oil-derived epoxy monomers and polymer blends: A comparative study with review. Express Polym. Lett. 2013, 7, 272–292. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Silva, S.S.; Reis, R.L. Challenges and opportunities on vegetable oils derived systems for biomedical applications. Biomater. Adv. 2022, 134, 112720. [Google Scholar] [CrossRef] [PubMed]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Adekunle, K. A Review of Vegetable Oil-Based Polymers: Synthesis and Applications. Open J. Polym. Chem. 2015, 5, 34–40. [Google Scholar] [CrossRef]
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of vegetable-oil-based polymers. J. Appl. Polym. Sci. 2014, 131, 40787. [Google Scholar] [CrossRef]
- Chakraborty, I.; Chatterjee, K. Polymers and Composites Derived from Castor Oil as Sustainable Materials and Degradable Biomaterials: Current Status and Emerging Trends. Biomacromolecules 2020, 21, 4639–4662. [Google Scholar] [CrossRef] [PubMed]
- Karak, N. 2-Biodegradable Polymers. In Vegetable Oil-Based Polymers; Karak, N., Ed.; Woodhead Publishing: Sawston, UK, 2012; pp. 31–53. ISBN 978-0-85709-710-1. [Google Scholar]
- Chong, K.L.; Lai, J.C.; Rahman, R.A.; Adrus, N.; Al-Saffar, Z.H.; Hassan, A.; Lim, T.H.; Wahit, M.U. A review on recent approaches to sustainable bio-based epoxy vitrimer from epoxidized vegetable oils. Ind. Crop. Prod. 2022, 189, 115857. [Google Scholar] [CrossRef]
- Di Mauro, C.; Malburet, S.; Genua, A.; Graillot, A.; Mija, A. Sustainable series of new epoxidized vegetable oils-based thermosets with chemical recycling properties. Biomacromolecules 2020, 21, 3923–3935. [Google Scholar] [CrossRef]
- Gan, Y.; Jiang, X. CHAPTER 1 Photo-Cured Materials from Vegetable Oils. In Green Materials from Plant Oils; The Royal Society of Chemistry: London, UK, 2015; pp. 1–27. ISBN 978-1-84973-901-6. [Google Scholar]
- Zhang, X. Sustainable Polymeric Materials Derived From Plant Oils: From Synthesis To Applications (Master’s Thesis). 2017. Available online: https://scholarcommons.sc.edu/etd/4540 (accessed on 18 November 2022).
- Hood, C.; Ghazani, S.M.; Marangoni, A.G.; Pensini, E. Flexible polymeric biomaterials from epoxidized soybean oil, epoxidized oleic acid, and citric acid as both a hardener and acid catalyst. J. Appl. Polym. Sci. 2022, 139, e53011. [Google Scholar] [CrossRef]
