Lipid Biomarkers and Atherosclerosis—Old and New in Cardiovascular Risk in Childhood
Abstract
:1. Introduction
2. Methods and Previous Studies
3. Lipid Determination Methods
4. Traditional Lipid Biomarkers
Hypercholesterolemia and Cardiovascular Risk in Children
5. Newer Lipid Biomarkers
5.1. Lipoprotein(a) and Apolipoprotein(a)
5.2. Apolipoproteins
5.2.1. Apolipoproteins A
5.2.2. Apolipoproteins B
5.2.3. Apolipoproteins C
5.2.4. Apolipoproteins E
6. Research Lipid Biomarkers
6.1. Oxidized Lipid Molecules
6.2. Lipoprotein X
6.3. Lipoprotein-Associated Phospholipase A2
6.4. Ceramides
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McNamara, K.; Alzubaidi, H.; Jackson, J.K. Cardiovascular disease as a leading cause of death: How are pharmacists getting involved? Integr. Pharm. Res. Pract. 2019, 8, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The Role of Lipids and Lipoproteins in Atherosclerosis. In Comprehensive Endocrinology Book; Endotext, Feingold, K.R., Anawalt, B., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK343489/ (accessed on 25 December 2022).
- Mayerl, C.; Lukasser, M.; Sedivy, R.; Niederegger, H.; Seiler, R.; Wick, G. Atherosclerosis research from past to present—On the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Arch. 2006, 449, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Guo, Y.L.; Zhao, X.; Zhang, Y.; Zhu, C.G.; Wu, N.Q.; Xu, R.X.; Qing, P.; Gao, Y.; Li, X.L.; et al. Novel and traditional lipid-related biomarkers and their combinations in predicting coronary severity. Sci. Rep. 2017, 7, 360. [Google Scholar] [CrossRef] [Green Version]
- Hinterwirth, H.; Stegemann, C.; Mayr, M. Lipidomics: Quest for molecular lipid biomarkers in cardiovascular disease. Circ. Cardiovasc. Genet. 2014, 7, 941–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGill, H.C., Jr.; McMahan, C.A.; Herderick, E.E.; Malcom, G.T.; Tracy, R.E.; Strong, J.P. Origin of atherosclerosis in childhood and adolescence. Am. J. Clin. Nutr. 2000, 72, 1307S–1315S. [Google Scholar]
- Milei, J.; Ottaviani, G.; Lavezzi, A.M.; Grana, D.R.; Stella, I.; Matturri, L. Perinatal and infant early atherosclerotic coronary lesions. Can. J. Cardiol. 2008, 24, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bridger, T. Childhood obesity and cardiovascular disease. Paediatr. Child Health 2009, 14, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Raghuveer, G. Lifetime cardiovascular risk of childhood obesity. Am. J. Clin. Nutr. 2010, 91, 1514S–1519S. [Google Scholar] [CrossRef] [Green Version]
- Li-Beisson, Y.; Nakamura, Y.; Harwood, J. Lipids: From Chemical Structures, Biosynthesis, and Analyses to Industrial Applications. Subcell. Biochem. 2016, 86, 1–18. [Google Scholar]
- Clish, C.B. Metabolomics: An emerging but powerful tool for precision medicine. Cold Spring Harb. Mol. Case Stud. 2015, 1, a000588. [Google Scholar] [CrossRef] [Green Version]
- Juhola, J.; Magnussen, C.G.; Viikari, J.S.; Kähönen, M.; Hutri-Kähönen, N.; Jula, A.; Lehtimäki, T.; Åkerblom, H.K.; Pietikäinen, M.; Laitinen, T.; et al. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: The Cardiovascular Risk in Young Finns Study. J. Pediatr. 2011, 159, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Feingold, K.R. Introduction to Lipids and Lipoproteins. In Comprehensive Endocrinology Book; Endotext, Feingold, K.R., Anawalt, B., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK305896/ (accessed on 25 December 2022).
- Berenson, G.S.; Srnivasan, S.R.; Bogalusa Heart Study Group. Cardiovascular risk factors in youth with implications for aging: The Bogalusa Heart Study. Neurobiol. Aging 2005, 26, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Steinberger, J.; Daniels, S.R.; American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young); American Heart Association Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Obesity, insulin resistance, diabetes, and cardiovascular risk in children: An American Heart Association scientific statement from the Atherosclerosis, Hypertension, and Obesity in the Young Committee (Council on Cardiovascular Disease in the Young) and the Diabetes Committee (Council on Nutrition, Physical Activity, and Metabolism). Circulation 2003, 107, 1448–1453. [Google Scholar]
