Potential Roles of YAP/TAZ Mechanotransduction in Spaceflight-Induced Liver Dysfunction
Abstract
1. Introduction
2. Microgravity Induces Liver Dysfunction
2.1. Inflight Studies
| Object | Method (Space Mission) | Duration | Outcome | Refs |
|---|---|---|---|---|
| PICM-19 pig liver stem cells | Space shuttle (STS-126) | 16 days | CYP450 N.S. Urea secretion N.S. Liver-specific genes N.S. | [10] |
| Japanese quail liver | Orbital station Mir | 5 days | Number of lipid droplets ↑ | [17] |
| Mouse liver | ISS (RR-1, RR-3) and Space shuttle (STS-135) | 13.5~42 days | Lipid deposition ↑ Lipid metabolism ↑ Lipotoxic pathways ↑ | [18] |
| Mouse liver | Space shuttle (STS-135) | 13.5 days | Lipid droplets ↑ HSCs activation ↑ ECM remodeling ↑ PPAR pathways ↑ Retinol storage in HSCs lipid droplets ↓ | [19] |
| Rat liver | Satellite (Cosmos 2044) | 14 days | Glycogen storage ↑ Tyrosine aminotransferase ↑ Tryptophan oxygenase ↑ Cholesterol and sphingolipids ↓ δ-aminolevulinic acid synthase ↓ CYP450 N.S. | [21] |
| Rat liver | Satellite (Cosmos 936) | 18.5 days | Glycogen storage ↑ Palmitoyl CoA desaturase ↑ Glycogen phosphorylase ↓ α-glycerol phosphate acyltransferase ↓ Diglyceride acyltransferase ↓ Aconitase ↓ 6-phosphogluconate dehydrogenase ↓ | [22] |
| Mouse liver | Space shuttle (STS-135) | 13.5 days | Fatty acid oxidation ↑ Viral infection defense ↑ Phagocytosis ↑ CD8+ T cells activation ↓ Glycolysis ↓ Glycogen storage ↓ | [23] |
| Rat liver | Space shuttle (SLS-2) | 14 days | CYP450 ↓ | [25] |
| Mouse liver | Satellite (Bion-M1) | 30 days | CYP1A2, 2C29, 2E1 ↑ | [26] |
| Mouse liver | Space shuttle (STS-108) | 12 days | CYP4A1 ↑ T cells activation ↑ CDK inhibitor 1A ↑ Apoptosis ↑ Cell death ↑ | [27] |
| Mouse liver | ISS (Kibo) | 35 days | Hepatic cells proliferation in offspring ↑ | [28] |
| Rat liver | Space shuttle (SLS-1) | 9 days | Glycogen storage ↑ Lipid deposition ↑ CYP4A1 ↑ Crip ↑ HSP90 ↓ p53 ↓ Glutathione-S-transferase N.S. | [25,29] |
| Mouse liver | Space shuttle (STS-135) | 13.5 days | Reactive oxygen species ↑ Autophagy ↑ Proteasome ↑ Lipid deposition ↑ Hepatocyte senescence ↑ Glutathione levels ↓ NFE2L2-mediated pathway ↓ | [30] |
| Human blood | ISS | 180 days | Total cholesterol ↑ Low-density lipoprotein ↑ High-density lipoprotein ↓ | [6] |
2.2. Ground-Based Studies
| Object | Method | Condition | Outcome | Refs |
|---|---|---|---|---|
| Rat liver | Tail suspension | 2 months | AST, ALT ↑ Apoptosis ↑ Serum glucose ↓ Glycogen storage ↓ | [32] |
| Rat liver | Tail suspension | 14~42 days | AST, ALT ↑ Alkaline phosphatase ↓ Hepatocyte proliferation ↓ | [33] |
| Human liver | Head-down | −15°, 12 h | Portal vein blood flow ↓ | [34] |
| Human liver | Head-down | −6°, 85 days | Portal vein blood flow ↑ Portal vein cross-section area ↑ | [35] |
| Porcine primary hepatocyte | RWV | 10~15 rpm, 12 days | Albumin ↑ Hepatocyte polarity ↑ α5 integrin ↑ | [39] |
| HepG2 cells | RWV | 20~30 rpm, 1~15 days | Lactate dehydrogenase ↑ Alpha-fetoprotein ↑ CD29, CD44, CD54 ↑ E-cadherin ↓ Glucose consumption ↓ | [40] |
| HepG2 cells | RWV | 1~10 days | Albumin ↑ α1 antitrypsin ↑ Proliferation ↑ CYP1A1, 1A2 ↓ | [41] |
| Mouse fetal liver cells | RWV | 15~18.5 rpm, 5~10 days | Albumin ↑ α1 antitrypsin ↑ Glucose-6-phosphatase ↑ Tryptophan-2,3-dioxygenase ↑ Asialoglycoprotein receptor ↑ Ornithine transcarbamylase ↑ Ammonia elimination ↑ CYP3A ↑ | [43] |
| HepG2 cells | RWV | 16~20 rpm, 6 h~7 days | Albumin ↑ CYP450 ↑ ECM ↓ APOA1, APOA2, APOB ↓ | [44,46] |
| Mouse primary hepatocytes | RWV | 16 rpm, 4 h~3 days | Albumin ↑ CYP1A1 ↑ Metabolic genes ↑ Mesenchymal genes ↓ Cytoskeletal genes ↓ Proliferation ↓ | [47] |
| CCL-13 cells | 3D clinostat | 72 h | Proliferation ↓ α-tubulin 3, β-actin ↓ | [48] |
| HepG2 cells | 3D clinostat | 0~3 days | Apoptosis ↑ Autophagy ↑ | [49] |
| HepG2/C3A cells | Clinostat | 22~25 days | Drug metabolism ↑ | [51] |
3. YAP/TAZ May Bridge Microgravity and Liver Dysfunction
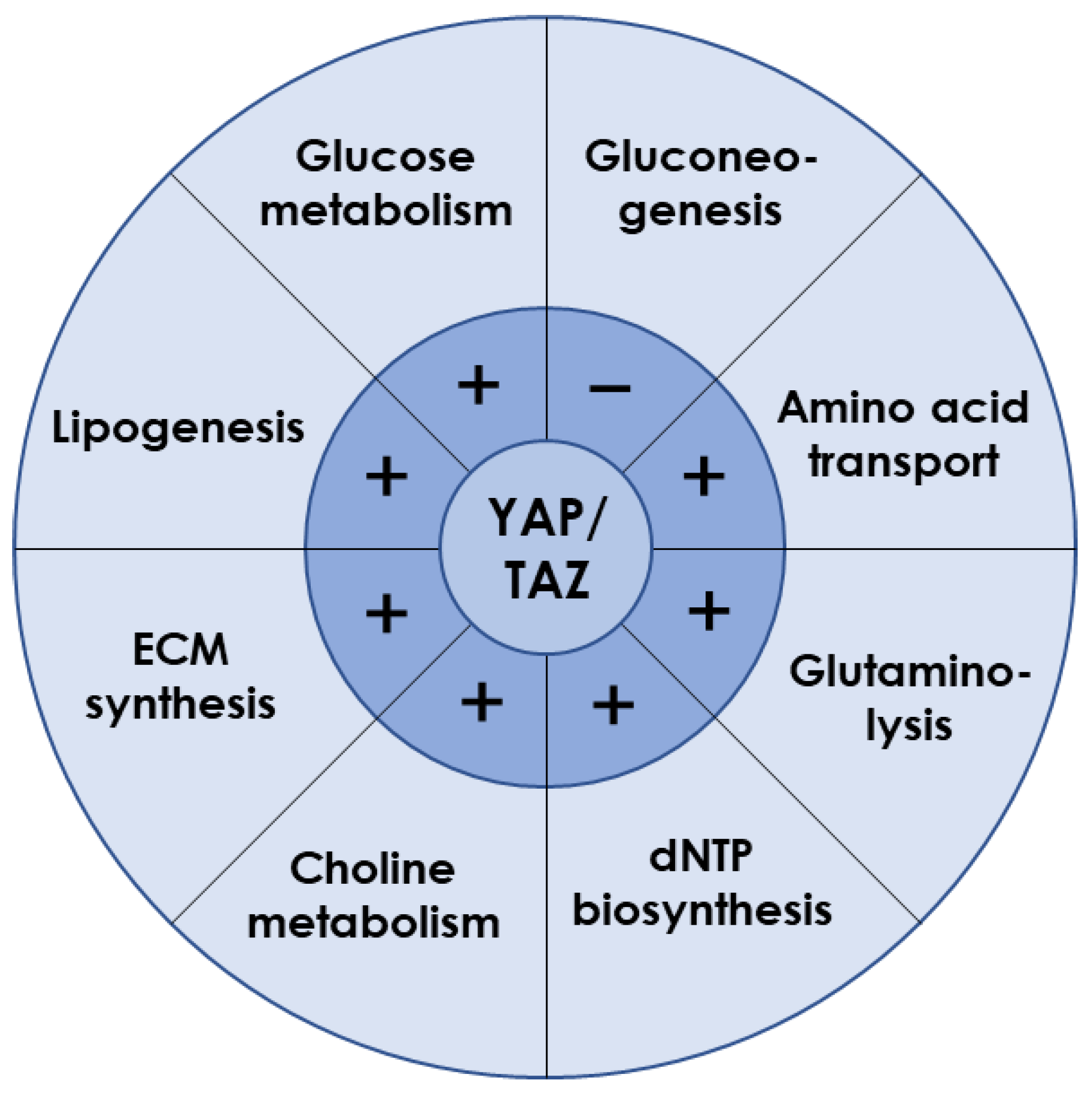
3.1. YAP/TAZ Is Essential for Liver Metabolism
3.2. Microgravity Regulates YAP/TAZ Activation
4. YAP/TAZ Pathway Could Be Specialized in Microgravity-Induced Liver Dysfunction
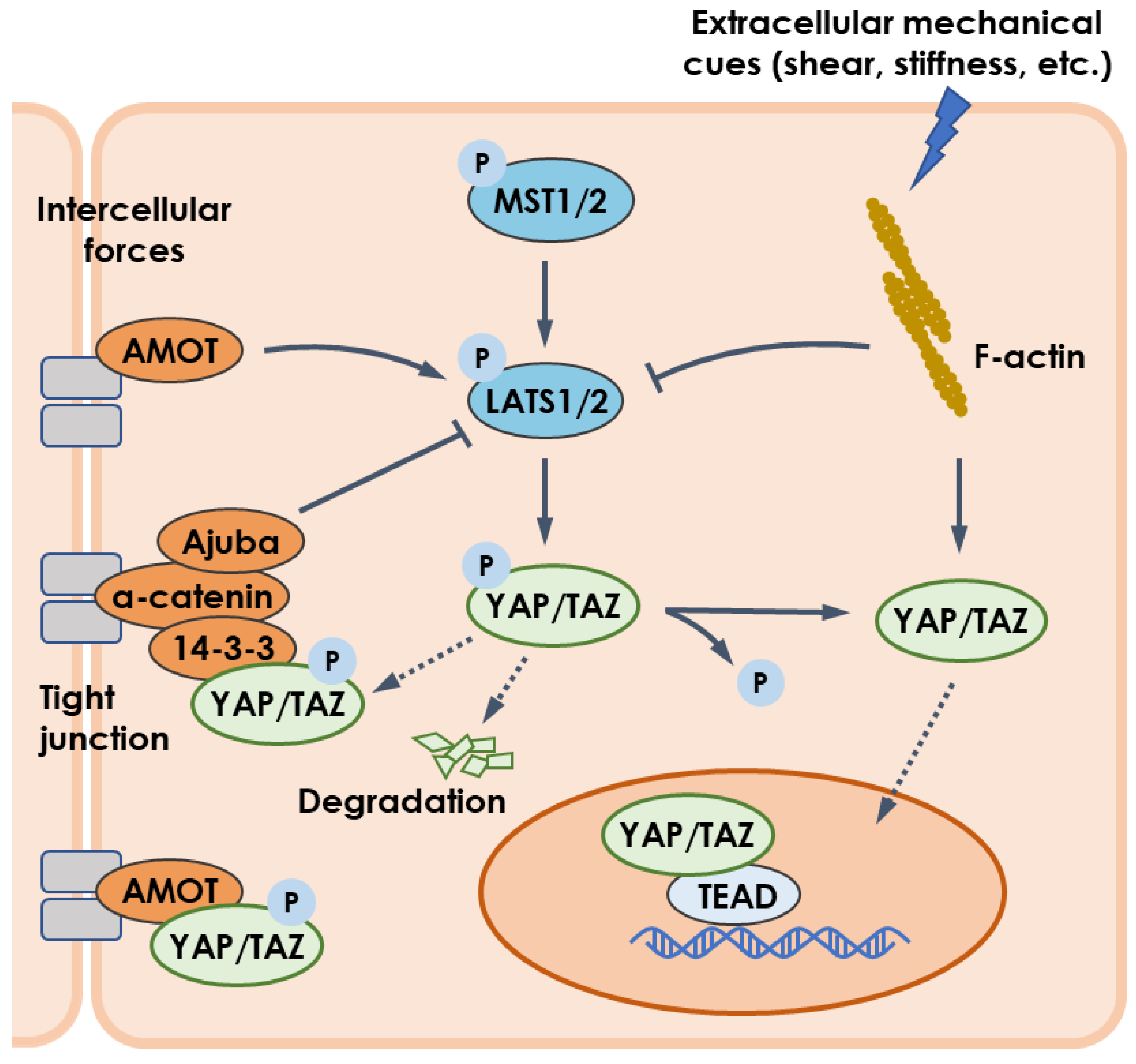
4.1. Mechanotransduction and YAP/TAZ Pathway
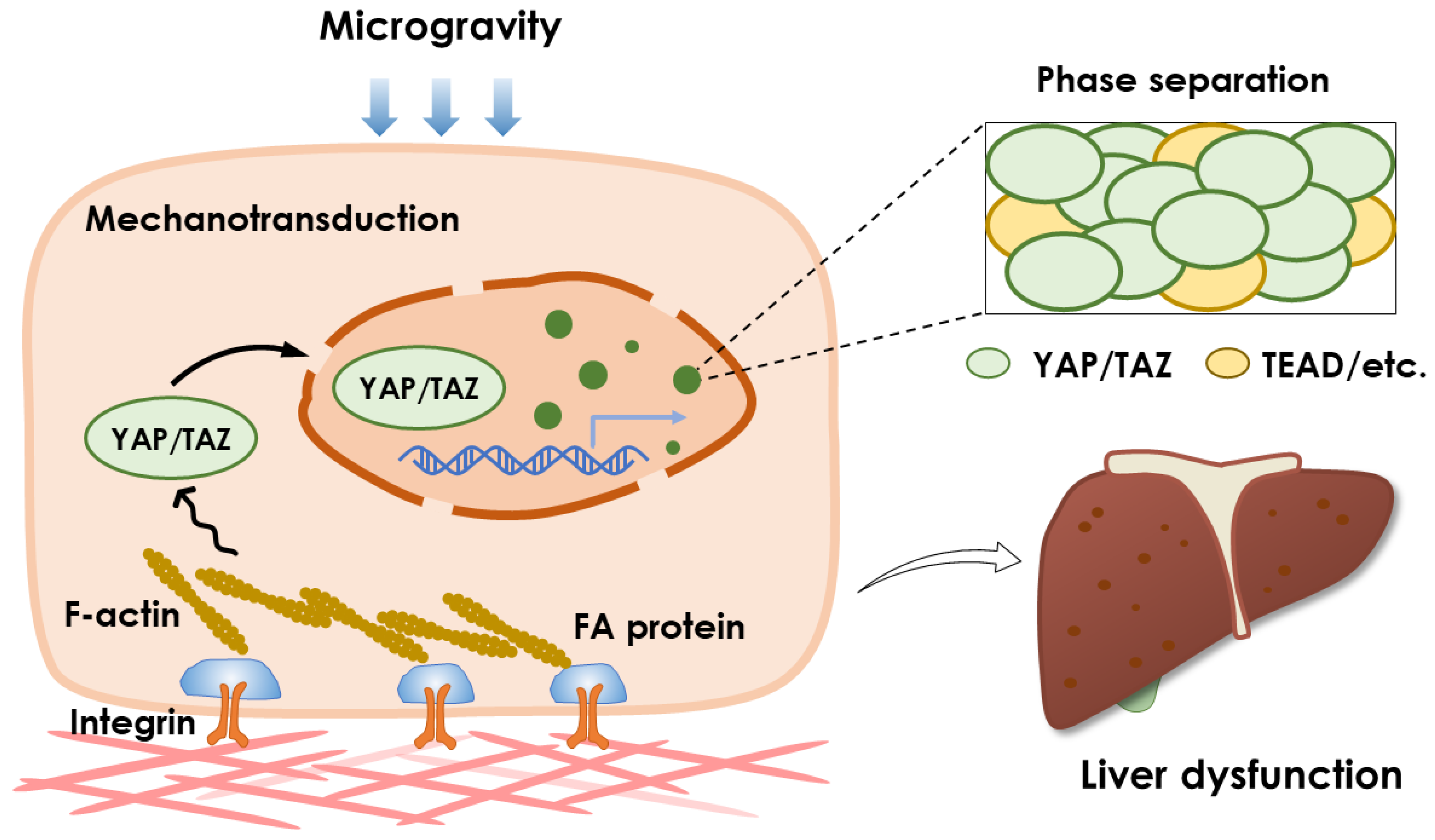
4.2. Phase Separation and YAP/TAZ Pathway
5. Conclusive Remarks and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Afshinnekoo, E.; Scott, R.T.; MacKay, M.J.; Pariset, E.; Cekanaviciute, E.; Barker, R.; Gilroy, S.; Hassane, D.; Smith, S.M.; Zwart, S.R.; et al. Fundamental biological features of spaceflight: Advancing the field to enable deep-space exploration. Cell 2020, 183, 1162–1184. [Google Scholar] [CrossRef] [PubMed]
- Garrett-Bakelman, F.E.; Darshi, M.; Green, S.J.; Gur, R.C.; Lin, L.; Macias, B.R.; McKenna, M.J.; Meydan, C.; Mishra, T.; Nasrini, J.; et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight. Science 2019, 364, eaau8650. [Google Scholar] [CrossRef] [PubMed]
