Understanding the Role of ATP Release through Connexins Hemichannels during Neurulation
Abstract
:1. Introduction
2. Results
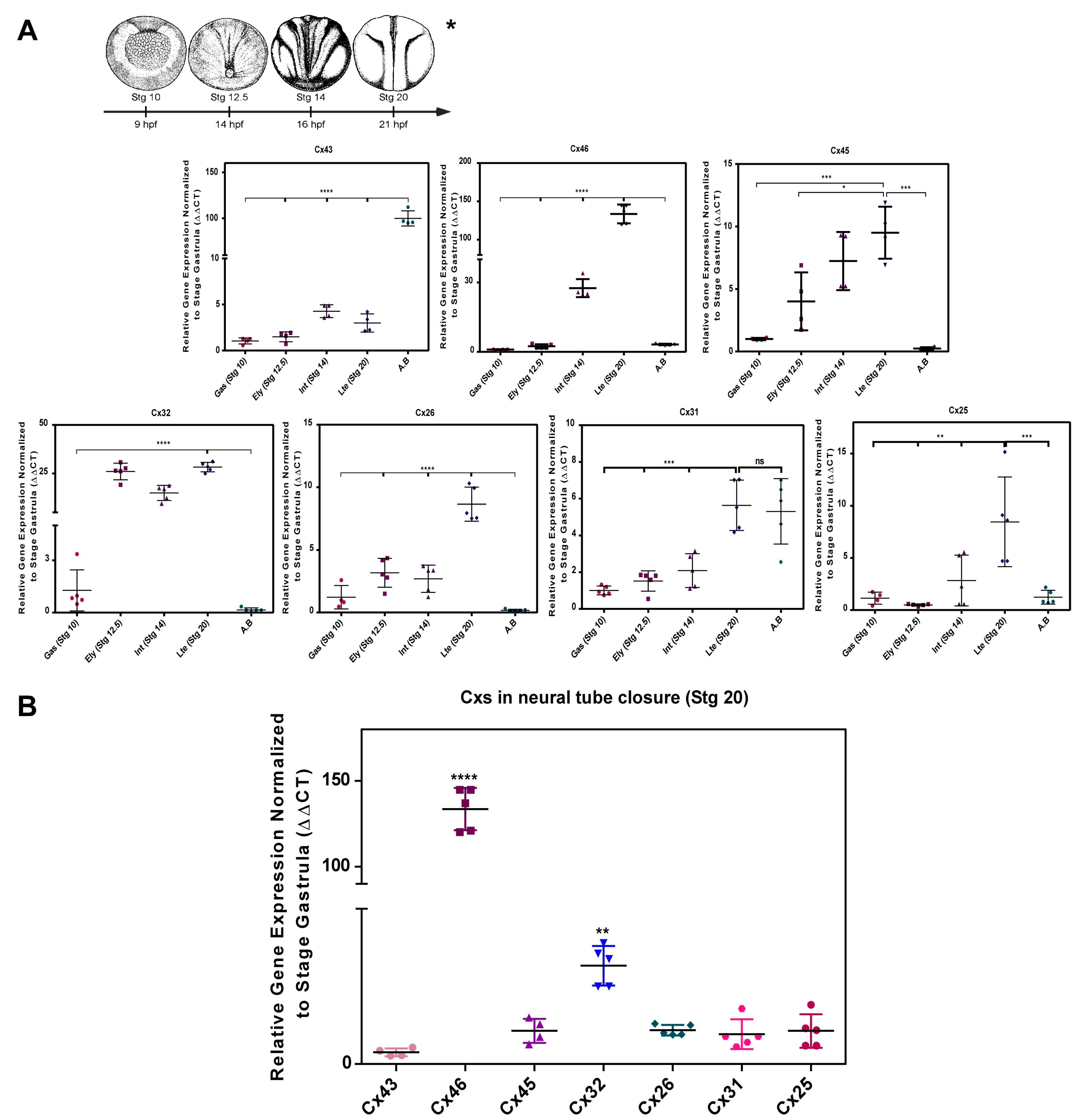
2.1. Transcript Transcript Profile of Cxs during Neurulation in Xenopus laevis
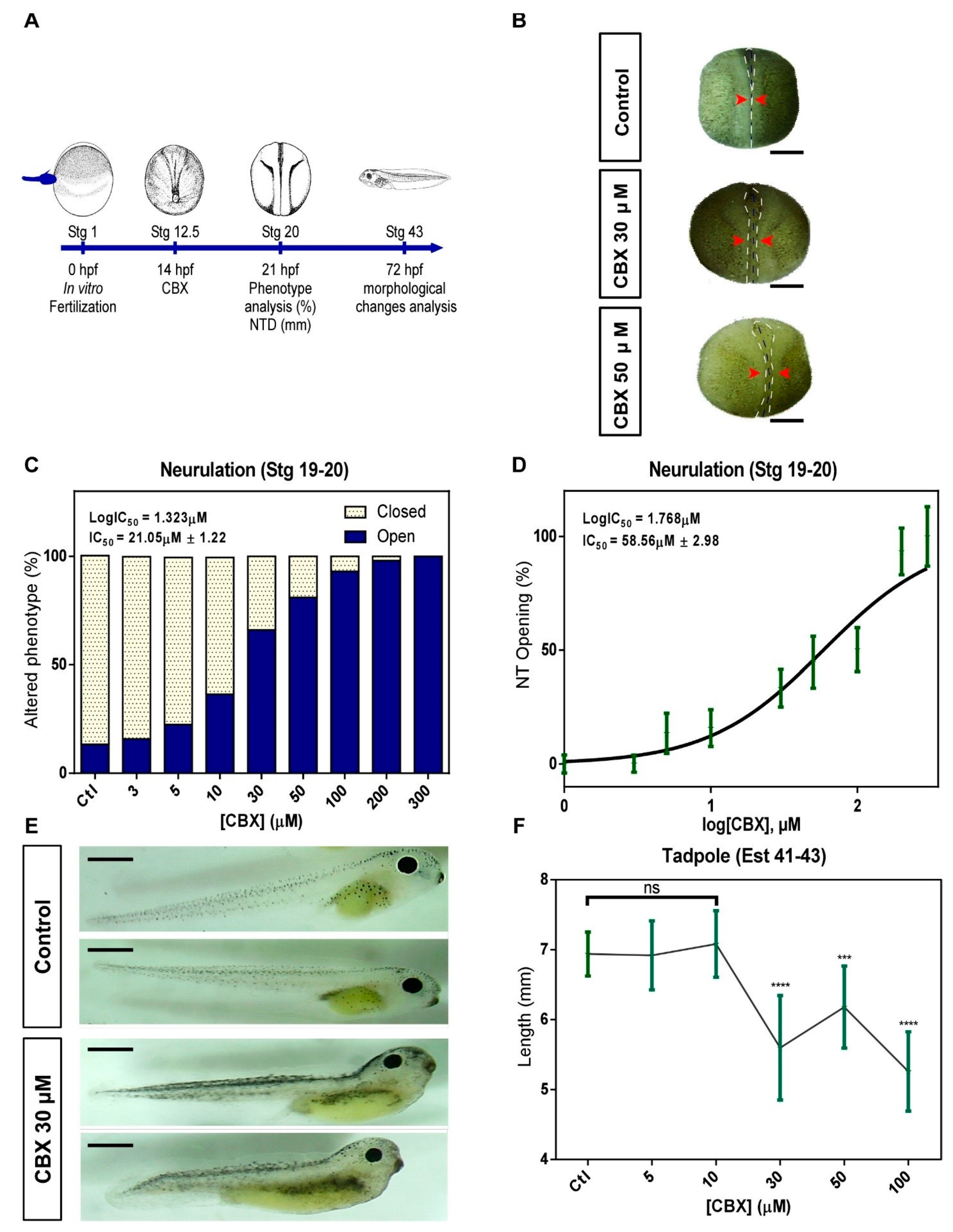
2.2. Pharmacological Blockade of Cxs in Neurulation Induces NTDs
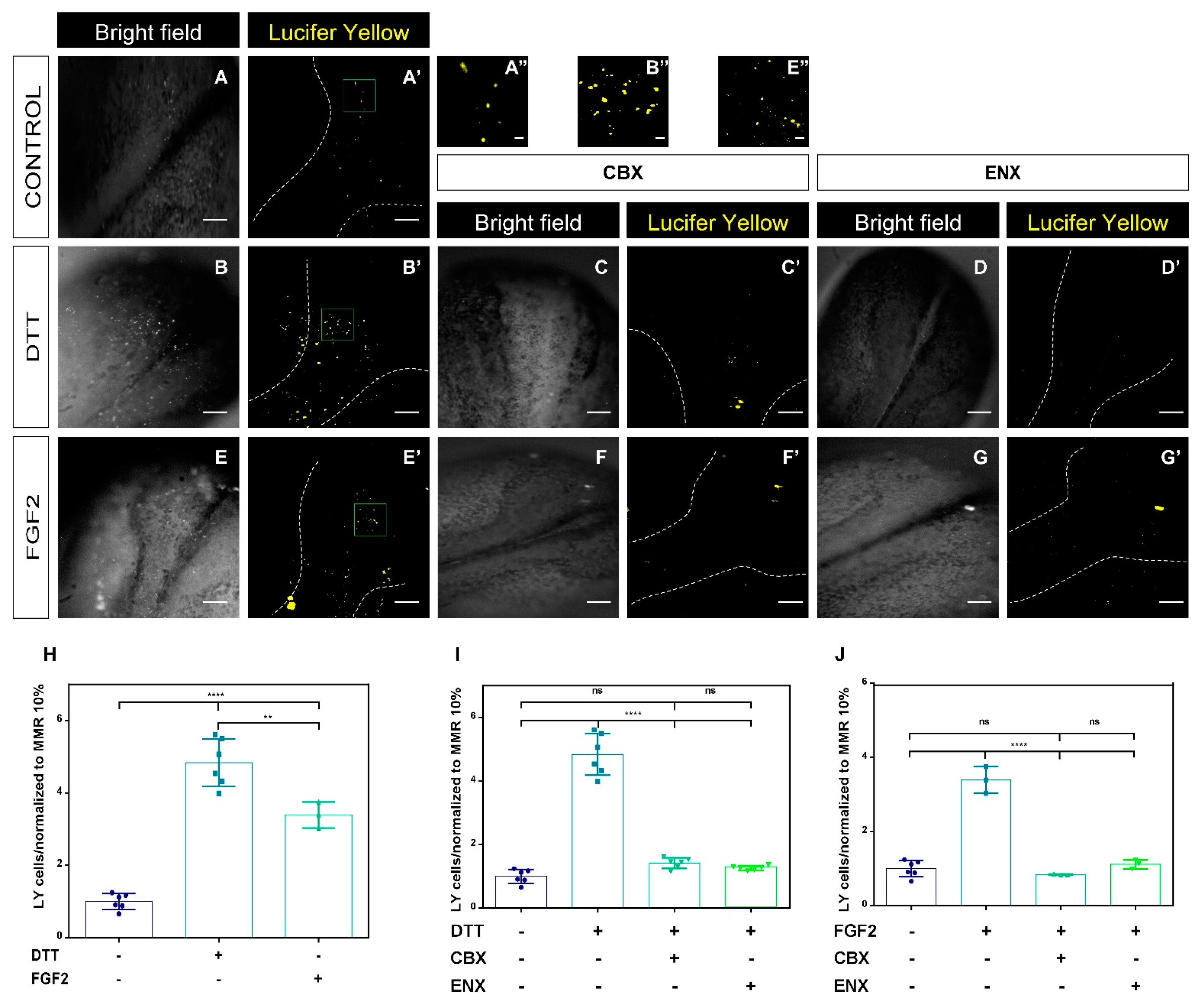
2.3. Functional Analyses of HCs-Cxs In Vivo by Redox Potentials and FGF2 Using Lucifer Yellow Permeability Assay
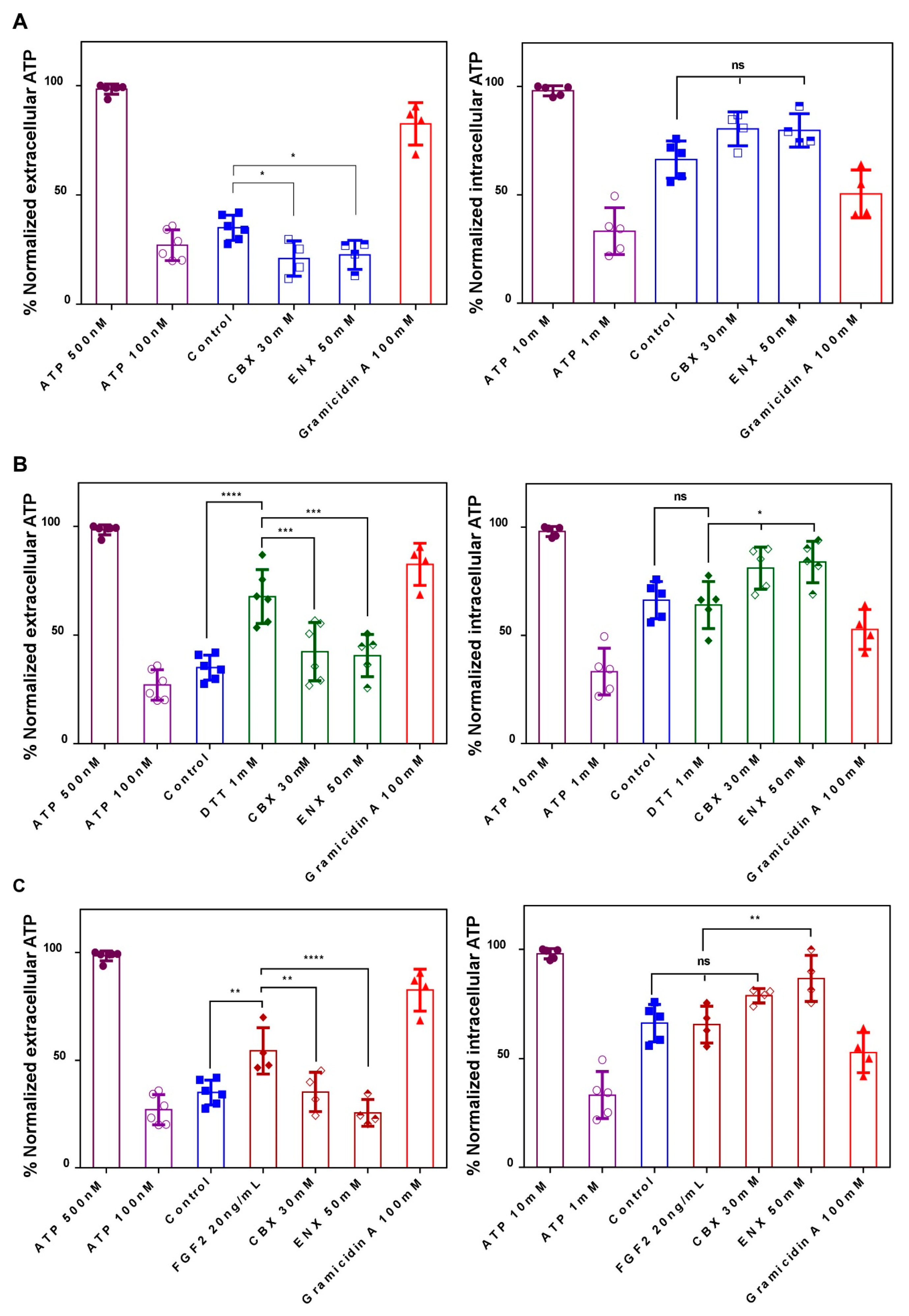
2.4. Evaluation of HCs-Cxs Functionality via ATP Release Induced by Redox Potentials and FGFs
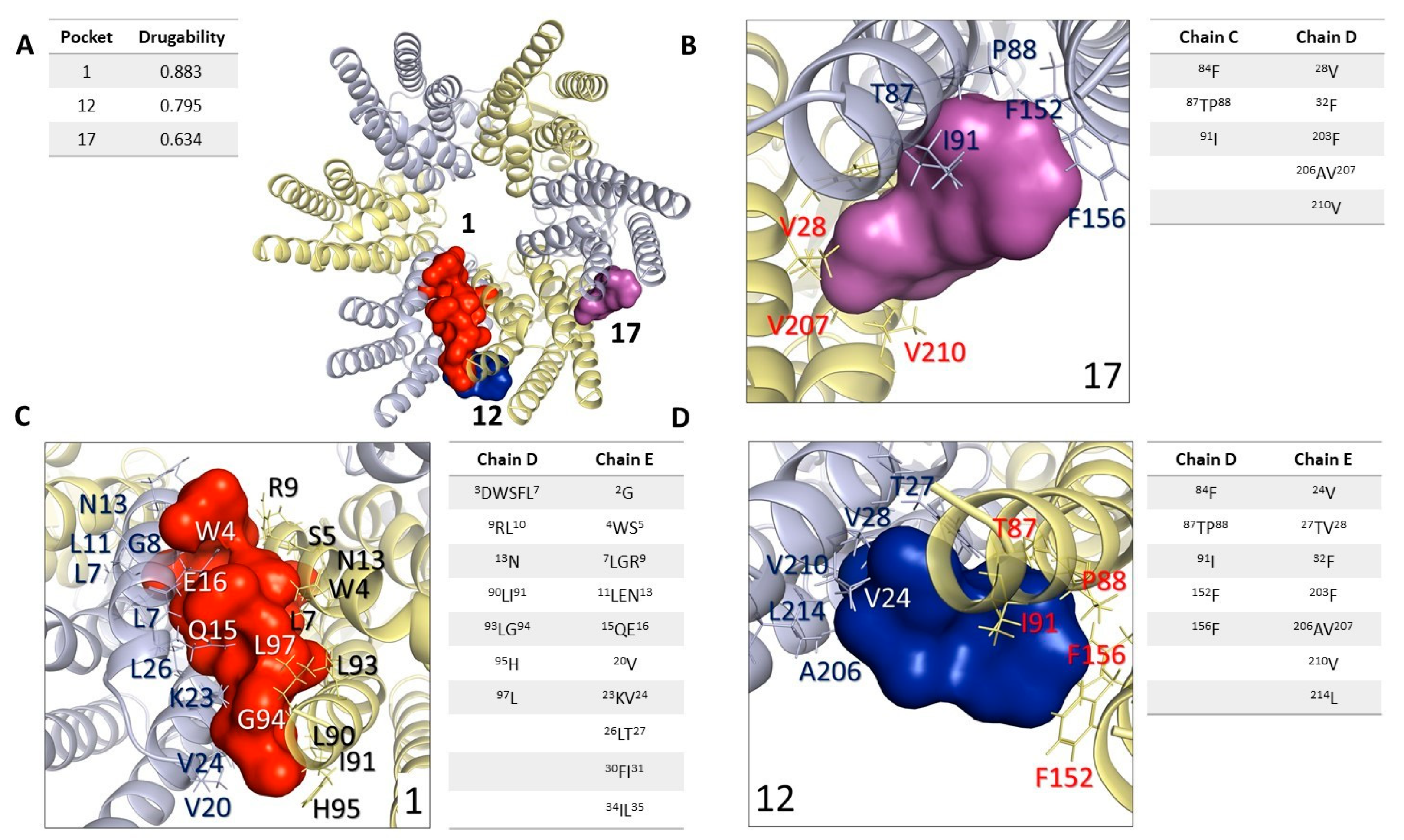
2.5. In Silico Analysis: Study and Analysis of Potential Interaction Sites in Connexin 46 Hemichannels
2.6. In Silico Analysis: Molecular Docking Simulations, Connexin 46 Hemichannel Interactions with Carbenoxolone/Enoxolone
3. Discussion
3.1. Connexins Expression and Function during the Neurulation in Xenopus laevis
3.2. FGF2 and Intracellular Redox Status Regulate the Opening of HCs-Cxs during the Neurulation Process in Xenopus laevis
3.3. Limitations and Caveats
3.4. In Silico Predictions: Binding Sites and Interactions of Widely Used Blockers to Modulate Cxs Activity
4. Materials and Methods
4.1. Ethical Statement and Experimental Animals
4.2. Obtaining Xenopus laevis Embryos
4.3. Conventional RT-PCR and Real-Time RT-qPCR
Total RNA Extraction
4.4. Reverse Transcription (RT) of Total RNA
4.5. cDNA Amplification by PCR
4.6. Agarose Gel Electrophoresis
4.7. Quantitative Real-Time RT-PCR (qRT-PCR)
4.8. Pharmacological Modulation of the Neurulation Process
4.9. Lucifer Yellow Uptake and Fluorescence Detection In Vivo
4.10. Quantification of Extracellular and Intracellular ATP
4.11. Western Blot Assay
Total Protein Extract
4.12. Polyacrylamide Gel Electrophoresis and Electrotransfer
4.13. Protein Immunodetection
4.14. Bioinformatic Analysis for Hemichannel Interaction Cx46-Carbenoxolone/Enoxolone Selection of Molecules
4.15. Preparation of Cx46
4.16. Prediction of Binding Sites in Cx46
4.17. Protein-Ligand Docking
4.18. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilde, J.J.; Petersen, J.R.; Niswander, L. Genetic, epigenetic, and environmental contributions to neural tube closure. Annu. Rev. Genet. 2014, 48, 583–611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, K.; Fortriede, J.D.; Lotay, V.S.; Burns, K.A.; Wang, D.Z.; Fisher, M.E.; Ponferrada, V.G. Xenbase: A genomic, epigenomic and transcriptomic model organism database. Nucleic Acids Res. 2018, 46, D861–D868. [Google Scholar] [CrossRef] [PubMed]
- O'Rahilly, R.; Müller, F. Neurulation in the normal human embryo. In Ciba Foundation Symposium 181-Neural Tube Defects: Neural Tube Defects; Ciba Foundation Symposium; John and Wiley and Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Böttcher, R.T.; Niehrs, C. Fibroblast growth factor signaling during early vertebrate development. Endocr. Rev. 2005, 26, 63–77. [Google Scholar] [CrossRef]
