Neural Tube Defects and Folate Deficiency: Is DNA Repair Defective?
Abstract
1. Introduction
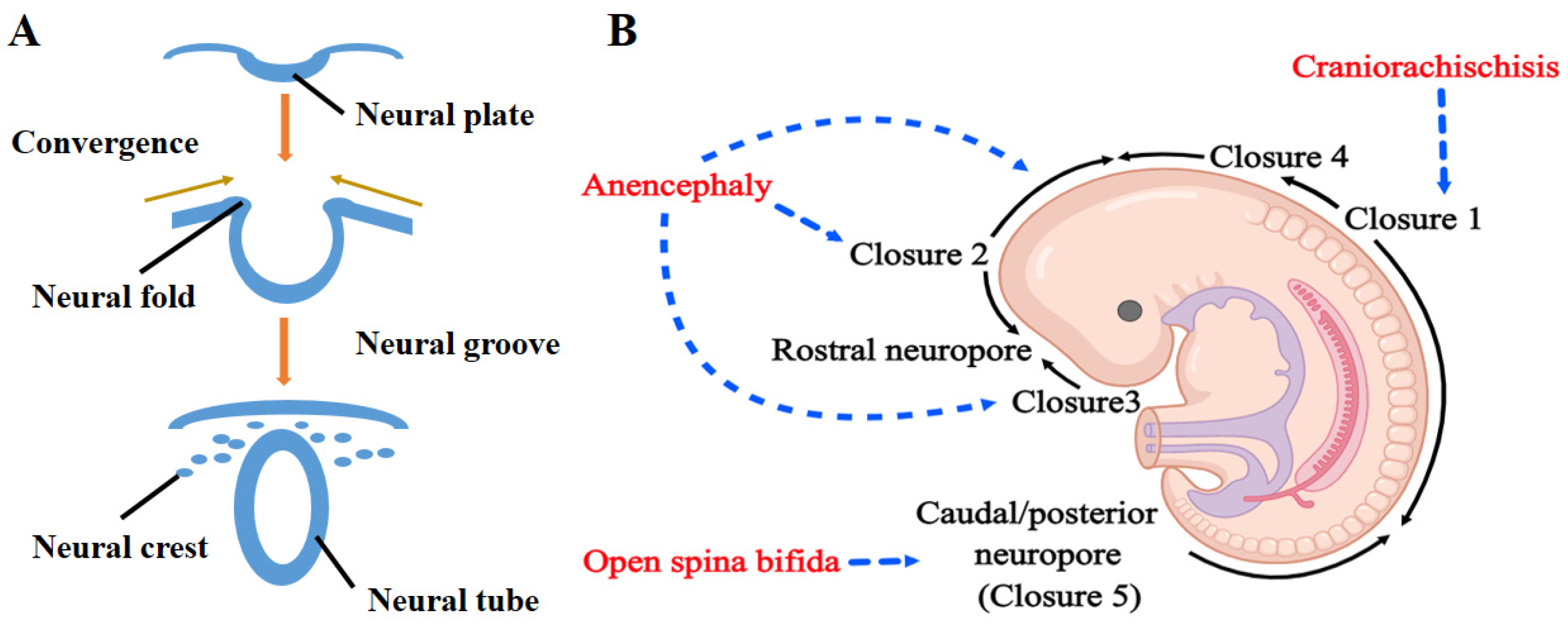
2. Neural Tube Development and NTDs
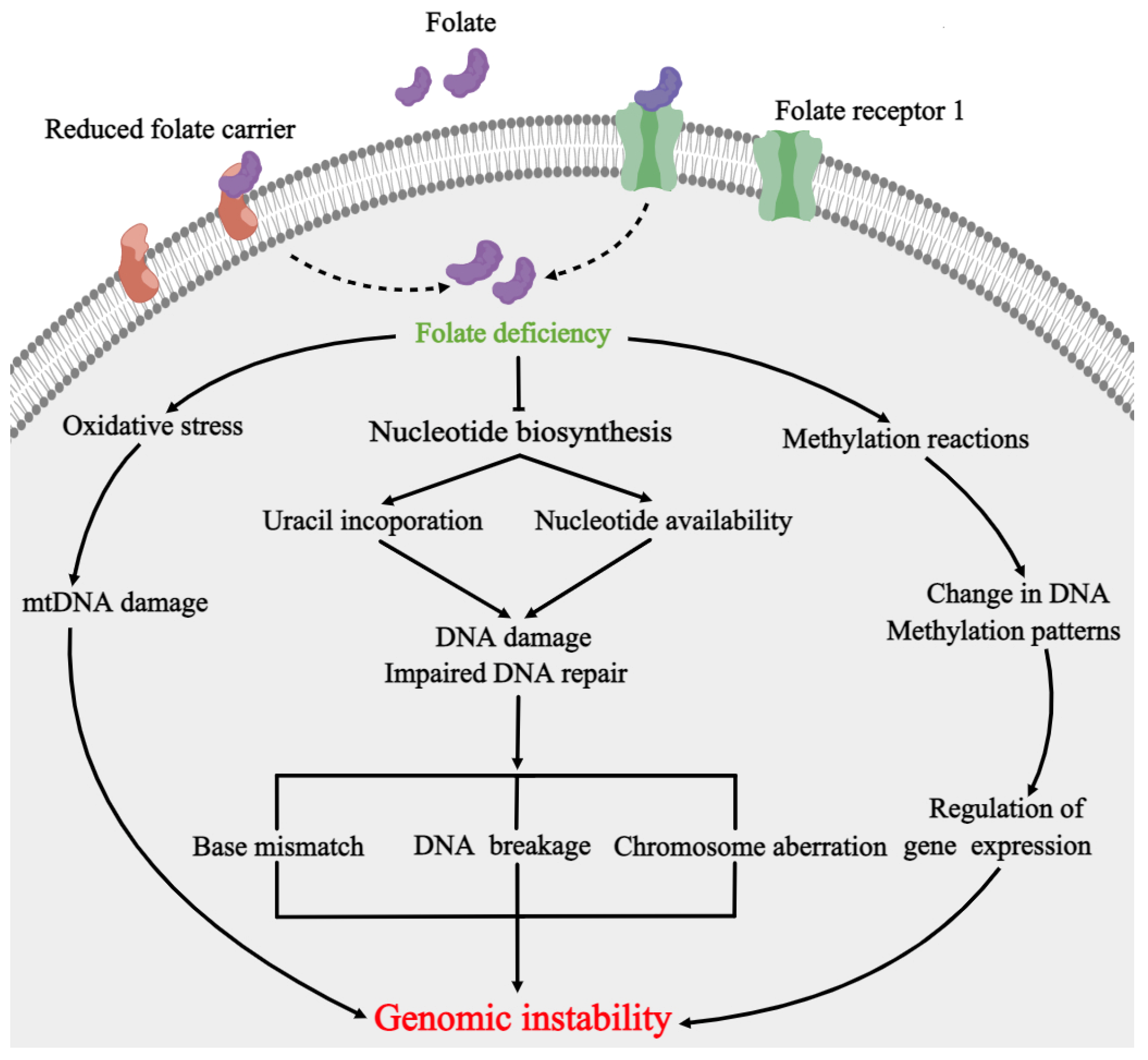
3. Folate Metabolism and NTDs
4. DNA Repair Pathways Involved in Folate Deficiency and NTDs
4.1. MMR and NTDs
4.2. BER and NTDs
4.3. DSBR and NTDs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Detrait, E.R.; George, T.M.; Etchevers, H.C.; Gilbert, J.R.; Vekemans, M.; Speer, M.C. Human neural tube defects: Developmental biology, epidemiology, and genetics. Neurotoxicol. Teratol. 2005, 27, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.D.; Copp, A.J. Neural tube defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef]
- Practice Bulletin No. 187: Neural Tube Defects. Obstet. Gynecol. 2017, 130, e279–e290. [CrossRef] [PubMed]
- Avagliano, L.; Massa, V.; George, T.M.; Qureshy, S.; Bulfamante, G.P.; Finnell, R.H. Overview on neural tube defects: From development to physical characteristics. Birth Defects Res. 2019, 111, 1455–1467. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.N.; Knowles, R.L.; Shawe, J.; Rankin, J.; Copp, A.J. Maternal ethnicity and the prevalence of British pregnancies affected by neural tube defects. Birth Defects Res. 2021, 113, 968–980. [Google Scholar] [CrossRef]
- Geneti, S.A.; Dimsu, G.G.; Sori, D.A.; Amente, L.D.; Kurmane, Z.M. Prevalence and patterns of birth defects among newborns in southwestern Ethiopia: A retrospective study. Pan Afr. Med. J. 2021, 40, 248. [Google Scholar] [CrossRef] [PubMed]
- Tsiklauri, R.; Jijeishvili, L.; Kherkheulidze, M.; Kvanchakhadze, R.; Kazakhashvili, N. Neural Tube Defects and Micronutrients Deficiency Prevalence in Georgia. Georgian Med. News 2020, 298, 61–66. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Chen, S.; Wang, F.; Zhang, T.; Niswander, L. Genetic contribution of retinoid-related genes to neural tube defects. Hum. Mutat. 2018, 39, 550–562. [Google Scholar] [CrossRef]
- Beaudin, A.E.; Abarinov, E.V.; Noden, D.M.; Perry, C.A.; Chu, S.; Stabler, S.P.; Allen, R.H.; Stover, P.J. Shmt1 and de novo thymidylate biosynthesis underlie folate-responsive neural tube defects in mice. Am. J. Clin. Nutr. 2011, 93, 789–798. [Google Scholar] [CrossRef]
- Cortellino, S.; Wang, C.; Wang, B.; Bassi, M.R.; Caretti, E.; Champeval, D.; Calmont, A.; Jarnik, M.; Burch, J.; Zaret, K.S.; et al. Defective ciliogenesis, embryonic lethality and severe impairment of the Sonic Hedgehog pathway caused by inactivation of the mouse complex A intraflagellar transport gene Ift122/Wdr10, partially overlapping with the DNA repair gene Med1/Mbd4. Dev. Biol. 2009, 325, 225–237. [Google Scholar] [CrossRef]
- Olshan, A.F.; Shaw, G.M.; Millikan, R.C.; Laurent, C.; Finnell, R.H. Polymorphisms in DNA repair genes as risk factors for spina bifida and orofacial clefts. Am. J. Med. Genet. A 2005, 135, 268–273. [Google Scholar] [CrossRef]
- Imbard, A.; Benoist, J.F.; Blom, H.J. Neural tube defects, folic acid and methylation. Int. J. Environ. Res. Public Health 2013, 10, 4352–4389. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Yu, H.L.; Zhao, H.F.; Liang, J.; Feng, J.F.; Wang, W. Developmental neurotoxicity role of cyclophosphamide on post-neural tube closure of rodents in vitro and in vivo. Int. J. Dev. Neurosci. 2007, 25, 531–537. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Guan, T.; Xiang, Q.; Wang, M.; Guan, Z.; Li, G.; Zhu, Z.; Xie, Q.; Zhang, T.; et al. Role of methotrexate exposure in apoptosis and proliferation during early neurulation. J. Appl. Toxicol. 