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatary, S. Biodiesel production from mixed oils: A sustainable approach towards industrial biofuel production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Tibbits, S. 4D Printing: Multi-Material Shape Change. Arch. Des. 2014, 84, 116–121. [Google Scholar] [CrossRef]
- Ahmed, A.; Arya, S.; Gupta, V.; Furukawa, H.; Khosla, A. 4D printing: Fundamentals, materials, applications and challenges. Polymer 2021, 228, 123926. [Google Scholar] [CrossRef]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Tan, S.G.; Chow, W.S. Biobased Epoxidized Vegetable Oils and Its Greener Epoxy Blends: A Review. Polym. Technol. Eng. 2010, 49, 1581–1590. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Y.; Gao, H.; Li, Q.; Xi, Z.; Zhao, L.; Peng, C.; Li, L. Bio-Based Epoxy Resin from Epoxidized Soybean Oil. In Soybean; Minobu Kasai, Ed.; IntechOpen: Rijeka, Croatia, 2018; p. Ch. 8. ISBN 978-1-78985-374-2. [Google Scholar]
- Prileschajew, N. Oxydation ungesättigter Verbindungen mittels organischer Superoxyde. Eur. J. Inorg. Chem. 1909, 42, 4811–4815. [Google Scholar] [CrossRef]
- Sammaiah, A.; Padmaja, K.V.; Narayna Prasad, R.B. Synthesis of Epoxy Jatropha Oil and Its Evaluation for Lubricant Properties. J. Oleo Sci. 2014, 63, 637–643. [Google Scholar] [CrossRef]
- Gurbanov, M.S.; Mamedov, B. Epoxidation of flax oil with hydrogen peroxide in a conjugate system in the presence of acetic acid and chlorinated cation exchanger KU-2×8 as catalyst. Russ. J. Appl. Chem. 2009, 82, 1483–1487. [Google Scholar] [CrossRef]
- Klaas, M.R.G.; Warwel, S. Complete and Partial Epoxidation of Plant Oils by Lipase-Catalyzed Perhydrolysis. Ind. Crop. Prod. 1999, 9, 125–132. [Google Scholar] [CrossRef]
- Gerbase, A.E.; Gregório, J.R.; Martinelli, M.; Brasil, M.C.; Mendes, A.N.F. Epoxidation of soybean oil by the methyltrioxorhenium-CH2 Cl2 /H2 O2 catalytic biphasic system. J. Am. Oil Chem. Soc. 2002, 79, 179–181. [Google Scholar] [CrossRef]
- Khot, S.N.; Lascala, J.J.; Can, E.; Morye, S.S.; Williams, G.I.; Palmese, G.R.; Kusefoglu, S.H.; Wool, R.P. Development and application of triglyceride-based polymers and composites. J. Appl. Polym. Sci. 2001, 82, 703–723. [Google Scholar] [CrossRef]