- Hébert, J.M. FGFs: Neurodevelopment’s Jack-of-all-trades–how do they do it? Front. Neurosci. 2011, 5, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichert, S.; Randall, R.A.; Hill, C.S. A BMP regulatory network controls ectodermal cell fate decisions at the neural plate border. Development 2013, 140, 4435–4444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copp, A.J.; Greene, N.D. Genetics and development of neural tube defects. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2010, 220, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Nikolopoulou, E.; Galea, G.L.; Rolo, A.; Greene, N.D.; Copp, A.J. Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development 2017, 144, 552–566. [Google Scholar] [CrossRef] [Green Version]
- Greene, N.D.; Copp, A.J. Neural tube defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef] [Green Version]
- Wallingford, J.B.; Niswander, L.A.; Shaw, G.M.; Finnell, R.H. The continuing challenge of understanding, preventing, and treating neural tube defects. Science 2013, 339, 122. [Google Scholar] [CrossRef] [Green Version]
- Seidahmed, M.Z.; Abdelbasit, O.B.; Shaheed, M.M.; Alhussein, K.A.; Miqdad, A.M.; Khalil, M.I.; Salih, M.A. Epidemiology of neural tube defects. Saudi Med. J. 2014, 35, S29. [Google Scholar]
- Blom, H.J. Folic acid, methylation and neural tube closure in humans. Birth Defects Res. Part A Clin. Mol. Teratol. 2009, 85, 295–302. [Google Scholar] [CrossRef]
- Cabrera, R.M.; Hill, D.S.; Etheredge, A.J.; Finnell, R.H. Investigations into the etiology of neural tube defects. Birth Defects Res. Part Embryo Today Rev. 2004, 72, 330–344. [Google Scholar] [CrossRef]
- Detrait, E.R.; George, T.M.; Etchevers, H.C.; Gilbert, J.R.; Vekemans, M.; Speer, M.C. Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicol. Teratol. 2005, 27, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Gleeson, J.G. Closing in on Mechanisms of Open Neural Tube Defects. Trends Neurosci. 2020, 43, 519–532. [Google Scholar] [CrossRef]
- Represa, A.; Ben-Ari, Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005, 28, 278–283. [Google Scholar] [CrossRef]
- Borodinsky, L.N.; O'Leary, D.; Neale, J.H.; Vicini, S.; Coso, O.A.; Fiszman, M.L. GABA-induced neurite outgrowth of cerebellar granule cells is mediated by GABAA receptor activation, calcium influx and CaMKII and erk1/2 pathways. J. Neurochem. 2003, 84, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Jansson, L.C.; Åkerman, K.E. The role of glutamate and its receptors in the proliferation, migration, differentiation and survival of neural progenitor cells. J. Neural Transm. 2014, 121, 819–836. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, N.C. Electrical activity in early neuronal development. Nature 2006, 444, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, H. Nucleotide signaling in nervous system development. Pflügers Arch. 2006, 452, 573–588. [Google Scholar] [CrossRef] [PubMed]
- Sequerra, E.B.; Goyal, R.; Castro, P.A.; Levin, J.B.; Borodinsky, L.N. NMDA receptor signaling is important for neural tube formation and for preventing antiepileptic drug-induced neural tube defects. J. Neurosci. 2018, 38, 4762–4773. [Google Scholar] [CrossRef] [Green Version]