- Prasad, B.; Grimm, D.; Strauch, S.M.; Erzinger, G.S.; Corydon, T.J.; Lebert, M.; Magnusson, N.E.; Infanger, M.; Richter, P.; Krüger, M. Influence of microgravity on apoptosis in cells, tissues, and other systems in vivo and in vitro. Int. J. Mol. Sci. 2020, 21, 9373. [Google Scholar] [CrossRef] [PubMed]
- ElGindi, M.; Sapudom, J.; Ibrahim, I.H.; Al-Sayegh, M.; Chen, W.; Garcia-Sabaté, A.; Teo, J.C.M. May the force be with you (or not): The immune system under microgravity. Cells 2021, 10, 1941. [Google Scholar] [CrossRef]
- Moser, D.; Sun, S.J.; Li, N.; Biere, K.; Hoerl, M.; Matzel, S.; Feuerecker, M.; Buchheim, J.I.; Strewe, C.; Thiel, C.S.; et al. Cells’ flow and immune cell priming under alternating g-forces in parabolic flight. Sci. Rep. 2019, 9, 11276. [Google Scholar] [CrossRef]
- da Silveira, W.A.; Fazelinia, H.; Rosenthal, S.B.; Laiakis, E.C.; Kim, M.S.; Meydan, C.; Kidane, Y.; Rathi, K.S.; Smith, S.M.; Stear, B.; et al. Comprehensive multi-omics analysis reveals mitochondrial stress as a central biological hub for spaceflight impact. Cell 2020, 183, 1185–1201.e20. [Google Scholar] [CrossRef]
- Kast, J.; Yu, Y.; Seubert, C.N.; Wotring, V.E.; Derendorf, H. Drugs in space: Pharmacokinetics and pharmacodynamics in astronauts. Eur. J. Pharm. Sci. 2017, 109, S2–S8. [Google Scholar] [CrossRef] [PubMed]
- Eyal, S. How do the pharmacokinetics of drugs change in astronauts in space? Expert Opin. Drug Metab. Toxicol. 2020, 16, 353–356. [Google Scholar] [CrossRef]
- Vinken, M. Hepatology in space: Effects of spaceflight and simulated microgravity on the liver. Liver Int. 2022, 42, 2599–2606. [Google Scholar] [CrossRef]
- Talbot, N.C.; Caperna, T.J.; Blomberg, L.; Graninger, P.G.; Stodieck, L.S. The effects of space flight and microgravity on the growth and differentiation of PICM-19 pig liver stem cells. Vitr. Cell. Dev. Biol. Anim. 2010, 46, 502–515. [Google Scholar] [CrossRef]
- Wuest, S.L.; Richard, S.; Kopp, S.; Grimm, D.; Egli, M. Simulated microgravity: Critical review on the use of random positioning machines for mammalian cell culture. BioMed Res. Int. 2015, 2015, 971474. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Wang, Y.; Zheng, H.; Shang, P.; Duan, E.; Lü, D. Mechano-biological coupling of cellular responses to microgravity. Microgravity Sci. Technol. 2015, 27, 505–514. [Google Scholar] [CrossRef]
- Zou, R.; Xu, Y.; Feng, Y.; Shen, M.; Yuan, F.; Yuan, Y. YAP nuclear-cytoplasmic translocation is regulated by mechanical signaling, protein modification, and metabolism. Cell Biol. Int. 2020, 44, 1416–1425. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Camberos, V.; Baio, J.; Bailey, L.; Hasaniya, N.; Lopez, L.V.; Kearns-Jonker, M. Effects of spaceflight and simulated microgravity on YAP1 expression in cardiovascular progenitors: Implications for cell-based repair. Int. J. Mol. Sci. 2019, 20, 2742. [Google Scholar] [CrossRef]
- Koo, J.H.; Guan, K.L. Interplay between YAP/TAZ and metabolism. Cell Metab. 2018, 28, 196–206. [Google Scholar] [CrossRef]
- Zibrin, M.; Cigankova, V.; Kocisova, J.; Tomajkova, E.; Komorova, T.; Boda, K.; Weismann, P.; Dadasheva, O.A.; Guryeva, T.S. Effect of short-term space flight on structure of liver, lungs, bone and bone marrow of Japanese quail hatched on orbital station Mir. Acta Vet. Brno 2005, 74, 167–174. [Google Scholar] [CrossRef]
- Beheshti, A.; Chakravarty, K.; Fogle, H.; Fazelinia, H.; da Silveira, W.A.; Boyko, V.; Polo, S.-H.L.; Saravia-Butler, A.M.; Hardiman, G.; Taylor, D.; et al. Multi-omics analysis of multiple missions to space reveal a theme of lipid dysregulation in mouse liver. Sci. Rep. 2019, 9, 19195. [Google Scholar] [CrossRef]
- Jonscher, K.R.; Alfonso-Garcia, A.; Suhalim, J.L.; Orlicky, D.J.; Potma, E.O.; Ferguson, V.L.; Bouxsein, M.L.; Bateman, T.A.; Stodieck, L.S.; Levi, M.; et al. Spaceflight activates lipotoxic pathways in mouse liver. PLoS ONE 2016, 11, e0152877. [Google Scholar]
- Anselm, V.; Novikova, S.; Zgoda, V. Re-adaption on Earth after spaceflights affects the mouse liver proteome. Int. J. Mol. Sci. 2017, 18, 1763. [Google Scholar] [CrossRef]
- Merrill, A.H.; Wang, E.; Larocque, R.; Mullins, R.E.; Morgan, E.T.; Hargrove, J.L.; Bonkovsky, H.L.; Popova, I.A. Differences in glycogen, lipids, and enzymes in livers from rats flown on COSMOS-2044. J. Appl. Physiol. 1992, 73, S142–S147. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.; Klein, H.P.; Lin, C.Y.; Volkmann, C. The effects of space flight on some rat liver enzymes regulating carbohydrate and lipid metabolism. Adv. Space Res. 1981, 1, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Pecaut, M.J.; Mao, X.W.; Bellinger, D.L.; Jonscher, K.R.; Stodieck, L.S.; Ferguson, V.L.; Bateman, T.A.; Mohney, R.P.; Gridley, D.S. Is spaceflight-induced immune dysfunction linked to systemic changes in metabolism? PLoS ONE 2017, 12, e0174174. [Google Scholar] [CrossRef]