- Lin, B.; Yang, L.; Dai, H.; Yi, A. Kinetic Studies on Oxirane Cleavage of Epoxidized Soybean Oil by Methanol and Characterization of Polyols. J. Am. Oil Chem. Soc. 2007, 85, 113–117. [Google Scholar] [CrossRef]
- Tran, T.-N.; Di Mauro, C.; Graillot, A.; Mija, A. Monitoring the structure–reactivity relationship in epoxidized perilla and safflower oil thermosetting resins. Polym. Chem. 2020, 11, 5088–5097. [Google Scholar] [CrossRef]
- Flory, P.J. Principles of Polymer Chemistry; Baker Lectures 1948; Cornell University Press: Ithaca, NY, USA, 1953; ISBN 978-0-8014-0134-3. [Google Scholar]
- Lang, M.; Hirner, S.; Wiesbrock, F.; Fuchs, P. A Review on Modeling Cure Kinetics and Mechanisms of Photopolymerization. Polymers 2022, 14, 2074. [Google Scholar] [CrossRef]
- Xia, Y.; Larock, R.C. Vegetable oil-based polymeric materials: Synthesis, properties, and applications. Green Chem. 2010, 12, 1893–1909. [Google Scholar] [CrossRef]
- Fan, X.-D.; Deng, Y.; Waterhouse, J.; Pfromm, P.; Carr, W.W. Development of an easily deinkable copy toner using soy-based copolyamides. J. Appl. Polym. Sci. 1999, 74, 1563–1570. [Google Scholar] [CrossRef]
- Russo, S.; Alongi, J. Le Polimerizzazioni Ioniche. In Sintesi di Materiali Polimerici; Edizioni Nuova Cultura: Rome, Italy, 2012; pp. 115–153. [Google Scholar]
- Shukla, V.; Bajpai, M.; Singh, D.; Singh, M.; Shukla, R. Review of basic chemistry of UV-curing technology. Pigment. Resin Technol. 2004, 33, 272–279. [Google Scholar] [CrossRef]
- Shi, S.; Croutxé-Barghorn, C.; Allonas, X. Photoinitiating systems for cationic photopolymerization: Ongoing push toward long wavelengths and low light intensities. Prog. Polym. Sci. 2017, 65, 1–41. [Google Scholar] [CrossRef]
- Štaffová, M.; Ondreáš, F.; Svatík, J.; Zbončák, M.; Jančář, J.; Lepcio, P. 3D printing and post-curing optimization of photopolymerized structures: Basic concepts and effective tools for improved thermomechanical properties. Polym. Test. 2022, 108, 107499. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Jiao, T.; Lu, G.; Liu, J. Photocurable modification of inorganic fillers and their application in photopolymers for 3D printing. Polym. Chem. 2019, 10, 6350–6359. [Google Scholar] [CrossRef]