- Benavides-Rivas, C.; Tovar, L.M.; Zúñiga, N.; Pinto-Borguero, I.; Retamal, C.; Yévenes, G.E.; Coddou, C. Altered Glutaminase 1 Activity During Neurulation and Its Potential Implications in Neural Tube Defects. Front. Pharmacol. 2020, 11, 900. [Google Scholar] [CrossRef]
- Abbracchio, M.P.; Burnstock, G.; Verkhratsky, A.; Zimmermann, H. Purinergic signalling in the nervous system: An overview. Trends Neurosci. 2009, 32, 19–29. [Google Scholar] [CrossRef]
- Boison, D. Adenosine as a neuromodulator in neurological diseases. Curr. Opin. Pharmacol. 2008, 8, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, K.; Chan, W.; Burnstock, G. Expression of P2X purinoceptors during rat brain development and their inhibitory role on motor axon outgrowth in neural tube explant cultures. Neuroscience 2005, 133, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hashimoto-Torii, K.; Torii, M.; Haydar, T.F.; Rakic, P. The role of ATP signaling in the migration of intermediate neuronal progenitors to the neocortical subventricular zone. Proc. Natl. Acad. Sci. USA 2008, 105, 11802–18071. [Google Scholar] [CrossRef] [Green Version]
- Webb, S.E.; Miller, A.L. Calcium signalling during embryonic development. Nat. Rev. Mol. Cell Biol. 2003, 4, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, Y.D.; Dale, L.; King, B.F.; Whittock, N.; Burnstock, G. Early expression of a novel nucleotide receptor in the neural plate of Xenopus embryos. J. Biol. Chem. 1997, 272, 12583–12591. [Google Scholar] [CrossRef] [Green Version]
- Burnstock, G.; Dale, N. Purinergic signalling during development and ageing. Purinergic Signal. 2015, 11, 277–305. [Google Scholar] [CrossRef] [Green Version]
- Massé, K.; Dale, N. Purines as potential morphogens during embryonic development. Purinergic Signal. 2012, 8, 503–521. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.; Sawada, K.; Morland, C.; Hiasa, M.; Ormel, L.; Moriyama, Y.; Gundersen, V. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb. Cortex 2012, 22, 1203–1214. [Google Scholar] [CrossRef] [Green Version]
- Sawada, K.; Echigo, N.; Juge, N.; Miyaji, T.; Otsuka, M.; Omote, H.; Moriyama, Y. Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. USA 2008, 105, 5683–5686. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef] [Green Version]
- Belousov, A.B. Novel model for the mechanisms of glutamate-dependent excitotoxicity: Role of neuronal gap junctions. Brain Res. 2012, 1487, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Mayorquin, L.C.; Rodriguez, A.V.; Sutachan, J.-J.; Albarracín, S.L. Connexin-mediated functional and metabolic coupling between astrocytes and neurons. Front. Mol. Neurosci. 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orellana, J.A.; Froger, N.; Ezan, P.; Jiang, J.X.; Bennett, M.V.; Naus, C.C.; Sáez, J.C. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011, 118, 826–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482–104881. [Google Scholar] [CrossRef] [Green Version]
- Weissman, T.A.; Riquelme, P.A.; Ivic, L.; Flint, A.C.; Kriegstein, A.R. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 2004, 43, 647–661. [Google Scholar] [CrossRef] [Green Version]