- Gröber-Grätz, D.; Widhalm, K.; de Zwaan, M.; Reinehr, T.; Blüher, S.; Schwab, K.O.; Wiegand, S.; Holl, R.W. Body mass index or waist circumference: Which is the better predictor for hypertension and dyslipidemia in overweight/obese children and adolescents? Association of cardiovascular risk related to body mass index or waist circumference. Horm. Res. Paediatr. 2013, 80, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Korsten-Reck, U.; Kromeyer-Hauschild, K.; Korsten, K.; Baumstark, M.W.; Dickhuth, H.H.; Berg, A. Frequency of secondary dyslipidemia in obese children. Vasc. Health Risk Manag. 2008, 4, 1089–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kavey, R.E. Combined dyslipidemia in childhood. J. Clin. Lipidol. 2015, 9, S41–S56. [Google Scholar] [CrossRef]
- Strong, J.P.; Malcom, G.T.; Newman, W.P., 3rd; Oalmann, M.C. Early lesions of atherosclerosis in childhood and youth: Natural history and risk factors. J. Am. Coll. Nutr. 1992, 11, 51S–54S. [Google Scholar] [CrossRef]
- Guardamagna, O.; Abello, F.; Saracco, P.; Baracco, V.; Rolfo, E.; Pirro, M. Endothelial activation, inflammation and premature atherosclerosis in children with familial dyslipidemia. Atherosclerosis 2009, 207, 471–475. [Google Scholar] [CrossRef]
- Morrison, K.M.; Dyal, L.; Conner, W.; Helden, E.; Newkirk, L.; Yusuf, S.; Lonn, E. Cardiovascular risk factors and non-invasive assessment of subclinical atherosclerosis in youth. Atherosclerosis 2010, 208, 501–505. [Google Scholar] [CrossRef]
- Litwin, S.E. Childhood obesity and adulthood cardiovascular disease: Quantifying the lifetime cumulative burden of cardiovascular risk factors. J. Am. Coll. Cardiol. 2014, 64, 1588–1590. [Google Scholar] [CrossRef] [Green Version]
- Magnussen, C.G.; Venn, A.; Thomson, R.; Juonala, M.; Srinivasan, S.R.; Viikari, J.S.; Berenson, G.S.; Dwyer, T.; Raitakari, O.T. The association of pediatric low- and high-density lipoprotein cholesterol dyslipidemia classifications and change in dyslipidemia status with carotid intima-media thickness in adulthood evidence from the cardiovascular risk in Young Finns study, the Bogalusa Heart study, and the CDAH (Childhood Determinants of Adult Health) study. J. Am. Coll. Cardiol. 2009, 53, 860–869. [Google Scholar] [PubMed] [Green Version]
- Li, S.; Chen, W.; Srinivasan, S.R.; Bond, M.G.; Tang, R.; Urbina, E.M.; Berenson, G.S. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: The Bogalusa Heart Study. JAMA 2003, 290, 2271–2276. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.R.; Frontini, M.G.; Xu, J.; Berenson, G.S. Utility of childhood non-high-density lipoprotein cholesterol levels in predicting adult dyslipidemia and other cardiovascular risks: The Bogalusa Heart Study. Pediatrics 2006, 118, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Dobiásová, M. AIP—Atherogenic index of plasma as a significant predictor of cardiovascular risk: From research to practice. Vnitr. Lek. 2006, 52, 64–71. [Google Scholar]
- Quijada, Z.; Paoli, M.; Zerpa, Y.; Camacho, N.; Cichetti, R.; Villarroel, V.; Arata-Bellabarba, G.; Lanes, R. The triglyceride/HDL-cholesterol ratio as a marker of cardiovascular risk in obese children; association with traditional and emergent risk factors. Pediatr. Diabetes 2008, 9, 464–471. [Google Scholar] [CrossRef]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef] [Green Version]
- Andersen, L.B.; Harro, M.; Sardinha, L.B.; Froberg, K.; Ekelund, U.; Brage, S.; Anderssen, S.A. Physical activity and clustered cardiovascular risk in children: A cross-sectional study (The European Youth Heart Study). Lancet 2006, 368, 299–304. [Google Scholar] [CrossRef]
- Cesa, C.C.; Sbruzzi, G.; Ribeiro, R.A.; Barbiero, S.M.; de Oliveira Petkowicz, R.; Eibel, B.; Machado, N.B.; Marques, R.; Tortato, G.; dos Santos, T.J.; et al. Physical activity and cardiovascular risk factors in children: Meta-analysis of randomized clinical trials. Prev. Med. 2014, 69, 54–62. [Google Scholar] [CrossRef]
- Avogaro, P.; Bon, G.B.; Cazzolato, G.; Quinci, G.B. Are apolipoproteins better discriminators than lipids for atherosclerosis? Lancet 1979, 1, 901–903. [Google Scholar] [CrossRef]
- Manfroi, W.C.; Zago, A.J.; Campos, M.; Alves, A.; Brisolara, M.L.; de Souza, J.; Candiago, R.H.; Kirschnick, L.; Ribeiro, L.; Ordovás, K.; et al. Are apolipoproteins A and B better than lipoproteins for assessing risk of obstructive coronary heart disease? Arq. Bras. Cardiol. 1999, 72, 657–668. [Google Scholar] [CrossRef]