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug metabolism in the liver. Clin. Liver Dis. 2017, 21, 1–20. [Google Scholar] [CrossRef]
- Rabot, S.; Szylit, O.; Nugon-Baudon, L.; Meslin, J.C.; Vaissade, P.; Popot, F.; Viso, M. Variations in digestive physiology of rats after short duration flights aboard the US space shuttle. Dig. Dis. Sci. 2000, 45, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Moskaleva, N.; Moysa, A.; Novikova, S.; Tikhonova, O.; Zgoda, V.; Archakov, A. Spaceflight effects on cytochrome P450 content in mouse liver. PLoS ONE 2015, 10, e0142374. [Google Scholar] [CrossRef]
- Hammond, T.G.; Allen, P.L.; Birdsall, H.H. Effects of space flight on mouse liver versus kidney: Gene pathway analyses. Int. J. Mol. Sci. 2018, 19, 4106. [Google Scholar] [CrossRef]
- Yoshida, K.; Fujita, S.-i.; Isotani, A.; Kudo, T.; Takahashi, S.; Ikawa, M.; Shiba, D.; Shirakawa, M.; Muratani, M.; Ishii, S. Intergenerational effect of short-term spaceflight in mice. Iscience 2021, 24, 102773. [Google Scholar] [CrossRef]
- Baba, T.; Nishimura, M.; Kuwahara, Y.; Ueda, N.; Naitoh, S.; Kume, M.; Yamamoto, Y.; Fujita, J.; Funae, Y.; Fukumoto, M. Analysis of gene and protein expression of cytochrome P450 and stress-associated molecules in rat liver after spaceflight. Pathol. Int. 2008, 58, 589–595. [Google Scholar] [CrossRef]
- Blaber, E.A.; Pecaut, M.J.; Jonscher, K.R. Spaceflight activates autophagy programs and the proteasome in mouse liver. Int. J. Mol. Sci. 2017, 18, 2062. [Google Scholar] [CrossRef]
- Tobin, B.W.; Uchakin, P.N.; Leeper-Woodford, S.K. Insulin secretion and sensitivity in space flight: Diabetogenic effects. Nutrition 2002, 18, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Ding, Y.; Zou, J.; Li, Z.; Tian, J.; She, R.; Wang, D.; Wang, H.; Lv, D.; Chang, L. Morphology and molecular mechanisms of hepatic injury in rats under simulated weightlessness and the protective effects of resistance training. PLoS ONE 2015, 10, e0127047. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Yang, C.; Zhang, H.; Wu, F.; Chen, J.; Li, K.; Wang, H.; Li, Y.; Li, Y.; et al. Upregulation of miR-223 in the rat liver inhibits proliferation of hepatocytes under simulated microgravity. Exp. Mol. Med. 2017, 49, e348. [Google Scholar] [CrossRef] [PubMed]
- Afonin, B.V.; Sedova, E.A.; Tikhonova, G.A.; Solov’eva, A.A.; Valuev, V.A. Evaluation of the liver functional changes due to modeling the hemodynamic effects of microgravity in bed rest studies. Aviakosm. Ekolog. Med. 2014, 48, 17–22. [Google Scholar]
- Arbeille, P.P.; Besnard, S.S.; Kerbeci, P.P.; Mohty, D.M. Portal vein cross-sectional area and flow and orthostatic tolerance: A 90-day bed rest study. J. Appl. Physiol. 2005, 99, 1853–1857. [Google Scholar] [CrossRef]
- Feuerecker, M.; Feuerecker, B.; Matzel, S.; Long, M.; Strewe, C.; Kaufmann, I.; Hoerl, M.; Schelling, G.; Rehm, M.; Choukèr, A. Five days of head-down-tilt bed rest induces noninflammatory shedding of L-selectin. J. Appl. Physiol. 2013, 115, 235–242. [Google Scholar] [CrossRef]
- Wang, C.; Li, N.; Zhang, C.; Sun, S.; Gao, Y.; Long, M. Effects of simulated microgravity on functions of neutrophil-like HL-60 cells. Microgravity Sci. Technol. 2015, 27, 515–527. [Google Scholar] [CrossRef]
- Khaoustov, V.I.; Risin, D.; Pellis, N.R.; Yoffe, B. Microarray analysis of genes differentially expressed in HepG2 cells cultured in simulated microgravity: Preliminary report. Vitr. Cell. Dev. Biol. Anim. 2001, 37, 84–88. [Google Scholar] [CrossRef]
- Nelson, L.J.; Walker, S.W.; Hayes, P.C.; Plevris, J.N. Low-shear modelled microgravity environment maintains morphology and differentiated functionality of primary porcine hepatocyte cultures. Cells Tissues Organs 2010, 192, 125–140. [Google Scholar] [CrossRef]
- Xu, D.-Y.; Yun, W.; Feng, M.-F. Studies on HepG2 growth under simulated microgravity: To establish a method for three-dimensional cultivation in vitro as an research model. Prog. Biochem. Biophys. 2007, 34, 146–153. [Google Scholar]
- Coward, S.M.; Selden, C.; Mantalaris, A.; Hodgson, H.J.F. Proliferation rates of HepG2 cells encapsulated in alginate are increased in a microgravity environment compared with static cultures. Artif. Organs 2005, 29, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.A.; Arterburn, L.M.; Miller, A.P.; Cowger, N.L.; Hartley, S.M.; Andrews, A.; Silber, P.M.; Li, A.P. Maintenance of liver functions in rat hepatocytes cultured as spheroids in a rotating wall vessel. Vitr. Cell. Dev. Biol. Anim. 2003, 39, 13–20. [Google Scholar] [CrossRef]
- Ishikawa, M.; Sekine, K.; Okamura, A.; Zheng, Y.-w.; Ueno, Y.; Koike, N.; Tanaka, J.; Taniguchi, H. Reconstitution of hepatic tissue architectures from fetal liver cells obtained from a three-dimensional culture with a rotating wall vessel bioreactor. J. Biosci. Bioeng. 2011, 111, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.T.; Hughes-Fulford, M. Monolayer and spheroid culture of human liver hepatocellular carcinoma cell line cells demonstrate distinct global gene expression patterns and functional phenotypes. Tissue Eng. Part A 2009, 15, 559–567. [Google Scholar] [CrossRef]