2014, 34, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, C.; Song, X.; Wu, L.; Jiang, Q.; Qiu, Z.; Cao, H.; Yu, K.; Wan, C.; Li, J.; et al. Folate deficiency facilitates recruitment of upstream binding factor to hot spots of DNA double-strand breaks of rRNA genes and promotes its transcription. Nucleic Acids Res. 2017, 45, 2472–2489. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guan, T.; Wang, J.; Xiang, Q.; Wang, M.; Wang, X.; Guan, Z.; Xie, Q.; Niu, B.; Zhang, T. Influence of the antifolate drug Methotrexate on the development of murine neural tube defects and genomic instability. J. Appl. Toxicol. 2013, 33, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liang, J.; Li, X.; Yu, H.; Li, X.; Xiao, R. Folic acid and soybean isoflavone combined supplementation protects the post-neural tube closure defects of rodents induced by cyclophosphamide in vivo and in vitro. Neurotoxicology 2010, 31, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Wang, X.; Zhang, J.; Guan, Z.; Xu, L.; Wang, J.; Zhang, T.; Niu, B. Raltitrexed’s effect on the development of neural tube defects in mice is associated with DNA damage, apoptosis, and proliferation. Mol. Cell. Biochem. 2015, 398, 223–231. [Google Scholar] [CrossRef]
- Tung, E.W.; Winn, L.M. Valproic acid-induced DNA damage increases embryonic p27(KIP1) and caspase-3 expression: A mechanism for valproic-acid induced neural tube defects. Reprod. Toxicol. 2011, 32, 255–260. [Google Scholar] [CrossRef]
- Copp, A.J.; Greene, N.D. Genetics and development of neural tube defects. J. Pathol. 2010, 220, 217–230. [Google Scholar] [CrossRef]
- Golden, J.A.; Chernoff, G.F. Intermittent pattern of neural tube closure in two strains of mice. Teratology 1993, 47, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Van Allen, M.I.; Kalousek, D.K.; Chernoff, G.F.; Juriloff, D.; Harris, M.; McGillivray, B.C.; Yong, S.L.; Langlois, S.; MacLeod, P.M.; Chitayat, D.; et al. Evidence for multi-site closure of the neural tube in humans. Am. J. Med. Genet. 1993, 47, 723–743. [Google Scholar] [CrossRef] [PubMed]
- Golden, J.A.; Chernoff, G.F. Multiple sites of anterior neural tube closure in humans: Evidence from anterior neural tube defects (anencephaly). Pediatrics 1995, 95, 506–510. [Google Scholar]
- Copp, A.J.; Greene, N.D. Neural tube defects—Disorders of neurulation and related embryonic processes. Wiley Interdiscip. Rev. Dev. Biol. 2013, 2, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Copp, A.J.; Adzick, N.S.; Chitty, L.S.; Fletcher, J.M.; Holmbeck, G.N.; Shaw, G.M. Spina bifida. Nat. Rev. Dis. Prim. 2015, 1, 15007. [Google Scholar] [CrossRef]
- Rowland, C.A.; Correa, A.; Cragan, J.D.; Alverson, C.J. Are encephaloceles neural tube defects? Pediatrics 2006, 118, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Janik, K.; Manire, M.A.; Smith, G.M.; Krynska, B. Spinal Cord Injury in Myelomeningocele: Prospects for Therapy. Front. Cell. Neurosci. 2020, 14, 201. [Google Scholar] [CrossRef]
- Greene, N.D.; Copp, A.J. Development of the vertebrate central nervous system: Formation of the neural tube. Prenat. Diagn. 2009, 29, 303–311. [Google Scholar] [CrossRef]
- Harris, M.J.; Juriloff, D.M. Mouse mutants with neural tube closure defects and their role in understanding human neural tube defects. Birth Defects Res. A Clin. Mol. Teratol. 2007, 79, 187–210. [Google Scholar] [CrossRef]