- Ye, Z.-C.; Wyeth, M.S.; Baltan-Tekkok, S.; Ransom, B.R. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J. Neurosci. 2003, 23, 3588–3596. [Google Scholar] [CrossRef] [Green Version]
- Hanani, M. Lucifer yellow–An angel rather than the devil. J. Cell. Mol. Med. 2012, 16, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Harris, A.L. Connexin channel permeability to cytoplasmic molecules. Prog. Biophys. Mol. Biol. 2007, 94, 120–143. [Google Scholar] [CrossRef] [Green Version]
- Kanaporis, G.; Mese, G.; Valiuniene, L.; White, T.W.; Brink, P.R.; Valiunas, V. Gap junction channels exhibit connexin-specific permeability to cyclic nucleotides. J. Gen. Physiol. 2008, 131, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Bai, D.; Wang, A.H. Extracellular domains play different roles in gap junction formation and docking compatibility. Biochem. J. 2014, 458, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, P.; Nichols, W.; Puhalla, S.; Oliver, T.; Berman, M.; Pleasure, D. Early migratory rat neural crest cells express functional gap junctions: Evidence that neural crest cell survival requires gap junction function. J. Neurosci. Res. 2000, 61, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Nadarajah, B.; Jones, A.; Evans, W.H.; Parnavelas, J. Differential expression of connexins during neocortical development and neuronal circuit formation. J. Neurosci. 1997, 17, 3096–3111. [Google Scholar] [CrossRef] [Green Version]
- Bittman, K.; LoTurco, J. Differential regulation of connexin 26 and 43 in murine neocortical precursors. Cereb. Cortex 1999, 9, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khodosevich, K.; Zuccotti, A.; Kreuzberg, M.M.; Le Magueresse, C.; Frank, M.; Willecke, K.; Monyer, H. Connexin45 modulates the proliferation of transit-amplifying precursor cells in the mouse subventricular zone. Proc. Natl. Acad. Sci. USA 2012, 109, 20107–20112. [Google Scholar] [CrossRef] [Green Version]
- Swayne, L.A.; Bennett, S.A. Connexins and pannexins in neuronal development and adult neurogenesis. BMC Cell Biol. 2016, 17, 39–49. [Google Scholar] [CrossRef] [Green Version]
- Evans, W.H.; De Vuyst, E.; Leybaert, L. The gap junction cellular internet: Connexin hemichannels enter the signalling limelight. Biochem. J. 2006, 397, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, D.A.; Paul, D.L. Beyond the gap: Functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 2003, 4, 285–295. [Google Scholar] [CrossRef]
- Sáez, J.C.; Schalper, K.A.; Retamal, M.A.; Orellana, J.A.; Shoji, K.F.; Bennett, M.V. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp. Cell Res. 2010, 316, 2377–2389. [Google Scholar] [CrossRef]
- De Vuyst, E.; Decrock, E.; De Bock, M.; Yamasaki, H.; Naus, C.C.; Evans, W.H.; Leybaert, L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol. Biol. Cell 2007, 18, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Garré, J.M.; Retamal, M.A.; Cassina, P.; Barbeito, L.; Bukauskas, F.F.; Sáez, J.C.; Abudara, V. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc. Natl. Acad. Sci. USA 2010, 107, 22659–22664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recabal, A.; Fernández, P.; López, S.; Barahona, M.J.; Ordenes, P.; Alejandra, P.; Caprile, T. The FGF2-induced tanycyte proliferation involves a connexin 43 hemichannel/purinergic-dependent pathway. J. Neurochem. 