- Kubisa, P. 4.08-Cationic Ring-Opening Polymerization of Cyclic Ethers. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 141–164. ISBN 978-0-08-087862-1. [Google Scholar]
- Novakov, T.; Jackson, M.; Robinson, G.; Ahmed, W.; Phoenix, D. Laser sintering of metallic medical materials—A review. Int. J. Adv. Manuf. Technol. 2017, 93, 2723–2752. [Google Scholar] [CrossRef]
- Crivello, J.V.; Liu, S. Photoinitiated cationic polymerization of epoxy alcohol monomers. J. Polym. Sci. Part Polym. Chem. 2000, 38, 389–401. [Google Scholar] [CrossRef]
- Decker, C.; Moussa, K. Kinetic study of the cationic photopolymerization of epoxy monomers. J. Polym. Sci. Part Polym. Chem. 1990, 28, 3429–3443. [Google Scholar] [CrossRef]
- Crivello, J.V.; Ortiz, R.A. Design and synthesis of highly reactive photopolymerizable epoxy monomers. J. Polym. Sci. Part Polym. Chem. 2001, 39, 2385–2395. [Google Scholar] [CrossRef]
- Crivello, J.V.; Varlemann, U. Mechanistic study of the reactivity of 3,4-epoxycyclohexylmethyl 3′,4′-epoxycyclohexancarboxylate in photoinitiated cationic polymerizations. J. Polym. Sci. Part Polym. Chem. 1995, 33, 2473–2486. [Google Scholar] [CrossRef]
- Decker, C.; Bianchi, C.; Decker, D.; Morel, F. Photoinitiated polymerization of vinyl ether-based systems. Prog. Org. Coatings 2001, 42, 253–266. [Google Scholar] [CrossRef]
- Abu-Abdoun, I.I. Aale-Ali Cationic Photopolymerization of Cyclohexene Oxide. Eur. Polym. J. 1992, 28, 73–78. [Google Scholar] [CrossRef]
- Crivello, J.V. Advances in the design of photoinitiators, photosensitizers and monomers for photoinitiated cationic polymerization. Des. Monomers Polym. 2002, 5, 141–154. [Google Scholar] [CrossRef]
- Tada, K.; Shirota, Y.; Mikawa, H. Mechanism of charge-transfer polymerization. V. Photopolymerization and photocyclodimerization of N-vinylcarbazole in the N-vinylcarbazole–oxygen–solvent system. J. Polym. Sci. Polym. Chem. Ed. 1973, 11, 2961–2974. [Google Scholar] [CrossRef]
- Morita, Y. Cationic polymerization of hydrogenated bisphenol-A glycidyl ether with cycloaliphatic epoxy resin and its thermal discoloration. J. Appl. Polym. Sci. 2005, 97, 1395–1400. [Google Scholar] [CrossRef]
- Rodrigues, M.R.; Neumann, M.G. Cationic photopolymerization of tetrahydrofuran: A mechanistic study on the use of a sulfonium salt-phenothiazine initiation system. J. Polym. Sci. Part Polym. Chem. 2000, 39, 46–55. [Google Scholar] [CrossRef]