- Finnell, R.H.; Caiaffa, C.D.; Kim, S.E.; Lei, Y.; Steele, J.; Cao, X.; Tukeman, G.; Lin, Y.L.; Cabrera, R.M.; Wlodarczyk, B.J. Gene Environment Interactions in the Etiology of Neural Tube Defects. Front. Genet. 2021, 12, 659612. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W., Jr.; Garcia, F.A.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Folic Acid Supplementation for the Prevention of Neural Tube Defects: US Preventive Services Task Force Recommendation Statement. JAMA 2017, 317, 183–189. [Google Scholar]
- Czeizel, A.E.; Dudas, I.; Vereczkey, A.; Banhidy, F. Folate deficiency and folic acid supplementation: The prevention of neural-tube defects and congenital heart defects. Nutrients 2013, 5, 4760–4775. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E.; Dudas, I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992, 327, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Alpers, D.H. Absorption and blood/cellular transport of folate and cobalamin: Pharmacokinetic and physiological considerations. Biochimie 2016, 126, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Chon, J.; Field, M.S.; Stover, P.J. Deoxyuracil in DNA and disease: Genomic signal or managed situation? DNA Repair 2019, 77, 36–44. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, D.; Ge, B.; Chen, H.; Du, Y.; Liu, S.; Ji, Y.; Sun, C.; Wang, G.; Gao, Y.; et al. Association of Folate Metabolites and Mitochondrial Function in Peripheral Blood Cells in Alzheimer’s Disease: A Matched Case-Control Study. J. Alzheimers Dis. 2019, 70, 1133–1142. [Google Scholar] [CrossRef]
- Zhang, T.; Xin, R.; Gu, X.; Wang, F.; Pei, L.; Lin, L.; Chen, G.; Wu, J.; Zheng, X. Maternal serum vitamin B12, folate and homocysteine and the risk of neural tube defects in the offspring in a high-risk area of China. Public Health Nutr. 2009, 12, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Salih, M.A.; Murshid, W.R.; Seidahmed, M.Z. Epidemiology, prenatal management, and prevention of neural tube defects. Saudi Med. J. 2014, 35 (Suppl. 1), S15–S28. [Google Scholar]
- Berry, R.J.; Li, Z.; Erickson, J.D.; Li, S.; Moore, C.A.; Wang, H.; Mulinare, J.; Zhao, P.; Wong, L.Y.; Gindler, J.; et al. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N. Engl. J. Med. 1999, 341, 1485–1490. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.; Guan, J.; Le, J.; Wu, L.; Zou, J.; Zhao, H.; Pei, L.; Zheng, X.; Zhang, T. Relation between hypomethylation of long interspersed nucleotide elements and risk of neural tube defects. Am. J. Clin. Nutr. 2010, 91, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Lai, X.; Xiang, K.; Han, X.; Yin, S.; Cabrera, R.M.; Steele, J.W.; Lei, Y.; Cao, X.; Finnell, R.H.; et al. Hypermethylation of PI3K-AKT signalling pathway genes is associated with human neural tube defects. Epigenetics 2022, 17, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Yang, S.; Jie, M.; Wang, S.; Sun, C.; Wu, L.; Chang, S.; Pei, P.; Wang, S.; Zhang, T.; et al. Folate deficiency disturbs PEG10 methylation modifications in human spina bifida. Pediatr. Res. 2022, 92, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Lu, X.; Wang, S.; Wang, Z.; Huo, J.; Huang, J.; Shangguan, S.; Li, S.; Zou, J.; Bao, Y.; et al. The effect of folic acid deficiency on FGF pathway via Brachyury regulation in neural tube defects. FASEB J. 2019, 33, 4688–4702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xue, P.; Li, H.; Bao, Y.; Wu, L.; Chang, S.; Niu, B.; Yang, F.; Zhang, T. Histone modification mapping in human brain reveals aberrant expression of histone H3 lysine 79 dimethylation in neural tube defects. Neurobiol. Dis. 2013, 54, 404–413. [Google Scholar] [CrossRef]