2020, 156, 182–199. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Palacios-Prado, N.; Retamal, M.A.; Shoji, K.F.; Martínez, A.D.; Sáez, J.C. Connexin hemichannel composition determines the FGF-1–induced membrane permeability and free [Ca2+] i responses. Mol. Biol. Cell 2008, 19, 3501–3513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schalper, K.A.; Riquelme, M.A.; Brañes, M.C.; Martínez, A.D.; Vega, J.L.; Berthoud, V.M.; Sáez, J.C. Modulation of gap junction channels and hemichannels by growth factors. Mol. BioSyst. 2012, 8, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Pogoda, K.; Kameritsch, P.; Retamal, M.A.; Vega, J.L. Regulation of gap junction channels and hemichannels by phosphorylation and redox changes: A revision. BMC Cell Biol. 2016, 17, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retamal, M.A. Connexin and Pannexin hemichannels are regulated by redox potential. Front. Physiol. 2014, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Retamal, M.A.; Schalper, K.A.; Shoji, K.F.; Bennett, M.V.; Sáez, J.C. Opening of connexin 43 hemichannels is increased by lowering intracellular redox potential. Proc. Natl. Acad. Sci. USA 2007, 104, 8322–8327. [Google Scholar] [CrossRef] [Green Version]
- Sáez, J.C.; Retamal, M.A.; Basilio, D.; Bukauskas, F.F.; Bennett, M.V. Connexin-based gap junction hemichannels: Gating mechanisms. Biochim. Biophys. Acta Biomembr. 2005, 1711, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Eckert, R. Gap-junctional single-channel permeability for fluorescent tracers in mammalian cell cultures. Biophys. J. 2006, 91, 565–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ek-Vitorin, J.F.; Burt, J.M. Structural basis for the selective permeability of channels made of communicating junction proteins. Biochim. Biophys. Acta (BBA)-Biomembr. 2013, 1828, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Elfgang, C.; Eckert, R.; Lichtenberg-Fraté, H.; Butterweck, A.; Traub, O.; Klein, R.A.; Willecke, K. Specific permeability and selective formation of gap junction channels in connexin-transfected HeLa cells. J. Cell Biol. 1995, 129, 805–817. [Google Scholar] [CrossRef] [PubMed]
- Willebrords, J.; Maes, M.; Yanguas, S.C.; Vinken, M. Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol. Ther. 2017, 180, 144–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koval, M.; Molina, S.A.; Burt, J.M. Mix and match: Investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett. 2014, 588, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ambrosi, C.; Qiu, F.; Jackson, D.G.; Sosinsky, G.; Dahl, G. The membrane protein Pannexin1 forms two open-channel conformations depending on the mode of activation. Sci. Signal. 2014, 7, ra69. [Google Scholar] [CrossRef] [Green Version]
- Cotrina, M.L.; Lin, J.H.-C.; Alves-Rodrigues, A.; Liu, S.; Li, J.; Azmi-Ghadimi, H.; Nedergaard, M. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. USA 1998, 95, 15735–15740. [Google Scholar] [CrossRef] [Green Version]