- Sangermano, M.; Malucelli, G.; Morel, F.; Decker, C.; Priola, A. Cationic photopolymerization of vinyl ether systems: Influence of the presence of hydrogen donor additives. Eur. Polym. J. 1999, 35, 639–645. [Google Scholar] [CrossRef]
- Sangermano, M.; Tonin, M.; Yagci, Y. Degradable epoxy coatings by photoinitiated cationic copolymerization of bisepoxide with ε-caprolactone. Eur. Polym. J. 2010, 46, 254–259. [Google Scholar] [CrossRef]
- Blyth, J.; Hofmann, A.W. Ueber das Styrol und einige seiner Zersetzungsproducte. Justus Liebigs Ann. Chem. 1845, 53, 289–329. [Google Scholar] [CrossRef]
- Crivello, J.V. Design of Network Polymers by Photopolymerization. Angew. Makromol. Chem. 1996, 240, 83–90. [Google Scholar] [CrossRef]
- Crivello, J.V. The Discovery and Development of Onium Salt Cationic Photoinitiators. J. Polym. Sci. Part Polym. Chem. 1999, 37, 4241–4254. [Google Scholar] [CrossRef]
- Penczek, S.; Kubisa, P. Cationic ring-opening polymerization: Ethers. In Comprehensive Polymer Science: The Synthesis, Characterization, Reactions & Applications of Polymers; Allen, G., Bevington, J.C., Eds.; Pergamon: Amsterdam, The Netherlands, 1989; Volume 3, pp. 751–786. [Google Scholar]
- Malik, M.S.; Schlögl, S.; Wolfahrt, M.; Sangermano, M. Review on UV-Induced Cationic Frontal Polymerization of Epoxy Monomers. Polymers 2020, 12, 2146. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef]
- Crivello, J.V.; Bulut, U. Indian Turmeric and its Use in Cationic Photopolymerizations. Macromol. Symp. 2006, 240, 1–11. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Rahdar, A. Composites of Vegetable Oil-Based Polymers and Carbon Nanomaterials. Macromol 2021, 1, 19. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Green Nanocomposites from Renewable Resources: Biodegradable Plant Oil-Silica Hybrid Coatings. Macromol. Rapid Commun. 2003, 24, 711–714. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Das, S.R.; Uz, M.; Ding, S.; Lentner, M.T.; Hondred, J.A.; Cargill, A.A.; Sakaguchi, D.S.; Mallapragada, S.; Claussen, J.C. Electrical Differentiation of Mesenchymal Stem Cells into Schwann-Cell-Like Phenotypes Using Inkjet-Printed Graphene Circuits. Adv. Heal. Mater. 2017, 6, 1601087. [Google Scholar] [CrossRef] [PubMed]