- Blount, B.C.; Mack, M.M.; Wehr, C.M.; MacGregor, J.T.; Hiatt, R.A.; Wang, G.; Wickramasinghe, S.N.; Everson, R.B.; Ames, B.N. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 3290–3295. [Google Scholar] [CrossRef]
- Anderson, D.D.; Woeller, C.F.; Stover, P.J. Small ubiquitin-like modifier-1 (SUMO-1) modification of thymidylate synthase and dihydrofolate reductase. Clin. Chem. Lab. Med. 2007, 45, 1760–1763. [Google Scholar] [CrossRef]
- Thomas, P.; Fenech, M. Methylenetetrahydrofolate reductase, common polymorphisms, and relation to disease. Vitam. Horm. 2008, 79, 375–392. [Google Scholar]
- Basten, G.P.; Duthie, S.J.; Pirie, L.; Vaughan, N.; Hill, M.H.; Powers, H.J. Sensitivity of markers of DNA stability and DNA repair activity to folate supplementation in healthy volunteers. Br. J. Cancer 2006, 94, 1942–1947. [Google Scholar] [CrossRef]
- Fenech, M. Folate (vitamin B9) and vitamin B12 and their function in the maintenance of nuclear and mitochondrial genome integrity. Mutat. Res. 2012, 733, 21–33. [Google Scholar] [CrossRef]
- Ingraham, H.A.; Tseng, B.Y.; Goulian, M. Nucleotide levels and incorporation of 5-fluorouracil and uracil into DNA of cells treated with 5-fluorodeoxyuridine. Mol. Pharmacol. 1982, 21, 211–216. [Google Scholar] [PubMed]
- Goulian, M.; Bleile, B.; Tseng, B.Y. Methotrexate-induced misincorporation of uracil into DNA. Proc. Natl. Acad. Sci. USA 1980, 77, 1956–1960. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Connor, E.E.; Berger, S.H.; Wyatt, M.D. Determination of apoptosis, uracil incorporation, DNA strand breaks, and sister chromatid exchanges under conditions of thymidylate deprivation in a model of BER deficiency. Biochem. Pharmacol. 2005, 70, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guan, Z.; Dong, Y.; Zhu, Z.; Wang, J.; Niu, B. Inhibition of thymidylate synthase affects neural tube development in mice. Reprod. Toxicol. 2018, 76, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, Y.; Gao, Y.; Zhao, H.; Sheng, X.; Zou, J.; Lip, V.; Xie, H.; Guo, J.; Shao, H.; et al. Detection of copy number variants reveals association of cilia genes with neural tube defects. PLoS ONE 2013, 8, e54492. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Passemard, S.; Kaindl, A.M.; Verloes, A. Microcephaly. Handb. Clin. Neurol. 2013, 111, 129–141. [Google Scholar]
- Kulkarni, A.; Wilson, D.M., 3rd. The involvement of DNA-damage and -repair defects in neurological dysfunction. Am. J. Hum. Genet. 2008, 82, 539–566. [Google Scholar] [CrossRef]
- Stead, E.R.; Bjedov, I. Balancing DNA repair to prevent ageing and cancer. Exp. Cell Res. 2021, 405, 112679. [Google Scholar] [CrossRef]
- Albino, D.; Brizzolara, A.; Moretti, S.; Falugi, C.; Mirisola, V.; Scaruffi, P.; Di Candia, M.; Truini, M.; Coco, S.; Bonassi, S.; et al. Gene expression profiling identifies eleven DNA repair genes down-regulated during mouse neural crest cell migration. Int. J. Dev. Biol. 2011, 55, 65–72. [Google Scholar] [CrossRef]
- Wood, R.D.; Mitchell, M.; Lindahl, T. Human DNA repair genes, 2005. Mutat. Res. 2005, 577, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A.; Lindsey-Boltz, L.A.; Unsal-Kacmaz, K.; Linn, S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, W.B.; Kaplan, D.R.; Miller, F.D. The p53 family in nervous system development and disease. J. Neurochem. 2006, 97, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Frappart, P.O.; Lee, Y.; Lamont, J.; McKinnon, P.J. BRCA2 is required for neurogenesis and suppression of medulloblastoma. EMBO J. 2007, 26, 2732–2742. [Google Scholar] [CrossRef] [PubMed]