- Tang, J.; Cui, J.; Chen, R.; Guo, K.; Kang, X.; Li, Y.; Gao, D.; Sun, L.; Xu, C.; Chen, J.; et al. A three-dimensional cell biology model of human hepatocellular carcinoma in vitro. Tumor Biol. 2011, 32, 469–479. [Google Scholar] [CrossRef]
- Clement, J.Q.; Lacy, S.M.; Wilson, B.L. Genome-wide gene expression profiling of microgravity effect on human liver cells. J. Gravit. Physiol. 2007, 14, P121–P122. [Google Scholar] [PubMed]
- Chang, T.T.; Hughes-Fulford, M. Molecular mechanisms underlying the enhanced functions of three-dimensional hepatocyte aggregates. Biomaterials 2014, 35, 2162–2171. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.N.Q.; Tran, M.T.; Doan, C.C.; Hoang, S.N.; Tran, D.H.; Le, L.T. Simulated microgravity inhibits the proliferation of Chang liver cells by attenuation of the major cell cycle regulators and cytoskeletal proteins. Int. J. Mol. Sci. 2021, 22, 4550. [Google Scholar] [CrossRef]
- Fukazawa, T.; Tanimoto, K.; Shrestha, L.; Imura, T.; Takahashi, S.; Sueda, T.; Hirohashi, N.; Hiyama, E.; Yuge, L. Simulated microgravity enhances CDDP-induced apoptosis signal via p53-independent mechanisms in cancer cells. PLoS ONE 2019, 14, e0219363. [Google Scholar] [CrossRef]
- Ikuzawa, M.; Asashima, M. Global expression of simulated microgravity-responsive genes in xenopus liver cells. Zool. Sci. 2008, 25, 828–837. [Google Scholar] [CrossRef]
- Stampar, M.; Frandsen, H.S.; Rogowska-Wrzesinska, A.; Wrzesinski, K.; Filipic, M.; Zegura, B. Hepatocellular carcinoma (HepG2/C3A) cell-based 3D model for genotoxicity testing of chemicals. Sci. Total Environ. 2021, 755, 143255. [Google Scholar] [CrossRef] [PubMed]
- Wrzesinski, K.; Fey, S.J. Metabolic reprogramming and the recovery of physiological functionality in 3d cultures in micro-bioreactors. Bioengineering 2018, 5, 22. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Chen, X.; Chen, L.; Wang, Z.; Wang, Y. Three-dimensional culture in a microgravity bioreactor improves the engraftment efficiency of hepatic tissue constructs in mice. J. Mater. Sci. Mater. Med. 2014, 25, 2699–2709. [Google Scholar] [CrossRef] [PubMed]
- Manning, S.A.; Kroeger, B.; Harvey, K.F. The regulation of Yorkie, YAP and TAZ: New insights into the Hippo pathway. Development 2020, 147, dev179069. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Tumaneng, K.; Wang, C.Y.; Guan, K.L. A coordinated phosphorylation by LATS and CK1 regulates YAP stability through SCF (beta-TrCP). Genes Dev. 2010, 24, 72–85. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zha, Z.Y.; Zhou, X.; Zhang, H.; Huang, W.; Zhao, D.; Li, T.; Chan, S.W.; Lim, C.J.; Hong, W.; et al. The Hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF (beta-TrCP) E3 ligase. J. Biol. Chem. 2010, 285, 37159–37169. [Google Scholar] [CrossRef]
- Park, J.; Hansen, C.G. Cellular feedback dynamics and multilevel regulation driven by the hippo pathway. Biochem. Soc. Trans. 2021, 49, 1515–1527. [Google Scholar] [CrossRef]
- Ibar, C.; Irvine, K.D. Integration of Hippo-YAP signaling with metabolism. Dev. Cell 2020, 54, 256–267. [Google Scholar] [CrossRef]
- Hao, L.; Guo, Y.; Peng, Q.; Zhang, Z.; Ji, J.; Liu, Y.; Xue, Y.; Li, C.; Zheng, K.; Shi, X. Dihydroartemisinin reduced lipid droplet deposition by YAP1 to promote the anti-PD-1 effect in hepatocellular carcinoma. Phytomedicine 2022, 96, 153913. [Google Scholar] [CrossRef]
- Jiao, T.; Yao, X.; Zhao, Y.; Zhou, Y.; Gao, Y.; Fan, S.; Chen, P.; Li, X.; Jiang, Y.; Yang, X.; et al. Dexamethasone-induced liver enlargement is related to PXR/YAP activation and lipid accumulation but not hepatocyte proliferation. Drug Metab. Dispos. 2020, 48, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Pei, Z.; Feng, Z.; Lin, P.; Wang, S.; Li, Y.; Huo, F.; Wang, Q.; Wang, Z.; Chen, Z.-N.; et al. Oncogenic activation of YAP signaling sensitizes ferroptosis of hepatocellular carcinoma via ALOXE3-mediated lipid peroxidation accumulation. Front. Cell Dev. Biol. 2021, 9, 751593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Qiu, H.; Ma, L.; Luo, J.; Sun, S.; Kang, K.; Gou, D.; Loor, J.J. miR-30e-5p and miR-15a synergistically regulate fatty acid metabolism in goat mammary epithelial cells via LRP6 and YAP1. Int. J. Mol. Sci. 2016, 17, 1909. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Ou, C.-H.; Huang, Y.-C.; Hou, P.-C.; Creighton, C.J.; Lin, Y.-S.; Hu, C.-Y.; Lin, S.-C. YAP1 overexpression contributes to the development of enzalutamide resistance by induction of cancer stemness and lipid metabolism in prostate cancer. Oncogene 2021, 40, 2407–2421. [Google Scholar] [CrossRef]