- Kausar, A. Fullerene Reinforced Polymeric Nanocomposites for Energy Storage—Status and Prognoses. Front. Mater. 2022, 9, 150. [Google Scholar] [CrossRef]
- Pikhurov, D.V.; Zuev, V.V. The Study of Mechanical and Tribological Performance of Fulleroid Materials Filled PA 6 Composites. Lubricants 2016, 4, 13. [Google Scholar] [CrossRef]
- Park, S.-J.; Jin, F.-L.; Lee, J.-R. Synthesis and Thermal Properties of Epoxidized Vegetable Oil. Macromol. Rapid Commun. 2004, 25, 724–727. [Google Scholar] [CrossRef]
- Park, S.-J.; Jin, F.-L.; Lee, J.-R.; Shin, J.-S. Cationic polymerization and physicochemical properties of a biobased epoxy resin initiated by thermally latent catalysts. Eur. Polym. J. 2005, 41, 231–237. [Google Scholar] [CrossRef]
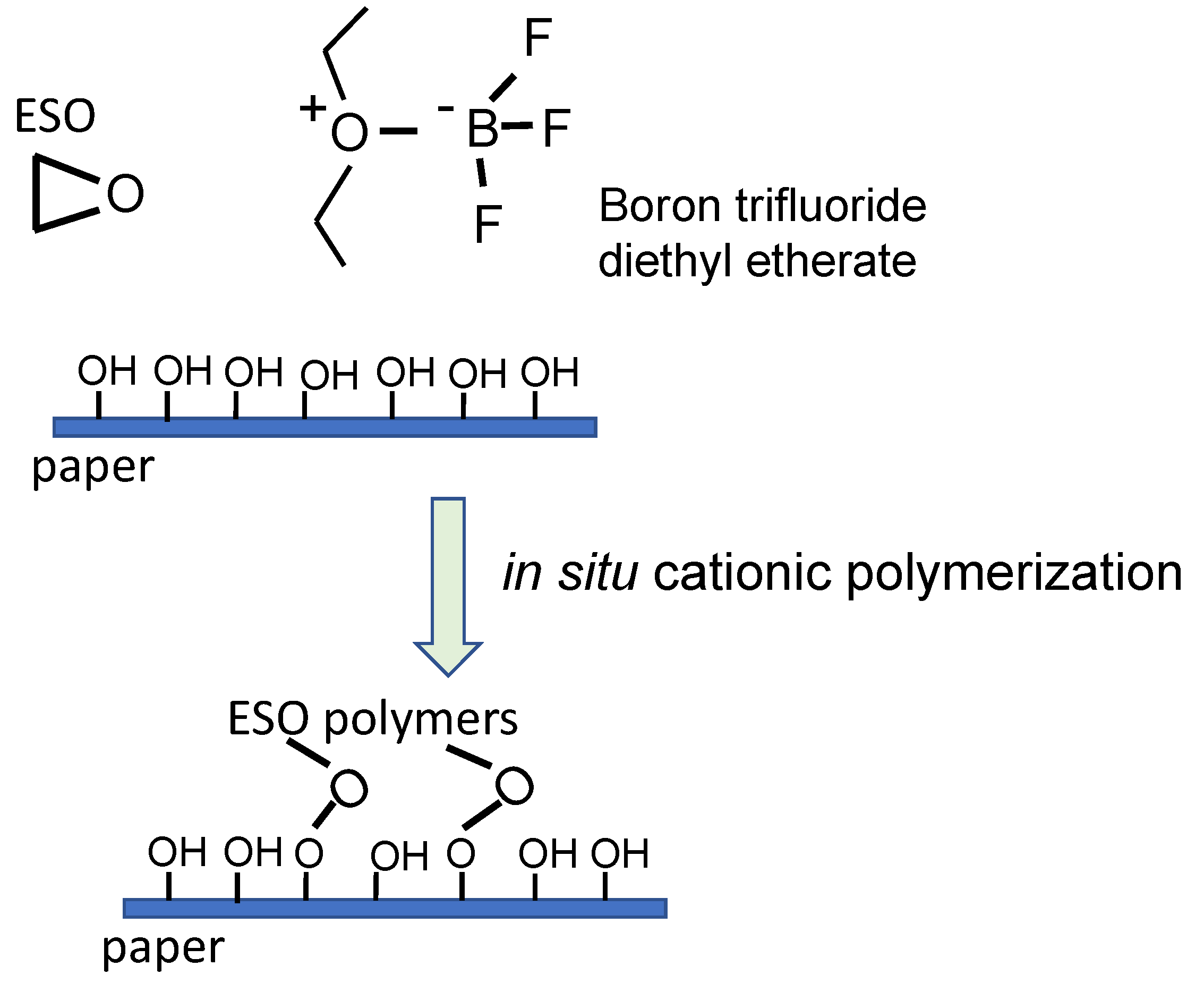
- Miao, S.; Liu, K.; Wang, P.; Su, Z.; Zhang, S. Preparation and characterization of epoxidized soybean oil-based paper composite as potential water-resistant materials. J. Appl. Polym. Sci. 2015, 132, 41575. [Google Scholar] [CrossRef]
- Albarrán-Preza, E.; Corona-Becerril, D.; Vigueras-Santiago, E.; Hernández-López, S. Sweet polymers: Synthesis and characterization of xylitol-based epoxidized linseed oil resins. Eur. Polym. J. 2016, 75, 539–551. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H. Full Biobased Polymeric Material from Plant Oil and Poly(lactic acid) with a Shape Memory Property. ACS Sustain. Chem. Eng. 2014, 2, 2057–2062. [Google Scholar] [CrossRef]
- Li, F.; Perrenoud, A.; Larock, R.C. Thermophysical and mechanical properties of novel polymers prepared by the cationic copolymerization of fish oils, styrene and divinylbenzene. Polymer 2001, 42, 10133–10145. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, Y.; Huang, H.; Lu, J. Recent advances in shape–memory polymers: Structure, mechanism, functionality, modeling and applications. Prog. Polym. Sci. 2012, 37, 1720–1763. [Google Scholar] [CrossRef]
- Serrano, M.C.; Ameer, G.A. Recent Insights Into the Biomedical Applications of Shape-memory Polymers. Macromol. Biosci. 2012, 12, 1156–1171. [Google Scholar] [CrossRef] [PubMed]