- Hartman, A.R.; Ford, J.M. BRCA1 and p53: Compensatory roles in DNA repair. J. Mol. Med. 2003, 81, 700–707. [Google Scholar] [CrossRef]
- Hales, B.F. DNA repair disorders causing malformations. Curr. Opin. Genet. Dev. 2005, 15, 234–240. [Google Scholar] [CrossRef]
- Lachenauer, E.R.; Stabler, S.P.; Field, M.S.; Stover, P.J. p53 Disruption Increases Uracil Accumulation in DNA of Murine Embryonic Fibroblasts and Leads to Folic Acid-Nonresponsive Neural Tube Defects in Mice. J. Nutr. 2020, 150, 1705–1712. [Google Scholar] [CrossRef]
- Gowen, L.C.; Johnson, B.L.; Latour, A.M.; Sulik, K.K.; Koller, B.H. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat. Genet. 1996, 12, 191–194. [Google Scholar] [CrossRef]
- Tran, S.; Wang, L.; Le, J.; Guan, J.; Wu, L.; Zou, J.; Wang, Z.; Wang, J.; Wang, F.; Chen, X.; et al. Altered methylation of the DNA repair gene MGMT is associated with neural tube defects. J. Mol. Neurosci. 2012, 47, 42–51. [Google Scholar] [CrossRef]
- Fukui, K.; Baba, S.; Kumasaka, T.; Yano, T. Structural Features and Functional Dependency on beta-Clamp Define Distinct Subfamilies of Bacterial Mismatch Repair Endonuclease MutL. J. Biol. Chem. 2016, 291, 16990–17000. [Google Scholar] [CrossRef]
- Kunkel, T.A.; Erie, D.A. Eukaryotic Mismatch Repair in Relation to DNA Replication. Annu. Rev. Genet. 2015, 49, 291–313. [Google Scholar] [CrossRef] [PubMed]
- Baretti, M.; Le, D.T. DNA mismatch repair in cancer. Pharmacol. Ther. 2018, 189, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Niv, Y. Microsatellite instability and MLH1 promoter hypermethylation in colorectal cancer. World J. Gastroenterol. 2007, 13, 1767–1769. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Goel, A. Microsatellite instability in colorectal cancer. Gastroenterology 2010, 138, 2073–2087.e3. [Google Scholar] [CrossRef]
- Cravo, M.L.; Albuquerque, C.M.; Salazar de Sousa, L.; Gloria, L.M.; Chaves, P.; Dias Pereira, A.; Nobre Leitao, C.; Quina, M.G.; Costa Mira, F. Microsatellite instability in non-neoplastic mucosa of patients with ulcerative colitis: Effect of folate supplementation. Am. J. Gastroenterol. 1998, 93, 2060–2064. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Z.; Li, Y.; Ouyang, S.; Chang, H.; Zhang, T.; Zheng, X.; Wu, J. Association of genomic instability, and the methylation status of imprinted genes and mismatch-repair genes, with neural tube defects. Eur. J. Hum. Genet. 2012, 20, 516–520. [Google Scholar] [CrossRef]
- Li, F.; Mao, G.; Tong, D.; Huang, J.; Gu, L.; Yang, W.; Li, G.M. The histone mark H3K36me3 regulates human DNA mismatch repair through its interaction with MutSalpha. Cell 2013, 153, 590–600. [Google Scholar] [CrossRef]
- Hu, M.; Sun, X.J.; Zhang, Y.L.; Kuang, Y.; Hu, C.Q.; Wu, W.L.; Shen, S.H.; Du, T.T.; Li, H.; He, F.; et al. Histone H3 lysine 36 methyltransferase Hypb/Setd2 is required for embryonic vascular remodeling. Proc. Natl. Acad. Sci. USA 2010, 107, 2956–2961. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Zhao, H.; Wang, F.; Bao, Y.; Guo, J.; Chang, S.; Wu, L.; Cheng, H.; Chen, S.; et al. Low folate concentration impacts mismatch repair deficiency in neural tube defects. Epigenomics 2020, 12, 5–18. [Google Scholar] [CrossRef]
- Riccio, A.; Aaltonen, L.A.; Godwin, A.K.; Loukola, A.; Percesepe, A.; Salovaara, R.; Masciullo, V.; Genuardi, M.; Paravatou-Petsotas, M.; Bassi, D.E.; et al. The DNA repair gene MBD4 (MED1) is mutated in human carcinomas with microsatellite instability. Nat. Genet. 1999, 23, 266–268. [Google Scholar] [CrossRef]