- Li, M.; Gao, Z.; Ding, H.; Wang, Z.; Mu, H.; Zhang, L.; Wei, J.; Ma, Z. FSCN1 promotes glycolysis and epithelial-mesenchymal transition in prostate cancer through a YAP/TAZ signaling pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 6245647. [Google Scholar] [CrossRef]
- Li, H.; Fu, L.; Lin, B.; Lin, X.; Dong, Q.; Wang, E. Ajuba overexpression regulates mitochondrial potential and glucose uptake through YAP/Bcl-xL/GLUT1 in human gastric cancer. Gene 2019, 693, 16–24. [Google Scholar] [CrossRef]
- Yu, T.; Liu, Y.; Xue, J.; Sun, X.; Zhu, D.; Ma, L.; Guo, Y.; Jin, T.; Cao, H.; Chen, Y.; et al. Gankyrin modulated non-small cell lung cancer progression via glycolysis metabolism in a YAP1-dependent manner. Cell Death Discov. 2022, 8, 312. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, S.; Wang, S.; Pan, X.; Zhang, Y.; Xu, J.; Jiang, Y.; Li, H.; Zhang, Q.; Gao, J.; et al. S1P/S1PR3 axis promotes aerobic glycolysis by YAP/c-MYC/PGAM1 axis in osteosarcoma. Ebiomedicine 2019, 40, 210–223. [Google Scholar] [CrossRef]
- Liu, Q.P.; Luo, Q.; Deng, B.; Ju, Y.; Song, G.B. Stiffer matrix accelerates migration of hepatocellular carcinoma cells through enhanced aerobic glycolysis via the MAPK-YAP signaling. Cancers 2020, 12, 490. [Google Scholar] [CrossRef]
- Li, Y.; Yang, S.; Liu, Y.; Yang, S. Deletion of Trp53 and Rb1 in Ctsk-expressing cells drives osteosarcoma progression by activating glucose metabolism and YAP signaling. MedComm 2022, 3, e131. [Google Scholar] [CrossRef]
- Wang, L.; Sun, J.; Gao, P.; Su, K.; Wu, H.; Li, J.; Lou, W. Wnt1-inducible signaling protein 1 regulates laryngeal squamous cell carcinoma glycolysis and chemoresistance via the YAP1/TEAD1/GLUT1 pathway. J. Cell. Physiol. 2019, 234, 15941–15950. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, T.; Mukai, R.; Oka, S.-i.; Zhai, P.; Nakada, Y.; Yang, Z.; Mizushima, W.; Nakahara, T.; Warren, J.S.; Abdellatif, M.; et al. YAP mediates compensatory cardiac hypertrophy through aerobic glycolysis in response to pressure overload. J. Clin. Investig. 2022, 132, e150595. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zhang, Q.; Yuan, W.; Li, X.; Chen, C.; Guo, Y.; Shao, B.; Dang, Q.; Zhou, Q.; Wang, Q.; et al. MiR-103a-3p promotes tumour glycolysis in colorectal cancer via hippo/YAP1/HIF1A axis. J. Exp. Clin. Cancer Res. 2020, 39, 250. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S. LncRNA GHET1 promotes hypoxia-induced glycolysis, proliferation, and invasion in triple-negative breast cancer through the Hippo/YAP signaling pathway. Front. Cell Dev. Biol. 2021, 9, 643515. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.-Y.; Wang, J.; Wang, Y.; Zhang, P.; Ma, N.; Mo, S.-J. Phosphorylation of 14-3-3 zeta links YAP transcriptional activation to hypoxic glycolysis for tumorigenesis. Oncogenesis 2019, 8, 31. [Google Scholar] [CrossRef]
- Jia, Y.; Li, H.-Y.; Wang, Y.; Wang, J.; Zhu, J.-W.; Wei, Y.-Y.; Lou, L.; Chen, X.; Mo, S.-J. Crosstalk between hypoxia-sensing ULK1/2 and YAP-driven glycolysis fuels pancreatic ductal adenocarcinoma development. Int. J. Biol. Sci. 2021, 17, 2772–2794. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Y.; Yang, L.; Wang, T.; Meng, X.; Zong, Z.; Sun, X.; Hua, X.; Li, H. Yes-associated protein (YAP) binds to HIF-1 alpha and sustains HIF-1 alpha protein stability to promote hepatocellular carcinoma cell glycolysis under hypoxic stress. J. Exp. Clin. Cancer Res. 2018, 37, 216. [Google Scholar] [CrossRef]
- Feng, Y.; Zou, R.; Zhang, X.; Shen, M.; Chen, X.; Wang, J.; Niu, W.; Yuan, Y.; Yuan, F. YAP promotes ocular neovascularization by modifying PFKFB3-driven endothelial glycolysis. Angiogenesis 2021, 24, 489–504. [Google Scholar] [CrossRef]
- Pocaterra, A.; Santinon, G.; Romani, P.; Brian, I.; Dimitracopoulos, A.; Ghisleni, A.; Carnicer-Lombarte, A.; Forcato, M.; Braghetta, P.; Montagner, M.; et al. F-actin dynamics regulates mammalian organ growth and cell fate maintenance. J. Hepatol. 2019, 71, 130–142. [Google Scholar] [CrossRef]
- Hu, Y.; Shin, D.J.; Pan, H.; Lin, Z.; Dreyfuss, J.M.; Camargo, F.D.; Miao, J.; Biddinger, S.B. YAP suppresses gluconeogenic gene expression through PGC1α. Hepatology 2017, 66, 2029–2041. [Google Scholar] [CrossRef]
- Han, D.J.; Aslam, R.; Misra, P.; Chiu, F.; Ojha, T.; Chowdhury, A.; Chan, C.K.; Sung, H.-K.; Yuen, D.A.; Luk, C.T. Disruption of adipocyte YAP improves glucose homeostasis in mice and decreases adipose tissue fibrosis. Mol. Metab. 2022, 66, 101594. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ma, K.; Kang, Y.; Liu, W.; Liu, X.; Long, X.; Hayashi, T.; Hattori, S.; Mizuno, K.; Fujisaki, H.; et al. Type I collagen reduces lipid accumulation during adipogenesis of preadipocytes 3T3-L1 via the YAP-mTOR-autophagy axis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159181. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.-M.; Liu, J.-Y.; Yu, C.-W.; Fan, J.-X.; Li, T.; Yang, J.-X.; Zheng, Y.-B.; Liu, F.-C.; He, Z.-T.; Yuan, H.-L.; et al. PLC epsilon knockdown prevents serine/glycine metabolism and proliferation of prostate cancer by suppressing YAP. Am. J. Cancer Res. 2020, 10, 196–210. [Google Scholar] [PubMed]