- Myers, J.B.; Haddad, B.G.; O’Neill, S.E.; Chorev, D.S.; Yoshioka, C.C.; Robinson, C.V.; Reichow, S.L. Structure of native lens connexin 46/50 intercellular channels by cryo-EM. Nature 2018, 564, 372–377. [Google Scholar] [CrossRef]
- Le Guilloux, V.; Schmidtke, P.; Tuffery, P. Fpocket: An open source platform for ligand pocket detection. BMC Bioinform. 2009, 10, 168. [Google Scholar] [CrossRef] [Green Version]
- Schmidtke, P.; Le Guilloux, V.; Maupetit, J.; Tuffery, P. Fpocket: Online tools for protein ensemble pocket detection and tracking. Nucleic Acids Res. 2010, 38, W582–W589. [Google Scholar] [CrossRef] [Green Version]
- Beyer, E.C.; Lipkind, G.M.; Kyle, J.W.; Berthoud, V.M. Structural organization of intercellular channels II. Amino terminal domain of the connexins: Sequence 2012, functional roles, and structure. Biochim. Biophys. Acta Biomembr. 2012, 1818, 1823–1830. [Google Scholar] [CrossRef] [Green Version]
- Bai, D.; Yue, B.; Aoyama, H. Crucial motifs and residues in the extracellular loops influence the formation and specificity of connexin docking. Biochim. Biophys. Acta Biomembr. 2018, 1860, 9–21. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Bai, D. Structural Analysis of Key Gap Junction Domains—Lessons From Genome data and Disease-Linked Mutants. In Seminars Cell & Developmental Biology; Academic Press: London, UK, 2016. [Google Scholar]
- Gilbert, S.F.; Singer, S. Formation of the neural tube. In Developmental Biology; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Oyamada, M.; Oyamada, Y.; Takamatsu, T. Regulation of connexin expression. Biochim. Biophys. Acta Biomembr. 2005, 1719, 6–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodenough, D.A.; Paul, D.L. Gap junctions. Cold Spring Harb. Perspect. Biol. 2009, 1, a002576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinken, M.; Decrock, E.; De Vuyst, E.; Ponsaerts, R.; D'hondt, C.; Bultynck, G.; Rogiers, V. Connexins: Sensors and regulators of cell cycling. Biochim. Biophys. Acta Rev. Cancer 2011, 1815, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Huettner, J.E.; Lu, A.; Qu, Y.; Wu, Y.; Kim, M.; McDonald, J.W., III. Gap junctions and connexon hemichannels in human embryonic stem cells. Stem Cells 2006, 24, 1654–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, N.M.; Gilula, N.B. The gap junction communication channel. Cell 1996, 84, 381–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, D.W. Life cycle of connexins in health and disease. Biochem. J. 2006, 394, 527–543. [Google Scholar] [CrossRef] [Green Version]
- Recabal, A.; Elizondo-Vega, R.; Philippot, C.; Salgado, M.; López, S.; Palma, A.; Caprile, T. Connexin-43 gap junctions are responsible for the hypothalamic tanycyte-coupled network. Front. Cell. Neurosci. 2018, 12, 406. [Google Scholar] [CrossRef]
- Hitomi, M.; Deleyrolle, L.P.; Mulkearns-Hubert, E.E.; Jarrar, A.; Li, M.; Sinyuk, M.; Hubert, C.G. Differential connexin function enhances self-renewal in glioblastoma. Cell Rep. 2015, 11, 1031–1042. [Google Scholar] [CrossRef] [Green Version]
- Marins, M.; Xavier, A.L.; Viana, N.B.; Fortes, F.S.; Fróes, M.M.; Menezes, J.R. Gap junctions are involved in cell migration in the early postnatal subventricular zone. Dev. Neurobiol. 2009, 69, 715–730. [Google Scholar] [CrossRef]