- Xie, T. Tunable polymer multi-shape memory effect. Nature 2010, 464, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, X.; Yu, K.; Fu, Y.Q.; Leng, J. A thermodynamic model for tunable multi-shape memory effect and cooperative relaxation in amorphous polymers. Smart Mater. Struct. 2019, 28, 025031. [Google Scholar] [CrossRef]
- Berthiaume, F.; Maguire, T.J.; Yarmush, M.L. Tissue Engineering and Regenerative Medicine: History, Progress, and Challenges. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 403–430. [Google Scholar] [CrossRef] [PubMed]
- Santos Jr, A.R. Bioresorbable Polymers for Tissue Engineering. In Tissue Engineering; Daniel Eberli, Ed.; IntechOpen: Rijeka, Croatia, 2010; pp. 225–246 Ch.11. [Google Scholar]
- Buonvino, S.; Ciocci, M.; Nanni, F.; Cacciotti, I.; Melino, S. New vegetable-waste biomaterials by Lupin albus L. as cellular scaffolds for applications in biomedicine and food. Biomaterials 2023, 293, 121984. [Google Scholar] [CrossRef]
- Cancelliere, R.; Zurlo, F.; Micheli, L.; Melino, S. Vegetable waste scaffolds for 3D-stem cell proliferating systems and low cost biosensors. Talanta 2020, 223, 121671. [Google Scholar] [CrossRef]
- Indurkar, A.; Pandit, A.; Jain, R.; Dandekar, P. Plant-based biomaterials in tissue engineering. Bioprinting 2021, 21, e00127. [Google Scholar] [CrossRef]
- Kolanthai, E.; Sarkar, K.; Meka, S.R.K.; Madras, G.; Chatterjee, K. Copolyesters from Soybean Oil for Use as Resorbable Biomaterials. ACS Sustain. Chem. Eng. 2015, 3, 880–891. [Google Scholar] [CrossRef]
- Sittinger, M.; Bujia, J.; Rotter, N.; Reitzel, D.; Minuth, W.W.; Burmester, G.R. Tissue engineering and autologous transplant formation: Practical approaches with resorbable biomaterials and new cell culture techniques. Biomaterials 1996, 17, 237–242. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef]
- Hong, C.K.; Wool, R.P. Development of a bio-based composite material from soybean oil and keratin fibers. J. Appl. Polym. Sci. 2005, 95, 1524–1538. [Google Scholar] [CrossRef]
- Mondal, D.; Srinivasan, A.; Comeau, P.; Toh, Y.-C.; Willett, T.L. Acrylated epoxidized soybean oil/hydroxyapatite-based nanocomposite scaffolds prepared by additive manufacturing for bone tissue engineering. Mater. Sci. Eng. C 2020, 118, 111400. [Google Scholar] [CrossRef] [PubMed]
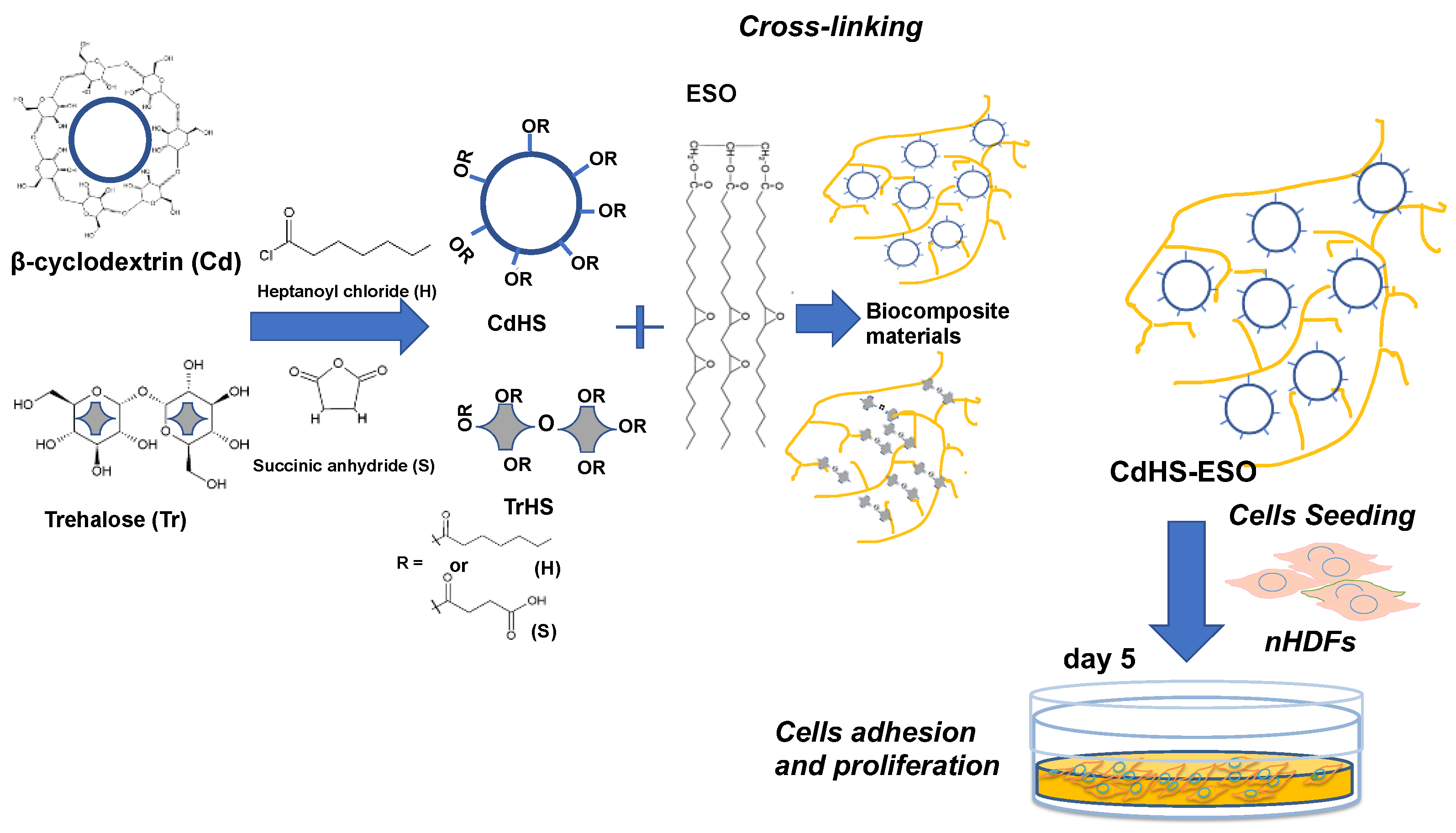