- Wu, Q.; Li, J.; Sun, S.; Chen, X.; Zhang, H.; Li, B.; Sun, S. YAP/TAZ-mediated activation of serine metabolism and methylation regulation is critical for LKB1-deficient breast cancer progression. Biosci. Rep. 2017, 37, BSR20171072. [Google Scholar] [CrossRef]
- Jeon, H.Y.; Choi, J.; Kraaier, L.; Kim, Y.H.; Eisenbarth, D.; Yi, K.; Kang, J.-G.; Kim, J.W.; Shim, H.S.; Lee, J.-H.; et al. Airway secretory cell fate conversion via YAP-mTORC1-dependent essential amino acid metabolism. EMBO J. 2022, 41, e109365. [Google Scholar] [CrossRef]
- Edwards, D.N.; Ngwa, V.M.; Wang, S.; Shiuan, E.; Brantley-Sieders, D.M.; Kim, L.C.; Reynolds, A.B.; Chen, J. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci. Signal. 2017, 10, eaan4667. [Google Scholar] [CrossRef]
- Cox, A.G.; Hwang, K.L.; Brown, K.K.; Evason, K.J.; Beltz, S.; Tsomides, A.; O’Connor, K.; Galli, G.G.; Yimlamai, D.; Chhangawala, S.; et al. Yap reprograms glutamine metabolism to increase nucleotide biosynthesis and enable liver growth. Nat. Cell Biol. 2016, 18, 886–896. [Google Scholar] [CrossRef]
- Santinon, G.; Brian, I.; Pocaterra, A.; Romani, P.; Franzolin, E.; Rampazzo, C.; Bicciato, S.; Dupont, S. dNTP metabolism links mechanical cues and YAP/TAZ to cell growth and oncogene-induced senescence. EMBO J. 2018, 37, e97780. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, S.; Kofuji, S.; Nishina, H. YAP drives cell competition by activating choline metabolism. Biochem. Biophys. Res. Commun. 2021, 572, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, Z.; Zhu, Y.; Yan, J.; Li, J.; Chen, J.; Zhou, J.; Zhang, Y.; Chen, W.; Xu, K.; et al. Bromodomain-containing protein 7 regulates matrix metabolism and apoptosis in human nucleus pulposus cells through the BRD7-PI3K-YAP1 signaling axis. Exp. Cell Res. 2021, 405, 112658. [Google Scholar] [CrossRef]
- Baio, J.; Martinez, A.F.; Silva, I.; Hoehn, C.V.; Countryman, S.; Bailey, L.; Hasaniya, N.; Pecaut, M.J.; Kearns-Jonker, M. Cardiovascular progenitor cells cultured aboard the International Space Station exhibit altered developmental and functional properties. NPJ Microgravity 2018, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Arun, R.P.; Sivanesan, D.; Patra, B.; Varadaraj, S.; Verma, R.S. Simulated microgravity increases polyploid giant cancer cells and nuclear localization of YAP. Sci. Rep. 2019, 9, 10684. [Google Scholar] [CrossRef] [PubMed]
- Silvani, G.; Bradbury, P.; Basirun, C.; Mehner, C.; Zalli, D.; Poole, K.; Chou, J. Testing 3D printed biological platform for advancing simulated microgravity and space mechanobiology research. NPJ Microgravity 2022, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.; Woods, K.; Newberg, J.; Oxford, J.T.; Uzer, G. Low-intensity vibration restores nuclear YAP levels and acute YAP nuclear shuttling in mesenchymal stem cells subjected to simulated microgravity. NPJ Microgravity 2020, 6, 35. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, Q.; Lin, C.; Song, G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells through down regulating the transcriptional co-activator TAZ. Biochem. Biophys. Res. Commun. 2015, 468, 21–26. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, Q.; Lin, C.; Kuang, D.; Song, G. Simulated microgravity inhibits osteogenic differentiation of mesenchymal stem cells via depolymerizing F-actin to impede TAZ nuclear translocation. Sci. Rep. 2016, 6, 30322. [Google Scholar] [CrossRef]
- De Cesari, C.; Barravecchia, I.; Pyankova, O.V.; Vezza, M.; Germani, M.M.; Scebba, F.; van Loon, J.J.W.A.; Angeloni, D. Hypergravity activates a pro-angiogenic homeostatic response by human capillary endothelial cells. Int. J. Mol. Sci. 2020, 21, 2354. [Google Scholar] [CrossRef]
- Balsamo, M.; Barravecchia, I.; Mariotti, S.; Merenda, A.; De Cesari, C.; Vukich, M.; Angeloni, D. Molecular and cellular characterization of space flight effects on microvascular endothelial cell function—Preparatory work for the SFEF project. Microgravity Sci. Technol. 2014, 26, 351–363. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Zhou, J.; Li, W.; Shu, X.; Wu, Y.; Long, M. Multiscale biomechanics and mechanotransduction from liver fibrosis to cancer. Adv. Drug Deliv. Rev. 2022, 188, 114448. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, G.; Su, X.; Jin, C.; Yu, B.; Yu, X.; Lv, Z.; Ma, H.; Zhang, M.; Wei, W.; et al. Maintenance of primary hepatocyte functions in vitro by inhibiting mechanical tension-induced YAP activation. Cell Rep. 2019, 29, 3212–3222.e4. [Google Scholar] [CrossRef]