- Dorey, K.; Amaya, E. FGF signalling: Diverse roles during early vertebrate embryogenesis. Development 2010, 137, 3731–3742. [Google Scholar] [CrossRef] [Green Version]
- Thisse, B.; Thisse, C. Functions and regulations of fibroblast growth factor signaling during embryonic development. Dev. Biol. 2005, 287, 390–402. [Google Scholar] [CrossRef]
- Lin, D.; Lobell, S.; Jewell, A.; Takemoto, D.J. Differential phosphorylation of connexin46 and connexin50 by H2O2 activation of protein kinase C gamma. Mol. Vis. 2004, 10, 688–695. [Google Scholar]
- Wang, Z.; Schey, K.L. Phosphorylation and truncation sites of bovine lens connexin 46 and connexin 50. Exp. Eye Res. 2009, 89, 898–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, T.; Tsuchida, M.; Lampe, P.D.; Murakami, M. Cardiomyocyte FGF signaling is required for Cx43 phosphorylation and cardiac gap junction maintenance. Exp. Cell Res. 2013, 319, 2152–2165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boswell, B.A.; Le, A.-C.N.; Musil, L.S. Upregulation and maintenance of gap junctional communication in lens cells. Exp. Eye Res. 2009, 88, 919–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbe, M.T.; Monyer, H.; Bruzzone, R. Cell-cell communication beyond connexins: The pannexin channels. Physiology 2006, 21, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Penuela, S.; Gehi, R.; Laird, D.W. The biochemistry and function of pannexin channels. Biochim. Biophys. Acta Biomembr. 2013, 1828, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Baroja-Mazo, A.; Barberà-Cremades, M.; Pelegrín, P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim. Biophys. Acta Biomembr. 2013, 1828, 79–93. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, M.; Sato, M.; Koyama, H.; Hara, Y.; Hayashi, K.; Yasue, N.; Campbell, R.E. Distinct intracellular Ca2+ dynamics regulate apical constriction and differentially contribute to neural tube closure. Development 2017, 144, 1307–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahan, O.; Gingold, H.; Pilpel, Y. Regulatory mechanisms and networks couple the different phases of gene expression. Trends Genet. 2011, 27, 316–322. [Google Scholar] [CrossRef]
- Schwanhäusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koussounadis, A.; Langdon, S.P.; Um, I.H.; Harrison, D.J.; Smith, V.A. Relationship between differentially expressed mRNA and mRNA-protein correlations in a xenograft model system. Sci. Rep. 2015, 5, 10775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, C.; Abreu Rde, S.; Ko, D.; Le, S.Y.; Shapiro, B.A.; Burns, S.C.; Sandhu, D.; Boutz, D.R.; Marcotte, E.M.; Penalva, L.O. Sequence signatures and mRNA concentration can explain two-thirds of protein abundance variation in a human cell line. Mol. Syst. Biol. 2010, 6, 400. [Google Scholar] [CrossRef] [PubMed]
- Kindt, L.M.; Coughlin, A.R.; Perosino, T.R.; Ersfeld, H.N.; Hampton, M.; Liang, J.O. Identification of transcripts potentially involved in neural tube closure using RNA sequencing. Genesis 2018, 56, e23096. [Google Scholar] [CrossRef]