- Welsh, C.; Celis-Morales, C.A.; Brown, R.; Mackay, D.F.; Lewsey, J.; Mark, P.B.; Gray, S.R.; Ferguson, L.D.; Anderson, J.J.; Lyall, D.M.; et al. Comparison of Conventional Lipoprotein Tests and Apolipoproteins in the Prediction of Cardiovascular Disease. Circulation 2019, 140, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Berglund, L.; Ramakrishnan, R. Lipoprotein (a): An elusive cardiovascular risk factor. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, G.; Sakka, E.; Blathra, E.; Kalivi, A.; Elisaf, M.; Liamis, G.; Liberopoulos, E. Lipoprotein (a): A Concealed Precursor of Increased Cardiovascular Risk? A Real-World Regional Lipid Clinic Experience. Arch. Med. Res. 2021, 52, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Gulayin, P.E.; Lozada, A.; Schreier, L.; Gutierrez, L.; López, G.; Poggio, R.; Mores, N.; Ponzo, J.; Calandrelli, M.; Lanas, F.; et al. Elevated Lipoprotein (a) prevalence and association with family history of premature cardiovascular disease in general population with moderate cardiovascular risk and increased LDL cholesterol. Int. J. Cardiol. Heart Vasc. 2022, 42, 101100. [Google Scholar] [CrossRef]
- Palmeira, Á.C.; Leal, A.A.; Ramos Nde, M.; Neto Jde, A.; Simões, M.O.; Medeiros, C.C. Lipoprotein (a) and cardiovascular risk factors in children and adolescents. Rev. Paul Pediatr. 2013, 31, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Glowinska, B.; Urban, M.; Koput, A. Cardiovascular risk factors in children with obesity, hypertension and diabetes: Lipoprotein (a) levels and body mass index correlate with family history of cardiovascular disease. Eur. J. Pediatr. 2002, 161, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Pederiva, C.; Capra, M.E.; Biasucci, G.; Banderali, G.; Fabrizi, E.; Gazzotti, M.; Casula, M.; Catapano, A.L.; LIPIGEN Paediatric Group. Lipoprotein (a) and family history for cardiovascular disease in paediatric patients: A new frontier in cardiovascular risk stratification. Data from the LIPIGEN paediatric group. Atherosclerosis 2022, 349, 233–239. [Google Scholar] [CrossRef]
- Guardamagna, O.; Abello, F.; Anfossi, G.; Pirro, M. Lipoprotein (a) and family history of cardiovascular disease in children with familial dyslipidemias. J. Pediatr. 2011, 159, 314–319. [Google Scholar] [CrossRef]
- Sultan, S.M.; Schupf, N.; Dowling, M.M.; Deveber, G.A.; Kirton, A.; Elkind, M.S. Review of lipid and lipoprotein (a) abnormalities in childhood arterial ischemic stroke. Int. J. Stroke 2014, 9, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, N.A.; Bernard, T.J.; Hillhouse, J.; Armstrong-Wells, J.; Galinkin, J.; Knapp-Clevenger, R.; Jacobson, L.; Marcovina, S.M.; Manco-Johnson, M.J. Elevated lipoprotein (a), small apolipoprotein (a), and the risk of arterial ischemic stroke in North American children. Haematologica 2013, 98, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Qayum, O.; Alshami, N.; Ibezim, C.F.; Reid, K.J.; Noel-MacDonnell, J.R.; Raghuveer, G. Lipoprotein (a): Examination of Cardiovascular Risk in a Pediatric Referral Population. Pediatr. Cardiol. 2018, 39, 1540–1546. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.P.; Koschinsky, M.L.; Moriarty, P.M. Expert position statements: Comparison of recommendations for the care of adults and youth with elevated lipoprotein (a). Curr. Opin. Endocrinol. Diabetes Obes. 2021, 28, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Tounian, P.; Aggoun, Y.; Dubern, B.; Varille, V.; Guy-Grand, B.; Sidi, D.; Girardet, J.P.; Bonnet, D. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: A prospective study. Lancet 2001, 358, 1400–1404. [Google Scholar] [CrossRef]
- Jaimungal, S.; Wehmeier, K.; Mooradian, A.D.; Haas, M.J. The emerging evidence for vitamin D-mediated regulation of apolipoprotein A-I synthesis. Nutr. Res. 2011, 31, 805–812. [Google Scholar] [CrossRef]
- Tamang, H.K.; Timilsina, U.; Singh, K.P.; Shrestha, S.; Raman, R.K.; Panta, P.; Karna, P.; Khadka, L.; Dahal, C. Apo B/Apo A-I Ratio is Statistically A Better Predictor of Cardiovascular Disease (CVD) than Conventional Lipid Profile: A Study from Kathmandu Valley, Nepal. J. Clin. Diagn. Res. 2014, 8, 34–36. [Google Scholar] [CrossRef]
- Walldius, G.; Jungner, I. Apolipoprotein B and apolipoprotein A-I: Risk indicators of coronary heart disease and targets for lipid-modifying therapy. J. Intern. Med. 2004, 255, 188–205. [Google Scholar] [CrossRef]
- Barroso, W.K.S.; Souza, A.L.L. Obesity, Overweight, Body Adiposity and Cardiovascular Risk in Children and Adolescents. Arq. Bras. Cardiol. 2020, 115, 172–173. [Google Scholar] [CrossRef]
- Savas Erdeve, S.; Simsek, E.; Dallar, Y.; Biyikli, Z. Utility of ApoB/ApoA1 ratio for the prediction of cardiovascular risk in children with metabolic syndrome. Indian J. Pediatr. 2010, 77, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Choi, S.H.; Lee, K.W.; Kim, D.J. Apolipoprotein B/A1 ratio is associated with free androgen index and visceral adiposity and may be an indicator of metabolic syndrome in male children and adolescents. Clin. Endocrinol. 2011, 74, 579–586. [Google Scholar] [CrossRef]