- Sorrentino, G.; Rezakhani, S.; Yildiz, E.; Nuciforo, S.; Heim, M.H.; Lutolf, M.P.; Schoonjans, K. Mechano-modulatory synthetic niches for liver organoid derivation. Nat. Commun. 2020, 11, 3416. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; He, J.; Su, G.; Wang, Y.; Fang, F.; Yang, W.; Gu, K.; Fu, N.; Wang, Y.; Shen, Y.; et al. Fluid shear stress activates YAP to promote epithelial-mesenchymal transition in hepatocellular carcinoma. Mol. Oncol. 2021, 15, 3164–3183. [Google Scholar] [CrossRef] [PubMed]
- Benham-Pyle, B.W.; Pruitt, B.L.; Nelson, W.J. Mechanical strain induces E-cadherin-dependent Yap1 and β-catenin activation to drive cell cycle entry. Science 2015, 348, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, R.; Schimizzi, G.V.; Zhang, K.; Loza, A.J.; Yabuta, N.; Nojima, H.; Longmore, G.D. Ajuba LIM proteins limit Hippo activity in proliferating cells by sequestering the Hippo core kinase complex in the cytosol. Mol. Cell. Biol. 2016, 36, 2526–2542. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Li, F.; Chan, S.W.; Lin, Z.; Wei, Z.; Yang, Z.; Guo, F.; Lim, C.J.; Xing, W.; et al. Angiomotin binding-induced activation of Merlin/NF2 in the Hippo pathway. Cell Res. 2015, 25, 801–817. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Lu, Q.; Wang, L.H.; Liu, C.Y.; Lei, Q.; Guan, K.L. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 2011, 25, 51–63. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Wang, L.; Wang, C.Y.; Yu, J.; Guan, K.L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012, 26, 54–68. [Google Scholar] [CrossRef]
- Maître, J.L.; Turlier, H.; Illukkumbura, R.; Eismann, B.; Niwayama, R.; Nédélec, F.; Hiiragi, T. Asymmetric division of contractile domains couples cell positioning and fate specification. Nature 2016, 536, 344–348. [Google Scholar] [CrossRef]
- Aragona, M.; Panciera, T.; Manfrin, A.; Giulitti, S.; Michielin, F.; Elvassore, N.; Dupont, S.; Piccolo, S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell 2013, 154, 1047–1059. [Google Scholar] [CrossRef]
- Guignandon, A.; Lafage-Proust, M.H.; Usson, Y.; Laroche, N.; Caillot-Augusseau, A.; Alexandre, C.; Vico, L. Cell cycling determines integrin-mediated adhesion in osteoblastic ROS 17/2.8 cells exposed to space-related conditions. FASEB J. 2001, 15, 2036–2038. [Google Scholar]
- Nabavi, N.; Khandani, A.; Camirand, A.; Harrison, R.E. Effects of microgravity on osteoclast bone resorption and osteoblast cytoskeletal organization and adhesion. Bone 2011, 49, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, C.; Sun, S.; Zhang, C.; Lü, D.; Chen, Q.; Long, M. Microgravity-induced alterations of inflammation-related mechanotransduction in endothelial cells on board SJ-10 satellite. Front. Physiol. 2018, 9, 1025. [Google Scholar] [CrossRef]
- Sun, S.; Wang, C.; Bi, Y.; Li, N.; Lü, D.; Chen, Q.; Chen, J.; Long, M. An integration design of gas exchange, bubble separation, and flow control in a space cell culture system on board the SJ-10 satellite. Rev. Sci. Instrum. 2019, 90, 075114. [Google Scholar] [CrossRef]
- Wu, X.T.; Yang, X.; Tian, R.; Li, Y.H.; Wang, C.Y.; Fan, Y.B.; Sun, L.W. Cells respond to space microgravity through cytoskeleton reorganization. FASEB J. 2022, 36, e22114. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Chen, S.; Hu, W.; Lü, S.; Long, M. Mechanical point loading induces cortex stiffening and actin reorganization. Biophys. J. 2019, 117, 1405–1418. [Google Scholar] [CrossRef]
- Guixé-Muntet, S.; Ortega-Ribera, M.; Wang, C.; Selicean, S.; Andreu, I.; Kechagia, J.Z.; Fondevila, C.; Roca-Cusachs, P.; Dufour, J.F.; Bosch, J.; et al. Nuclear deformation mediates liver cell mechanosensing in cirrhosis. JHEP Rep. 2020, 2, 100145. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Mofrad, M.R.K. On the nuclear pore complex and its emerging role in cellular mechanotransduction. APL Bioeng. 2022, 6, 011504. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A.; Andreu, I.; Beedle, A.E.M.; Lezamiz, A.; Uroz, M.; Kosmalska, A.J.; Oria, R.; Kechagia, J.Z.; Rico-Lastres, P.; Le Roux, A.L.; et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 2017, 171, 1397–1410.e14. [Google Scholar] [CrossRef]
- Driscoll, T.P.; Cosgrove, B.D.; Heo, S.J.; Shurden, Z.E.; Mauck, R.L. Cytoskeletal to nuclear strain transfer regulates YAP signaling in mesenchymal stem cells. Biophys. J. 2015, 108, 2783–2793. [Google Scholar] [CrossRef]
- Li, H.; Chen, J.; Zhang, Y.; Sun, S.; Tao, Z.; Long, M. Effects of oriented substrates on cell morphology, the cell cycle, and the cytoskeleton in Ros 17/2.8 cells. Sci. China Life Sci. 2010, 53, 1085–1091. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, L.; Zhang, F.; Lü, D.; Li, N.; Zheng, L.; Xu, Y.; Li, Z.; Sun, S.; Long, M. Mechanical remodeling of normally sized mammalian cells under a gravity vector. FASEB J. 2017, 31, 802–813. [Google Scholar] [CrossRef]