- Chau, K.F.; Shannon, M.L.; Fame, R.M.; Fonseca, E.; Mullan, H.; Johnson, M.B.; Sendamarai, A.K.; Springel, M.W.; Laurent, B.; Lehtinen, M.K. Downregulation of ribosome biogenesis during early forebrain development. eLife 2018, 7, e3. [Google Scholar] [CrossRef]
- Vogel, C.; Marcotte, E.M. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet. 2012, 13, 227–232. [Google Scholar] [CrossRef] [Green Version]
- Eden, E.; Geva-Zatorsky, N.; Issaeva, I.; Cohen, A.; Dekel, E.; Danon, T.; Cohen, L.; Mayo, A.; Alon, U. Proteome half-life dynamics in living human cells. Science 2011, 331, 764–768. [Google Scholar] [CrossRef] [Green Version]
- Mughal, B.B.; Leemans, M.; Spirhanzlova, P.; Demeneix, B.; Fini, J.-B. Reference gene identification and validation for quantitative real-time PCR studies in developing Xenopus laevis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Close, B.; Banister, K.; Baumans, V.; Bernoth, E.-M.; Bromage, N.; Bunyan, J.; Hackbarth, H. Recommendations for euthanasia of experimental animals: Part 2. Lab. Anim. 1997, 31, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nieuwkoop, P.D.; Faber, J. Normal Table of Xenopus Laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis; Garland Pub: Redhill, UK, 1994. [Google Scholar]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Shipley, G.L. The MIQE Guidelines: Minimum Information for Publication of Q uantitative Real-Time PCR Experiments; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- DeLano, W.L. Pymol: An open-source molecular graphics tool. CCP4 Newsl. Protein Crystallogr. 2002, 40, 82–92. [Google Scholar]
- Olsson, M.H.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent treatment of internal and surface residues in empirical p K a predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tovar, L.M.; Burgos, C.F.; Yévenes, G.E.; Moraga-Cid, G.; Fuentealba, J.; Coddou, C.; Bascunan-Godoy, L.; Catrupay, C.; Torres, A.; Castro, P.A. Understanding the Role of ATP Release through Connexins Hemichannels during Neurulation. Int. J. Mol. Sci. 2023, 24, 2159. https://doi.org/10.3390/ijms24032159
Tovar LM, Burgos CF, Yévenes GE, Moraga-Cid G, Fuentealba J, Coddou C, Bascunan-Godoy L, Catrupay C, Torres A, Castro PA. Understanding the Role of ATP Release through Connexins Hemichannels during Neurulation. International Journal of Molecular Sciences. 2023; 24(3):2159. https://doi.org/10.3390/ijms24032159
Chicago/Turabian StyleTovar, Lina Mariana, Carlos Felipe Burgos, Gonzalo E. Yévenes, Gustavo Moraga-Cid, Jorge Fuentealba, Claudio Coddou, Luisa Bascunan-Godoy, Claudio Catrupay, Angel Torres, and Patricio A. Castro. 2023. "Understanding the Role of ATP Release through Connexins Hemichannels during Neurulation" International Journal of Molecular Sciences 24, no. 3: 2159. https://doi.org/10.3390/ijms24032159
APA StyleTovar, L. M., Burgos, C. F., Yévenes, G. E., Moraga-Cid, G., Fuentealba, J., Coddou, C., Bascunan-Godoy, L., Catrupay, C., Torres, A., & Castro, P. A. (2023). Understanding the Role of ATP Release through Connexins Hemichannels during Neurulation. International Journal of Molecular Sciences, 24(3), 2159. https://doi.org/10.3390/ijms24032159