- Pinto, M.; Wu, Y.; Suriano, G.; Mensink, R.G.; Duval, A.; Oliveira, C.; Carvalho, B.; Hamelin, R.; Seruca, R.; Hofstra, R.M. MBD4 mutations are rare in gastric carcinomas with microsatellite instability. Cancer Genet. Cytogenet. 2003, 145, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Bellacosa, A. Role of MED1 (MBD4) Gene in DNA repair and human cancer. J. Cell. Physiol. 2001, 187, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Krokan, H.E.; Bjoras, M. Base excision repair. Cold Spring Harb. Perspect. Biol. 2013, 5, a012583. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Wilson, D.M., 3rd. Overview of base excision repair biochemistry. Curr. Mol. Pharmacol. 2012, 5, 3–13. [Google Scholar] [CrossRef]
- Grundy, G.J.; Parsons, J.L. Base excision repair and its implications to cancer therapy. Essays Biochem. 2020, 64, 831–843. [Google Scholar]
- Nemec, A.A.; Wallace, S.S.; Sweasy, J.B. Variant base excision repair proteins: Contributors to genomic instability. Semin. Cancer Biol. 2010, 20, 320–328. [Google Scholar] [CrossRef]
- Wiederhold, L.; Leppard, J.B.; Kedar, P.; Karimi-Busheri, F.; Rasouli-Nia, A.; Weinfeld, M.; Tomkinson, A.E.; Izumi, T.; Prasad, R.; Wilson, S.H.; et al. AP endonuclease-independent DNA base excision repair in human cells. Mol. Cell 2004, 15, 209–220. [Google Scholar] [CrossRef]
- Svilar, D.; Goellner, E.M.; Almeida, K.H.; Sobol, R.W. Base excision repair and lesion-dependent subpathways for repair of oxidative DNA damage. Antioxid. Redox Signal. 2011, 14, 2491–2507. [Google Scholar] [CrossRef]
- Woodhouse, B.C.; Dianova, I.I.; Parsons, J.L.; Dianov, G.L. Poly(ADP-ribose) polymerase-1 modulates DNA repair capacity and prevents formation of DNA double strand breaks. DNA Repair 2008, 7, 932–940. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kim, K. Excision of deoxyribose phosphate residues by DNA polymerase beta during DNA repair. Science 1995, 269, 699–702. [Google Scholar] [CrossRef]
- Odell, I.D.; Barbour, J.E.; Murphy, D.L.; Della-Maria, J.A.; Sweasy, J.B.; Tomkinson, A.E.; Wallace, S.S.; Pederson, D.S. Nucleosome disruption by DNA ligase III-XRCC1 promotes efficient base excision repair. Mol. Cell. Biol. 2011, 31, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, E.; Taylor, R.; Cevasco, M.; Abbondandolo, A.; Caldecott, K.; Frosina, G. Involvement of XRCC1 and DNA ligase III gene products in DNA base excision repair. J. Biol. Chem. 1997, 272, 23970–23975. [Google Scholar] [CrossRef] [PubMed]
- Frosina, G.; Fortini, P.; Rossi, O.; Carrozzino, F.; Raspaglio, G.; Cox, L.S.; Lane, D.P.; Abbondandolo, A.; Dogliotti, E. Two pathways for base excision repair in mammalian cells. J. Biol. Chem. 1996, 271, 9573–9578. [Google Scholar] [CrossRef]
- Podlutsky, A.J.; Dianova, I.I.; Podust, V.N.; Bohr, V.A.; Dianov, G.L. Human DNA polymerase beta initiates DNA synthesis during long-patch repair of reduced AP sites in DNA. EMBO J. 2001, 20, 1477–1482. [Google Scholar] [CrossRef]
- Bianchi, F.T.; Berto, G.E.; Di Cunto, F. Impact of DNA repair and stability defects on cortical development. Cell. Mol. Life Sci. 2018, 75, 3963–3976. [Google Scholar] [CrossRef] [PubMed]
- Cabelof, D.C.; Raffoul, J.J.; Nakamura, J.; Kapoor, D.; Abdalla, H.; Heydari, A.R. Imbalanced base excision repair in response to folate deficiency is accelerated by polymerase beta haploinsufficiency. J. Biol. Chem. 2004, 279, 36504–36513. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, A.; Prychitko, T.M.; Patel, H.V.; Chowdhury, M.E.; Pilling, A.B.; Ventrella-Lucente, L.F.; Papakonstantinou, E.V.; Cabelof, D.C.; Heydari, A.R. Folate deficiency regulates expression of DNA polymerase beta in response to oxidative stress. Free Radic. Biol. Med. 2011, 50, 270–280. [Google Scholar] [CrossRef]