- Koivistoinen, T.; Hutri-Kähönen, N.; Juonala, M.; Kööbi, T.; Aatola, H.; Lehtimäki, T.; Viikari, J.S.; Raitakari, O.T.; Kähönen, M. Apolipoprotein B is related to arterial pulse wave velocity in young adults: The Cardiovascular Risk in Young Finns Study. Atherosclerosis 2011, 214, 220–224. [Google Scholar] [CrossRef]
- Juonala, M.; Viikari, J.S.; Kähönen, M.; Solakivi, T.; Helenius, H.; Jula, A.; Marniemi, J.; Taittonen, L.; Laitinen, T.; Nikkari, T.; et al. Childhood levels of serum apolipoproteins B and A-I predict carotid intima-media thickness and brachial endothelial function in adulthood: The cardiovascular risk in young Finns study. J. Am. Coll. Cardiol. 2008, 52, 293–299. [Google Scholar] [CrossRef] [Green Version]
- Retnakaran, R.; Zinman, B.; Connelly, P.W.; Harris, S.B.; Hanley, A.J. Nontraditional cardiovascular risk factors in pediatric metabolic syndrome. J. Pediatr. 2006, 148, 176–182. [Google Scholar] [CrossRef]
- Glowinska, B.; Urban, M.; Koput, A.; Galar, M. New atherosclerosis risk factors in obese, hypertensive and diabetic children and adolescents. Atherosclerosis 2003, 167, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Frontini, M.G.; Srinivasan, S.R.; Xu, J.; Tang, R.; Bond, M.G.; Berenson, G.S. Usefulness of childhood non-high density lipoprotein cholesterol levels versus other lipoprotein measures in predicting adult subclinical atherosclerosis: The Bogalusa Heart Study. Pediatrics 2008, 121, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.F.; Yung, T.C.; Tam, S.C.; Ho, M.H.; Chau, A.K. Novel and traditional cardiovascular risk factors in children after Kawasaki disease: Implications for premature atherosclerosis. J. Am. Coll. Cardiol. 2004, 43, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.J.; Yang, S.I.; Kim, K.H.; Kim, J.N.; Kil, H.R. Cardiovascular risk factors of early atherosclerosis in school-aged children after Kawasaki disease. Korean J. Pediatr. 2014, 57, 217–221. [Google Scholar] [CrossRef]
- Czeck, M.A.; Northrop, E.F.; Evanoff, N.G.; Dengel, D.R.; Rudser, K.D.; Kelly, A.S.; Ryder, J.R. Relationship of Apolipoproteins with Subclinical Cardiovascular Risk in Youth. J. Pediatr. 2020, 227, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Guardiola, M.; Ribalta, J.; Gómez-Coronado, D.; Lasunción, M.A.; de Oya, M.; Garcés, C. The apolipoprotein A5 (APOA5) gene predisposes Caucasian children to elevated triglycerides and vitamin E (Four Provinces Study). Atherosclerosis 2010, 212, 543–547. [Google Scholar] [CrossRef] [PubMed]
- Andersen, L.H.; Miserez, A.R.; Ahmad, Z.; Andersen, R.L. Familial defective apolipoprotein B-100: A review. J. Clin. Lipidol. 2016, 10, 1297–1302. [Google Scholar] [CrossRef]
- Musso, C.; Graffigna, M.; Soutelo, J.; Honfi, M.; Ledesma, L.; Miksztowicz, V.; Pazos, M.; Migliano, M.; Schreier, L.E.; Berg, G.A. Cardiometabolic risk factors as apolipoprotein B, triglyceride/HDL-cholesterol ratio and C-reactive protein, in adolescents with and without obesity: Cross-sectional study in middle class suburban children. Pediatr. Diabetes 2011, 12, 229–234. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Verma, S.; Fung, M.; McQueen, M.J.; Anderson, T.J.; Lonn, E.M. Association of Apolipoproteins B and A-1 With Markers of Vascular Health or Cardiovascular Events. Can. J. Cardiol. 2017, 33, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Kallio, K.; Jokinen, E.; Saarinen, M.; Hämäläinen, M.; Volanen, I.; Kaitosaari, T.; Rönnemaa, T.; Viikari, J.; Raitakari, O.T.; Simell, O. Arterial intima-media thickness, endothelial function, and apolipoproteins in adolescents frequently exposed to tobacco smoke. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 196–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, G.S.; Cromwell, W.C.; Ali, S.; Chin, W.; Flaim, J.D.; Davidson, M. Mipomersen, an apolipoprotein B synthesis inhibitor, reduces atherogenic lipoproteins in patients with severe hypercholesterolemia at high cardiovascular risk: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 2013, 62, 2178–2184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivieri, O.; Bassi, A.; Stranieri, C.; Trabetti, E.; Martinelli, N.; Pizzolo, F.; Girelli, D.; Friso, S.; Pignatti, P.F.; Corrocher, R. Apolipoprotein C-III, metabolic syndrome, and risk of coronary artery disease. J. Lipid Res. 2003, 44, 2374–2381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.L.; Tain, Y.L.; Chen, H.E.; Hsu, C.N. Cardiovascular Disease Risk in Children With Chronic Kidney Disease: Impact of Apolipoprotein C-II and Apolipoprotein C-III. Front. Pediatr. 2021, 9, 706323. [Google Scholar] [CrossRef] [PubMed]