- Zhang, C.; Lü, D.; Zhang, F.; Wu, Y.; Zheng, L.; Zhang, X.; Li, Z.; Sun, S.; Long, M. Gravity-vector induces mechanical remodeling of rMSCs via combined substrate stiffness and orientation. Front. Bioeng. Biotechnol. 2021, 9, 724101. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, C.; Zhang, F.; Lü, S.; Sun, S.; Lü, D.; Long, M. Theoretical modeling of mechanical homeostasis of a mammalian cell under gravity-directed vector. Biomech. Model. Mechanobiol. 2018, 17, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Hughes-Fulford, M. Physiological effects of microgravity on osteoblast morphology and cell biology. Adv. Space Biol. Med. 2002, 8, 129–157. [Google Scholar] [PubMed]
- Su, Q.; Mehta, S.; Zhang, J. Liquid-liquid phase separation: Orchestrating cell signaling through time and space. Mol. Cell 2021, 81, 4137–4146. [Google Scholar] [CrossRef]
- Alberti, S. Phase separation in biology. Curr. Biol. 2017, 27, R1097–R1102. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, H.; Wen, W. Phase separation and mechanical forces in regulating asymmetric cell division of neural stem cells. Int. J. Mol. Sci. 2021, 22, 10267. [Google Scholar] [CrossRef]
- Loh, D.; Reiter, R.J. Melatonin: Regulation of biomolecular condensates in neurodegenerative disorders. Antioxidants 2021, 10, 1483. [Google Scholar] [CrossRef]
- Franklin, J.M.; Guan, K.-L. YAP/TAZ phase separation for transcription. Nat. Cell Biol. 2020, 22, 357–358. [Google Scholar] [CrossRef]
- Cai, D.; Feliciano, D.; Dong, P.; Flores, E.; Gruebele, M.; Porat-Shliom, N.; Sukenik, S.; Liu, Z.; Lippincott-Schwartz, J. Phase separation of YAP reorganizes genome topology for long-term YAP target gene expression. Nat. Cell Biol. 2019, 21, 1578–1589. [Google Scholar] [CrossRef]
- Yu, M.; Peng, Z.; Qin, M.; Liu, Y.; Wang, J.; Zhang, C.; Lin, J.; Dong, T.; Wang, L.; Li, S.; et al. Interferon-gamma induces tumor resistance to anti-PD-1 immunotherapy by promoting YAP phase separation. Mol. Cell 2021, 81, 1216–1230.e9. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wu, T.; Gutman, O.; Lu, H.; Zhou, Q.; Henis, Y.I.; Luo, K. Phase separation of TAZ compartmentalizes the transcription machinery to promote gene expression. Nat. Cell Biol. 2020, 22, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-H.; Tian, T.; Ge, Q.-W.; He, X.-Y.; Shi, C.-Y.; Li, J.-H.; Zhang, Z.; Liu, F.-Z.; Sang, L.-J.; Yang, Z.-Z.; et al. A phosphatidic acid-binding lncRNA SNHG9 facilitates LATS1 liquid-liquid phase separation to promote oncogenic YAP signaling. Cell Res. 2021, 31, 1088–1105. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, J.; Liu, Y.; Xie, S.A.; Zhang, J.; Zhao, C.; Zhou, Y.; Pang, W.; Yao, W.; Peng, Q.; et al. LLPS of DDR1 counteracts the Hippo Pathway to orchestrate arterial stiffening. Circ. Res. 2023, 132, 87–105. [Google Scholar] [CrossRef]
- Sun, X.; Ren, Z.; Cun, Y.; Zhao, C.; Huang, X.; Zhou, J.; Hu, R.; Su, X.; Ji, L.; Li, P.; et al. Hippo-YAP signaling controls lineage differentiation of mouse embryonic stem cells through modulating the formation of super-enhancers. Nucleic Acids Res. 2020, 48, 7182–7196. [Google Scholar] [CrossRef] [PubMed]
- Linsenmeier, M.; Kopp, M.R.G.; Grigolato, F.; Emmanoulidis, L.; Liu, D.; Zürcher, D.; Hondele, M.; Weis, K.; Capasso Palmiero, U.; Arosio, P. Dynamics of synthetic membraneless organelles in microfluidic droplets. Angew. Chem. Int. Ed. Engl. 2019, 58, 14489–14494. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Zhang, R.; Tsukamoto, K.; Li, A. Microgravity influence on the instability of phase separation in protein solution. Appl. Phys. Lett. 2015, 107, 123701. [Google Scholar] [CrossRef]
- Nichols, H.L.; Zhang, N.; Wen, X. Proteomics and genomics of microgravity. Physiol. Genom. 2006, 26, 163–171. [Google Scholar] [CrossRef]
- Grimm, D.; Wise, P.; Lebert, M.; Richter, P.; Baatout, S. How and why does the proteome respond to microgravity? Expert Rev. Proteom. 2011, 8, 13–27. [Google Scholar] [CrossRef]
- Najrana, T.; Sanchez-Esteban, J. Mechanotransduction as an adaptation to gravity. Front. Pediatr. 2016, 4, 140. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Shu, X.; Zhang, X.; Zhang, Z.; Sun, S.; Li, N.; Long, M. Potential Roles of YAP/TAZ Mechanotransduction in Spaceflight-Induced Liver Dysfunction. Int. J. Mol. Sci. 2023, 24, 2197. https://doi.org/10.3390/ijms24032197
Li W, Shu X, Zhang X, Zhang Z, Sun S, Li N, Long M. Potential Roles of YAP/TAZ Mechanotransduction in Spaceflight-Induced Liver Dysfunction. International Journal of Molecular Sciences. 2023; 24(3):2197. https://doi.org/10.3390/ijms24032197
Chicago/Turabian StyleLi, Wang, Xinyu Shu, Xiaoyu Zhang, Ziliang Zhang, Shujin Sun, Ning Li, and Mian Long. 2023. "Potential Roles of YAP/TAZ Mechanotransduction in Spaceflight-Induced Liver Dysfunction" International Journal of Molecular Sciences 24, no. 3: 2197. https://doi.org/10.3390/ijms24032197
APA StyleLi, W., Shu, X., Zhang, X., Zhang, Z., Sun, S., Li, N., & Long, M. (2023). Potential Roles of YAP/TAZ Mechanotransduction in Spaceflight-Induced Liver Dysfunction. International Journal of Molecular Sciences, 24(3), 2197. https://doi.org/10.3390/ijms24032197