- Duthie, S.J.; Hawdon, A. DNA instability (strand breakage, uracil misincorporation, and defective repair) is increased by folic acid depletion in human lymphocytes in vitro. FASEB J. 1998, 12, 1491–1497. [Google Scholar] [CrossRef]
- Wang, X.; Yue, H.; Li, S.; Guo, J.; Guan, Z.; Zhu, Z.; Niu, B.; Zhang, T.; Wang, J. Genetic Polymorphisms in DNA Repair Gene APE1/Ref-1 and the Risk of Neural Tube Defects in a High-Risk Area of China. Reprod. Sci. 2021, 28, 2592–2601. [Google Scholar] [CrossRef]
- Li, G.; Wang, X.; Wang, X.; Guan, Z.; Guo, J.; Wang, F.; Zhang, J.; Niu, B.; Zhang, T.; Wang, J.; et al. Polymorphism rs1052536 in Base Excision Repair Gene Is a Risk Factor in a High-Risk Area of Neural Tube Defects in China. Med. Sci. Monit. 2018, 24, 5015–5026. [Google Scholar] [CrossRef]
- Larsen, D.H.; Stucki, M. Nucleolar responses to DNA double-strand breaks. Nucleic Acids Res. 2016, 44, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Jeggo, P.A. DNA double-strand break repair in a cellular context. Clin. Oncol. (R. Coll. Radiol.) 2014, 26, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Spies, J.; Polasek-Sedlackova, H.; Lukas, J.; Somyajit, K. Homologous Recombination as a Fundamental Genome Surveillance Mechanism during DNA Replication. Genes 2021, 12, 1960. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Sun, H.; Huang, Y.; Wang, Y.; Liu, Y.; Chen, X. Pathways and assays for DNA double-strand break repair by homologous recombination. Acta Biochim. Biophys. Sin. 2019, 51, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Huselid, E.; Bunting, S.F. The Regulation of Homologous Recombination by Helicases. Genes 2020, 11, 498. [Google Scholar] [CrossRef]
- Beucher, A.; Birraux, J.; Tchouandong, L.; Barton, O.; Shibata, A.; Conrad, S.; Goodarzi, A.A.; Krempler, A.; Jeggo, P.A.; Lobrich, M. ATM and Artemis promote homologous recombination of radiation-induced DNA double-strand breaks in G2. EMBO J. 2009, 28, 3413–3427. [Google Scholar] [CrossRef]
- Lobrich, M.; Jeggo, P. A Process of Resection-Dependent Nonhomologous End Joining Involving the Goddess Artemis. Trends Biochem. Sci. 2017, 42, 690–701. [Google Scholar] [CrossRef]
- Madabhushi, R.; Pan, L.; Tsai, L.H. DNA damage and its links to neurodegeneration. Neuron 2014, 83, 266–282. [Google Scholar] [CrossRef]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Lieber, M.R. The mechanism of double-strand DNA break repair by the nonhomologous DNA end-joining pathway. Annu. Rev. Biochem. 2010, 79, 181–211. [Google Scholar] [CrossRef]
- Alt, F.W.; Schwer, B. DNA double-strand breaks as drivers of neural genomic change, function, and disease. DNA Repair 2018, 71, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Peng, M.; Yuan, H.; Liu, C.; Zhang, Y.; Fang, Y.; Su, Y.; Zhang, X.; Zhang, H.; Tang, Y.; et al. Studying the mechanism of sperm DNA damage caused by folate deficiency. J. Cell. Mol. Med. 2022, 26, 776–788. [Google Scholar] [CrossRef]
- Pei, P.; Cheng, X.; Yu, J.; Shen, J.; Li, X.; Wu, J.; Wang, S.; Zhang, T. Folate deficiency induced H2A ubiquitination to lead to downregulated expression of genes involved in neural tube defects. Epigenet. Chromatin 2019, 12, 69. [Google Scholar] [CrossRef]
- Wilson, M.P.; Hugge, C.; Bielinska, M.; Nicholas, P.; Majerus, P.W.; Wilson, D.B. Neural tube defects in mice with reduced levels of inositol 1,3,4-trisphosphate 5/6-kinase. Proc. Natl. Acad. Sci. USA 2009, 106, 9831–9835. [Google Scholar] [CrossRef]