- Marais, A.D. Apolipoprotein E in lipoprotein metabolism, health and cardiovascular disease. Pathology 2019, 51, 165–176. [Google Scholar] [CrossRef]
- Lehtimäki, T.; Porkka, K.; Viikari, J.; Ehnholm, C.; Akerblom, H.K.; Nikkari, T. Apolipoprotein E phenotypes and serum lipids in newborns and 3-year-old children: The Cardiovascular Risk in Young Finns Study. Pediatrics 1994, 94, 489–493. [Google Scholar] [CrossRef]
- Srinivasan, S.R.; Ehnholm, C.; Wattigney, W.A.; Bao, W.; Berenson, G.S. The relation of apolipoprotein E polymorphism to multiple cardiovascular risk in children: The Bogalusa Heart Study. Atherosclerosis 1996, 123, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Rego, C.; Castro, E.M.; Seixas, S.; Rocha, J. Influence of apolipoprotein e polymorphism on cardiovascular risk factors in obese children. Ann. Nutr. Metab. 2003, 47, 49–54. [Google Scholar] [CrossRef]
- Grönroos, P.; Raitakari, O.T.; Kähönen, M.; Hutri-Kähönen, N.; Marniemi, J.; Viikari, J.; Lehtimäki, T. Influence of apolipoprotein E polymorphism on serum lipid and lipoprotein changes: A 21-year follow-up study from childhood to adulthood. The Cardiovascular Risk in Young Finns Study. Clin. Chem. Lab. Med. 2007, 45, 592–598. [Google Scholar] [CrossRef]
- Ciftdoğan, D.Y.; Coskun, S.; Ulman, C.; Tıkız, H. The association of apolipoprotein E polymorphism and lipid levels in children with a family history of premature coronary artery disease. J. Clin. Lipidol. 2012, 6, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Taimela, S.; Lehtimäki, T.; Porkka, K.V.; Räsänen, L.; Viikari, J.S. The effect of physical activity on serum total and low-density lipoprotein cholesterol concentrations varies with apolipoprotein E phenotype in male children and young adults: The Cardiovascular Risk in Young Finns Study. Metabolism 1996, 45, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Tammi, A.; Rönnemaa, T.; Miettinen, T.A.; Gylling, H.; Rask-Nissilä, L.; Viikari, J.; Tuominen, J.; Marniemi, J.; Simell, O. Effects of gender, apolipoprotein E phenotype and cholesterol-lowering by plant stanol esters in children: The STRIP study. Special Turku Coronary Risk Factor Intervention Project. Acta Paediatr. 2002, 91, 1155–1162. [Google Scholar] [CrossRef]
- Blackman, J.A.; Worley, G.; Strittmatter, W.J. Apolipoprotein E and brain injury: Implications for children. Dev. Med. Child Neurol. 2005, 47, 64–70. [Google Scholar] [CrossRef]
- Taylor, A.E.; Guthrie, P.A.; Smith, G.D.; Golding, J.; Sattar, N.; Hingorani, A.D.; Deanfield, J.E.; Day, I.N. IQ, educational attainment, memory and plasma lipids: Associations with apolipoprotein E genotype in 5995 children. Biol. Psychiatry 2011, 70, 152–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acevedo, S.F.; Piper, B.J.; Craytor, M.J.; Benice, T.S.; Raber, J. Apolipoprotein E4 and sex affect neurobehavioral performance in primary school children. Pediatr. Res. 2010, 67, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimikas, S. Oxidized low-density lipoprotein biomarkers in atherosclerosis. Curr. Atheroscler. Rep. 2006, 8, 55–61. [Google Scholar] [CrossRef]
- Tsimikas, S. Oxidative biomarkers in the diagnosis and prognosis of cardiovascular disease. Am. J. Cardiol. 2006, 98, 9P–17P. [Google Scholar] [CrossRef]
- Fraley, A.E.; Tsimikas, S. Clinical applications of circulating oxidized low-density lipoprotein biomarkers in cardiovascular disease. Curr. Opin. Lipidol. 2006, 17, 502–509. [Google Scholar] [CrossRef]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 2015, 52, 70–85. [Google Scholar] [CrossRef]
- Norris, A.L.; Steinberger, J.; Steffen, L.M.; Metzig, A.M.; Schwarzenberg, S.J.; Kelly, A.S. Circulating oxidized LDL and inflammation in extreme pediatric obesity. Obesity 2011, 19, 1415–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, A.S.; Jacobs, D.R., Jr.; Sinaiko, A.R.; Moran, A.; Steffen, L.M.; Steinberger, J. Relation of circulating oxidized LDL to obesity and insulin resistance in children. Pediatr. Diabetes 2010, 11, 552–555. [Google Scholar] [CrossRef] [Green Version]