- Zhang, Q.; Phillips, H.R.; Purchel, A.; Hexum, J.K.; Reineke, T.M. Sustainable and Degradable Epoxy Resins from Trehalose, Cyclodextrin, and Soybean Oil Yield Tunable Mechanical Performance and Cell Adhesion. ACS Sustain. Chem. Eng. 2018, 6, 14967–14978. [Google Scholar] [CrossRef]
- Majeed, M.H.; Alsaheb, N.K.A. Morphological Evaluation of PLA/Soybean Oil Epoxidized Acrylate Three-Dimensional Scaffold in Bone Tissue Engineering. J. Renew. Mater. 2022, 10, 2391–2408. [Google Scholar] [CrossRef]
- Moreno, M.; Armentano, I.; Fortunati, E.; Mattioli, S.; Torre, L.; Lligadas, G.; Ronda, J.C.; Galia, M.; Cadíz, V. CelluloseNano-Bio-composites from High Oleic Sunflower Oil-Derived Thermosets. Eur. Polym. J. 2016, 79, 109–120. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Wound Healing Bionanocomposites Based on Castor Oil Polymeric Films Reinforced with Chitosan-Modified ZnO Nanoparticles. Biomacromolecules 2015, 16, 2631–2644. [Google Scholar] [CrossRef]
- Veloso-Fernández, A.; Laza, J.M.; Ruiz-Rubio, L.; Martín, A.; Taguado, M.; Benito-Vicente, A.; Martín, C.; Vilas, J.L. Towards a new generation of non-cytotoxic shape memory thermoplastic polyurethanes for biomedical applications. Mater. Today Commun. 2022, 33, 104730. [Google Scholar] [CrossRef]

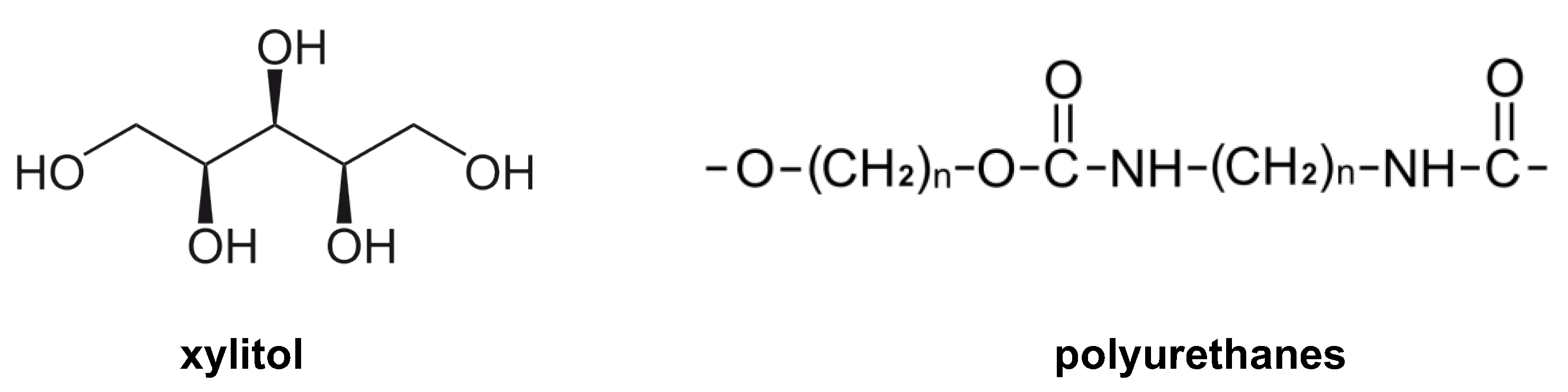

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurchi, C.; Buonvino, S.; Arciero, I.; Melino, S. Sustainable Vegetable Oil-Based Biomaterials: Synthesis and Biomedical Applications. Int. J. Mol. Sci. 2023, 24, 2153. https://doi.org/10.3390/ijms24032153
Nurchi C, Buonvino S, Arciero I, Melino S. Sustainable Vegetable Oil-Based Biomaterials: Synthesis and Biomedical Applications. International Journal of Molecular Sciences. 2023; 24(3):2153. https://doi.org/10.3390/ijms24032153
Chicago/Turabian StyleNurchi, Chiara, Silvia Buonvino, Ilaria Arciero, and Sonia Melino. 2023. "Sustainable Vegetable Oil-Based Biomaterials: Synthesis and Biomedical Applications" International Journal of Molecular Sciences 24, no. 3: 2153. https://doi.org/10.3390/ijms24032153
APA StyleNurchi, C., Buonvino, S., Arciero, I., & Melino, S. (2023). Sustainable Vegetable Oil-Based Biomaterials: Synthesis and Biomedical Applications. International Journal of Molecular Sciences, 24(3), 2153. https://doi.org/10.3390/ijms24032153