- Hanakahi, L.A.; West, S.C. Specific interaction of IP6 with human Ku70/80, the DNA-binding subunit of DNA-PK. EMBO J. 2002, 21, 2038–2044. [Google Scholar] [CrossRef]
- Hanakahi, L.A.; Bartlet-Jones, M.; Chappell, C.; Pappin, D.; West, S.C. Binding of inositol phosphate to DNA-PK and stimulation of double-strand break repair. Cell 2000, 102, 721–729. [Google Scholar] [CrossRef]
- Herrera, E.; Samper, E.; Blasco, M.A. Telomere shortening in mTR−/− embryos is associated with failure to close the neural tube. EMBO J. 1999, 18, 1172–1181. [Google Scholar] [CrossRef]
- Bekaert, S.; Derradji, H.; Baatout, S. Telomere biology in mammalian germ cells and during development. Dev. Biol. 2004, 274, 15–30. [Google Scholar] [CrossRef]
- Tebbs, R.S.; Flannery, M.L.; Meneses, J.J.; Hartmann, A.; Tucker, J.D.; Thompson, L.H.; Cleaver, J.E.; Pedersen, R.A. Requirement for the Xrcc1 DNA base excision repair gene during early mouse development. Dev. Biol. 1999, 208, 513–529. [Google Scholar] [CrossRef]
- Xanthoudakis, S.; Smeyne, R.J.; Wallace, J.D.; Curran, T. The redox/DNA repair protein, Ref-1, is essential for early embryonic development in mice. Proc. Natl. Acad. Sci. USA 1996, 93, 8919–8923. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, P.; Wang, F.; Yang, J.; Yang, Z.; Qin, H. The relationship between early embryo development and tumourigenesis. J. Cell Mol. Med. 2010, 14, 2697–2701. [Google Scholar] [CrossRef] [PubMed]
- Pieroth, R.; Paver, S.; Day, S.; Lammersfeld, C. Folate and Its Impact on Cancer Risk. Curr. Nutr. Rep. 2018, 7, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Naghibalhossaini, F.; Mokarram, P.; Khalili, I.; Vasei, M.; Hosseini, S.V.; Ashktorab, H.; Rasti, M.; Abdollahi, K. MTHFR C677T and A1298C variant genotypes and the risk of microsatellite instability among Iranian colorectal cancer patients. Cancer Genet. Cytogenet. 2010, 197, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Paschalis, A.; Sheehan, B.; Riisnaes, R.; Rodrigues, D.N.; Gurel, B.; Bertan, C.; Ferreira, A.; Lambros, M.B.K.; Seed, G.; Yuan, W.; et al. Prostate-specific Membrane Antigen Heterogeneity and DNA Repair Defects in Prostate Cancer. Eur. Urol. 2019, 76, 469–478. [Google Scholar] [CrossRef]
- Li, G.; Wu, J.; Li, L.; Jiang, P. p53 deficiency induces MTHFD2 transcription to promote cell proliferation and restrain DNA damage. Proc. Natl. Acad. Sci. USA 2021, 118, e2019822118. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, T.T.; Sharma, S.; Reyes, G.X.; Kolodziejczak, A.; Wagner, T.; Luke, B.; Hofer, A.; Chabes, A.; Hombauer, H. Inactivation of folylpolyglutamate synthetase Met7 results in genome instability driven by an increased dUTP/dTTP ratio. Nucleic Acids Res. 2020, 48, 264–277. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Yu, J.; Wang, J. Neural Tube Defects and Folate Deficiency: Is DNA Repair Defective? Int. J. Mol. Sci. 2023, 24, 2220. https://doi.org/10.3390/ijms24032220
Wang X, Yu J, Wang J. Neural Tube Defects and Folate Deficiency: Is DNA Repair Defective? International Journal of Molecular Sciences. 2023; 24(3):2220. https://doi.org/10.3390/ijms24032220
Chicago/Turabian StyleWang, Xiuwei, Jialu Yu, and Jianhua Wang. 2023. "Neural Tube Defects and Folate Deficiency: Is DNA Repair Defective?" International Journal of Molecular Sciences 24, no. 3: 2220. https://doi.org/10.3390/ijms24032220
APA StyleWang, X., Yu, J., & Wang, J. (2023). Neural Tube Defects and Folate Deficiency: Is DNA Repair Defective? International Journal of Molecular Sciences, 24(3), 2220. https://doi.org/10.3390/ijms24032220