- Manco, M.; Nobili, V.; Alisi, A.; Panera, N.; Handberg, A. Arterial Stiffness, Thickness and Association to Suitable Novel Markers of Risk at the Origin of Cardiovascular Disease in Obese Children. Int. J. Med. Sci. 2017, 14, 711–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Järvisalo, M.J.; Lehtimäki, T.; Raitakari, O.T. Determinants of arterial nitrate-mediated dilatation in children: Role of oxidized low-density lipoprotein, endothelial function, and carotid intima-media thickness. Circulation 2004, 109, 2885–2889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demirel, F.; Bideci, A.; Cinaz, P.; Camurdan, M.O.; Biberoğlu, G.; Yesilkaya, E.; Hasanoğlu, A. Serum leptin, oxidized low density lipoprotein and plasma asymmetric dimethylarginine levels and their relationship with dyslipidaemia in adolescent girls with polycystic ovary syndrome. Clin. Endocrinol. 2007, 67, 129–134. [Google Scholar] [CrossRef]
- Nascimento, H.; Alves, A.I.; Coimbra, S.; Catarino, C.; Gomes, D.; Bronze-da-Rocha, E.; Costa, E.; Rocha-Pereira, P.; Aires, L.; Mota, J.; et al. Bilirubin is independently associated with oxidized LDL levels in young obese patients. Diabetol. Metab. Syndr. 2015, 7, 4. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, L.; Emmerechts, J.; Hoylaerts, M.F.; Mathieu, C.; Hoet, P.H.; Nemery, B.; Nawrot, T.S. Traffic air pollution and oxidized LDL. PLoS ONE 2011, 6, e16200. [Google Scholar] [CrossRef] [Green Version]
- Kelishadi, R.; Hashemi, M.; Mohammadifard, N.; Asgary, S.; Khavarian, N. Association of changes in oxidative and proinflammatory states with changes in vascular function after a lifestyle modification trial among obese children. Clin. Chem. 2008, 54, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Mansikkaniemi, K.; Juonala, M.; Taimela, S.; Hirvensalo, M.; Telama, R.; Huupponen, R.; Saarikoski, L.; Hurme, M.; Mallat, Z.; Benessiano, J.; et al. Cross-sectional associations between physical activity and selected coronary heart disease risk factors in young adults. The Cardiovascular Risk in Young Finns Study. Ann. Med. 2012, 44, 733–744. [Google Scholar] [CrossRef]
- Kelishadi, R.; Hashemipour, M.; Adeli, K.; Tavakoli, N.; Movahedian-Attar, A.; Shapouri, J.; Poursafa, P.; Rouzbahani, A. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab. Syndr. Relat. Disord. 2010, 8, 505–510. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Hosseinpanah, F.; Rajab, A.; Asghari, G.; Azizi, F. Broccoli sprouts powder could improve serum triglyceride and oxidized LDL/LDL-cholesterol ratio in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. Diabetes Res. Clin. Pract. 2012, 96, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.T.; Dasari, P.S.; Tryggestad, J.B.; Aston, C.E.; Teague, A.M.; Short, K.R. Oxidized HDL and LDL in adolescents with type 2 diabetes compared to normal weight and obese peers. J. Diabetes Complicat. 2015, 29, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Taleb, A.; Witztum, J.L.; Tsimikas, S. Oxidized phospholipids on apoB-100-containing lipoproteins: A biomarker predicting cardiovascular disease and cardiovascular events. Biomark. Med. 2011, 5, 673–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimikas, S.; Kiechl, S.; Willeit, J.; Mayr, M.; Miller, E.R.; Kronenberg, F.; Xu, Q.; Bergmark, C.; Weger, S.; Oberhollenzer, F.; et al. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: Five-year prospective results from the Bruneck study. J. Am. Coll. Cardiol. 2006, 47, 2219–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergmark, C.; Dewan, A.; Orsoni, A.; Merki, E.; Miller, E.R.; Shin, M.J.; Binder, C.J.; Hörkkö, S.; Krauss, R.M.; Chapman, M.J.; et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J. Lipid Res. 2008, 49, 2230–2239. [Google Scholar] [CrossRef] [Green Version]
- Kiechl, S.; Willeit, J.; Mayr, M.; Viehweider, B.; Oberhollenzer, M.; Kronenberg, F.; Wiedermann, C.J.; Oberthaler, S.; Xu, Q.; Witztum, J.L.; et al. Oxidized phospholipids, lipoprotein (a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: Prospective results from the Bruneck study. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1788–1795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Codoñer-Franch, P.; Tavárez-Alonso, S.; Murria-Estal, R.; Tortajada-Girbés, M.; Simó-Jordá, R.; Alonso-Iglesias, E. Elevated advanced oxidation protein products (AOPPs) indicate metabolic risk in severely obese children. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 237–243. [Google Scholar] [CrossRef]
- Amar, M.J.A.; Freeman, L.A.; Nishida, T.; Sampson, M.L.; Pryor, M.; Vaisman, B.L.; Neufeld, E.B.; Karathanasis, S.K.; Remaley, A.T. LCAT protects against Lipoprotein-X formation in a murine model of drug-induced intrahepatic cholestasis. Pharmacol. Res. Perspect. 2019, 8, e00554. [Google Scholar] [CrossRef] [Green Version]
- Zidan, H.; Lo, S.; Wiebe, D.; Talano, J.; Alemzadeh, R. Severe hypercholesterolemia mediated by lipoprotein X in a pediatric patient with chronic graft-versus-host disease of the liver. Pediatr. Blood Cancer 2008, 50, 1280–1281. [Google Scholar] [CrossRef]
- Colantuono, R.; Pavanello, C.; Pietrobattista, A.; Turri, M.; Francalanci, P.; Spada, M.; Vajro, P.; Calabresi, L.; Mandato, C. Case report: Unusual and extremely severe lipoprotein X-mediated hypercholesterolemia in extrahepatic pediatric cholestasis. Front. Pediatr. 2022, 10, 969081. [Google Scholar] [CrossRef]
- Miida, T.; Hirayama, S. Controversy over the atherogenicity of lipoprotein-X. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Pantazi, D.; Tellis, C.; Tselepis, A.D. “SI: PAF” Oxidized phospholipids and lipoprotein-associated phospholipase A2 (Lp-PLA2) in atherosclerotic cardiovascular disease: An update. Biofactors, 2022; epub ahead of print. [Google Scholar]
- Dimitroglou, Y.; Sakalidis, A.; Mavroudis, A.; Kalantzis, C.; Valatsou, A.; Andrikou, I.; Christofi, A.; Mantzouranis, E.; Kachrimanidis, I.; Bei, E.; et al. Lipoprotein-associated phospholipase A2 in coronary artery disease. Curr. Top. Med. Chem. 2022; epub ahead of print. [Google Scholar]
- Colley, K.J.; Wolfert, R.L.; Cobble, M.E. Lipoprotein associated phospholipase A(2): Role in atherosclerosis and utility as a biomarker for cardiovascular risk. EPMA J. 2011, 2, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Alkuraishy, H.M.; Al-Gareeb, A.I.; Waheed, H.J. Lipoprotein-Associated Phospholipase A2 is Linked with Poor Cardio-Metabolic Profile in Patients with Ischemic Stroke: A Study of Effects of Statins. J. Neurosci. Rural Pract. 2018, 9, 496–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öngen, B.; Kalkan Uçar, S.; Levent, E.; Azarsız, E.; Koloğlu, T.; Çoker, M.; Sözmen, E.; Sağın, F.G. Lipoprotein-associated phospholipase A2: A new marker to determine cardiovascular risk in hypercholesterolemic dyslipidaemic children. Ann. Clin. Biochem. 2017, 54, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.K.; Hutten, B.A.; Vissers, M.N.; Wiegman, A.; Kastelein, J.J.; Tsimikas, S. Lipoprotein-associated phospholipase A2 mass and activity in children with heterozygous familial hypercholesterolemia and unaffected siblings: Effect of pravastatin. J. Clin. Lipidol. 2011, 5, 50–56. [Google Scholar] [CrossRef]
- Krebs, A.; Doerfer, J.; Krause, A.; Grulich-Henn, J.; Holder, M.; Hecker, W.; Lichte, K.; Schmidt-Trucksaess, A.; Winkler, K.; Schwab, K.O. Lipoprotein-associated phospholipase A2 activity and low-density lipoprotein subfractions after a 2-year treatment with atorvastatin in adolescents with type 1 diabetes. J. Pediatr. Endocrinol. Metab. 2016, 29, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Ferraro, F.; Martín, M.; Verona, J.; Gilligan, L.; Verona, M.F.; Botta, E.; Tetzlaff, W.; Lozano Chiappe, E.; Boero, L.; Brites, F. Increased Cholesteryl Ester Transfer Protein and Lipoprotein-Associated Phospholipase A2 Activities in Children and Adolescents Presenting High Triglyceride/High-Density Lipoprotein Cholesterol (TG/HDL-C) Ratio. Indian J. Pediatr. 2021, 88, 1180–1186. [Google Scholar] [CrossRef]
- Sakka, S.; Siahanidou, T.; Voyatzis, C.; Pervanidou, P.; Kaminioti, C.; Lazopoulou, N.; Kanaka-Gantenbein, C.; Chrousos, G.P.; Papassotiriou, I. Elevated circulating levels of lipoprotein-associated phospholipase A2 in obese children. Clin. Chem. Lab. Med. 2015, 53, 1119–1125. [Google Scholar] [CrossRef]
- da Silva, I.T.; Timm Ade, S.; Damasceno, N.R. Influence of obesity and cardiometabolic makers on lipoprotein-associated phospholipase A2 (Lp-PLA2) activity in adolescents: The healthy young cross-sectional study. Lipids Health Dis. 2013, 12, 19. [Google Scholar] [CrossRef] [Green Version]
- Kheirandish-Gozal, L.; Philby, M.F.; Qiao, Z.; Khalyfa, A.; Gozal, D. Endothelial Dysfunction in Children With Obstructive Sleep Apnea Is Associated With Elevated Lipoprotein-Associated Phospholipase A2 Plasma Activity Levels. J. Am. Heart Assoc. 2017, 6, e004923. [Google Scholar] [CrossRef]
- Cheng, Z.; Weng, H.; Zhang, J.; Yi, Q. The Relationship Between Lipoprotein-Associated Phospholipase-A2 and Coronary Artery Aneurysm in Children with Kawasaki Disease. Front. Pediatr. 2022, 10, 854079. [Google Scholar] [CrossRef] [PubMed]
- Hirschler, V.; Meroño, T.; Maccallini, G.; Gomez Rosso, L.; Aranda, C.; Brites, F. Association of lipoprotein-associated phospholipase A2 activity with components of the metabolic syndrome in apparently healthy boys. Cardiovasc. Hematol. Agents Med. Chem. 2011, 9, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Alexandropoulou, I.; Lantzanaki-Syrpou, M.; Grammatikopoulou, M.G.; Goulis, D.G. Ceramides as Dietary Biomarkers. In Biomarkers in Nutrition. Biomarkers in Disease: Methods, Discoveries and Applications; Patel, V.B., Preedy, V.R., Eds.; Springer: Cham, Switzerland, 2022; pp. 1–15. [Google Scholar]
- Summers, S.A.; Chaurasia, B.; Holland, W.L. Metabolic Messengers: Ceramides. Nat. Metab. 2019, 1, 1051–1058. [Google Scholar] [CrossRef] [PubMed]
- Song, J.H.; Kim, G.T.; Park, K.H.; Park, W.J.; Park, T.S. Bioactive Sphingolipids as Major Regulators of Coronary Artery Disease. Biomol. Ther. 2021, 29, 373–383. [Google Scholar] [CrossRef]
- McGurk, K.A.; Keavney, B.D.; Nicolaou, A. Circulating ceramides as biomarkers of cardiovascular disease: Evidence from phenotypic and genomic studies. Atherosclerosis 2021, 327, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Mandal, N.; Grambergs, R.; Mondal, K.; Basu, S.K.; Tahia, F.; Dagogo-Jack, S. Role of ceramides in the pathogenesis of diabetes mellitus and its complications. J. Diabetes Complicat. 2021, 35, 107734. [Google Scholar] [CrossRef]
- Laaksonen, R.; Ekroos, K.; Sysi-Aho, M.; Hilvo, M.; Vihervaara, T.; Kauhanen, D.; Suoniemi, M.; Hurme, R.; März, W.; Scharnagl, H.; et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J. 2016, 37, 1967–1976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meeusen, J.W.; Donato, L.J.; Bryant, S.C.; Baudhuin, L.M.; Berger, P.B.; Jaffe, A.S. Plasma Ceramides. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1933–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasilewska, N.; Bobrus-Chociej, A.; Harasim-Symbor, E.; Tarasów, E.; Wojtkowska, M.; Chabowski, A.; Lebensztejn, D.M. Increased serum concentration of ceramides in obese children with nonalcoholic fatty liver disease. Lipids Health Dis. 2018, 17, 216. [Google Scholar] [CrossRef] [Green Version]
- Olson, E.; Suh, J.H.; Schwarz, J.M.; Noworolski, S.M.; Jones, G.M.; Barber, J.R.; Erkin-Cakmak, A.; Mulligan, K.; Lustig, R.H.; Mietus-Snyder, M. Effects of Isocaloric Fructose Restriction on Ceramide Levels in Children with Obesity and Cardiometabolic Risk: Relation to Hepatic De Novo Lipogenesis and Insulin Sensitivity. Nutrients 2022, 14, 1432. [Google Scholar] [CrossRef]
- León-Aguilar, L.F.; Croyal, M.; Ferchaud-Roucher, V.; Huang, F.; Marchat, L.A.; Barraza-Villarreal, A.; Romieu, I.; Ramakrishnan, U.; Krempf, M.; Ouguerram, K.; et al. Maternal obesity leads to long-term altered levels of plasma ceramides in the offspring as revealed by a longitudinal lipidomic study in children. Int. J. Obes. 2019, 43, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Mitsnefes, M.; Scherer, P.E.; Friedman, L.A.; Gordillo, R.; Furth, S.; Warady, B.A.; CKiD Study Group. Ceramides and cardiac function in children with chronic kidney disease. Pediatr. Nephrol. 2014, 29, 415–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sands, M.S. Farber disease: Understanding a fatal childhood disorder and dissecting ceramide biology. EMBO Mol. Med. 2013, 5, 799–801. [Google Scholar] [CrossRef] [PubMed]
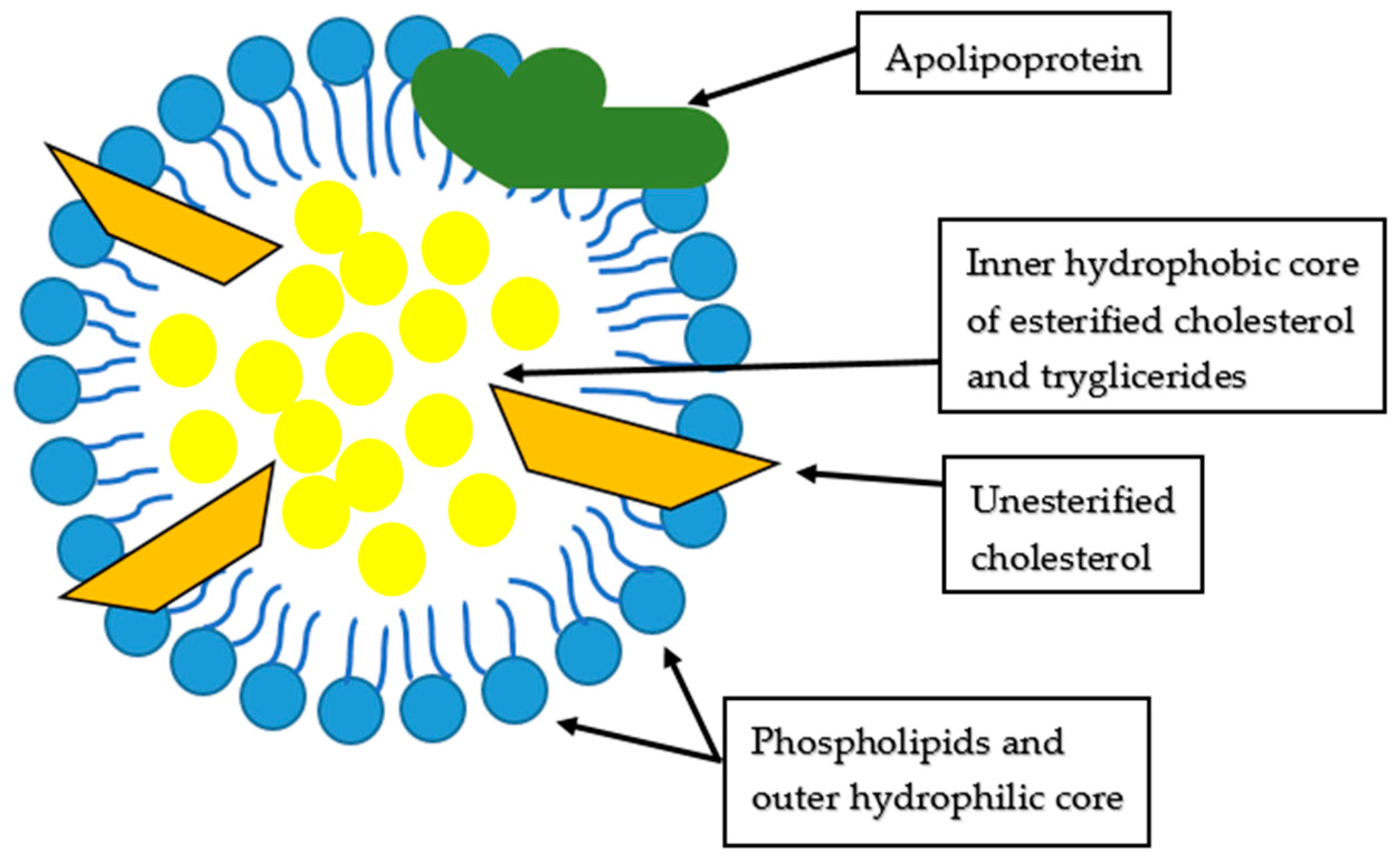
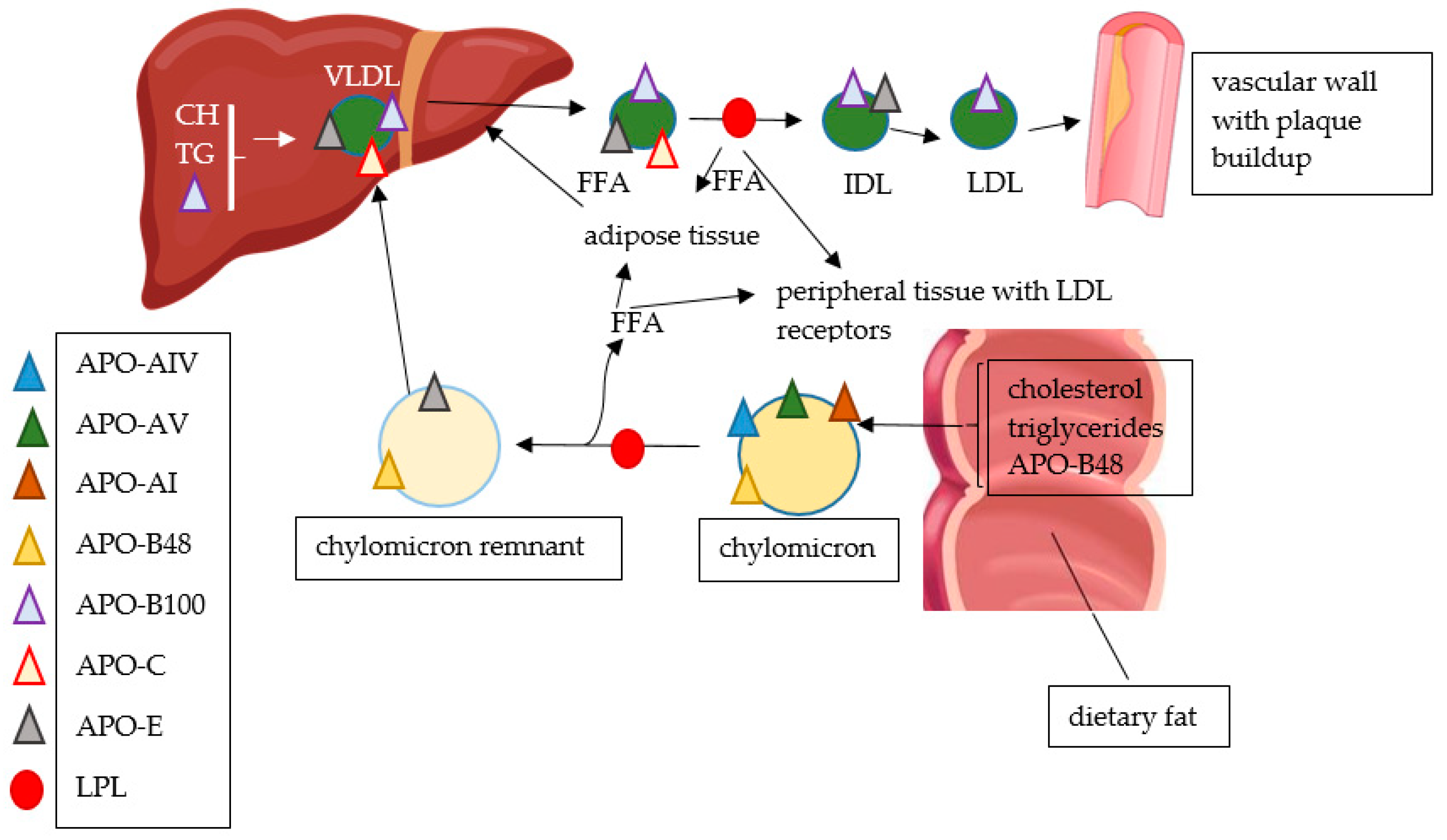
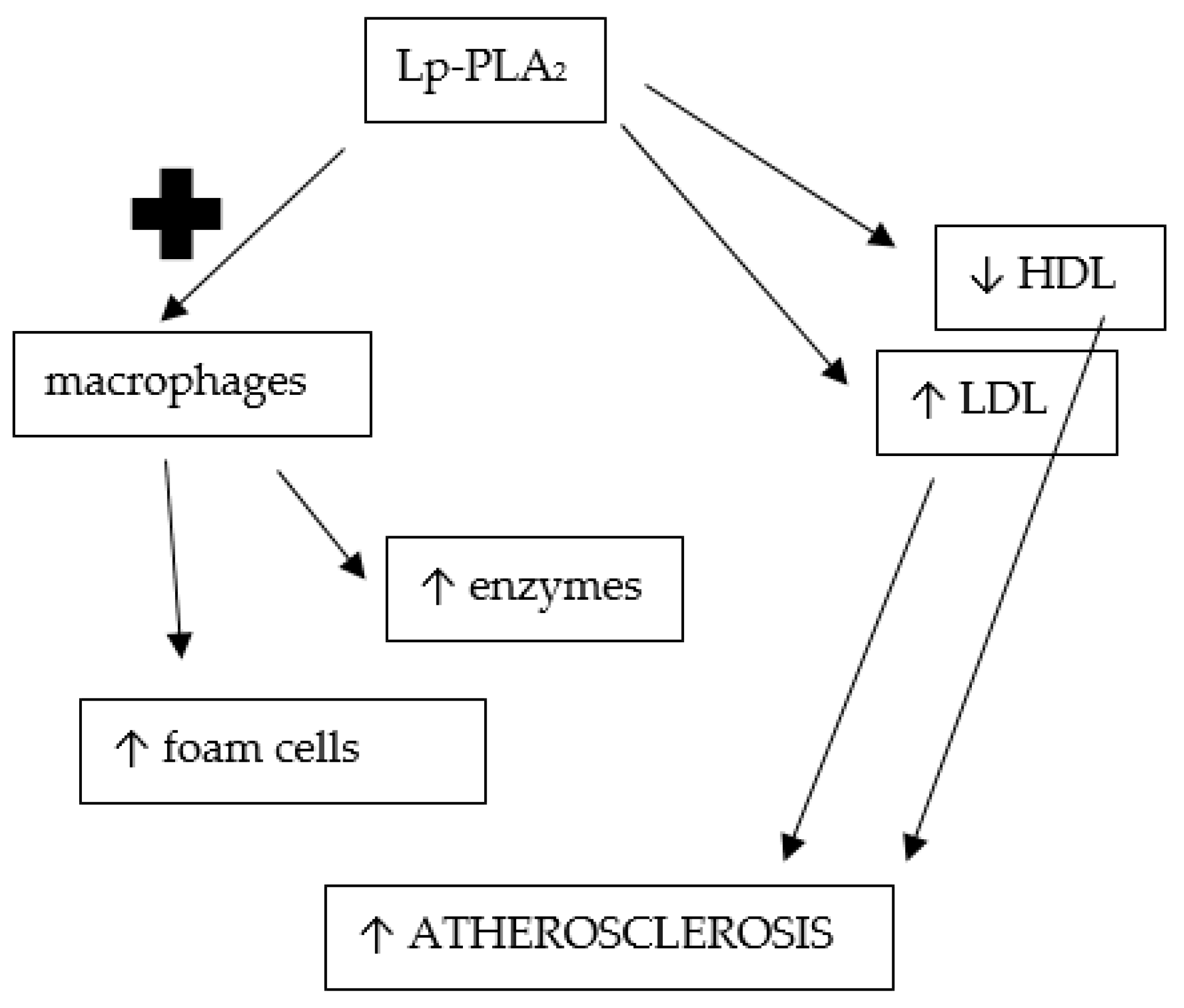
| Review/Original Paper | Research Methods | Applicability of the Results | Comparison to Our Review |
|---|---|---|---|
| Li et al. [4] | Original paper; lipid biomarkers determination in pateints with coronary artery disease | Simultaneous determination of traditional and novel lipid biomarkers showing their association with coronary severity in men | Similarities: simultaneous determination of lipid biomarkers, used for cardiovascular risk stratification |
| Differences: research study, adult population | |||
| Hinterwirth et al. [5] | Review; lipids classification, lipidomics studies in cohorts of patients | The knowledge of lipid biomarkers lacking, the association between several lipid biomarkers and cardiovascular diseases in adults | Similarities: wide range of biomarkers, review of some disease states |
| Differences: applicability to adult population, focus on lipidomic studies | |||
| Juhola et al. [12] | Multicenter study; correlation between serum lipid levels in childhood and adulthood | The importance of lipid biomarkers in children | Similarities: the importance of lipid biomarkers in paediatric population |
| Differences: our review provides additional novel and research biomarkers, not included previously | |||
| Feingold et al. [13] | Review; a comprehensive explanation of lipoprotein and apolipoprotein’s structure with their physiologic function | Physiologic function of several biomarkers | Similarities: comprehensive review |
| Differences: inclusion of lipids, other than lipoproteins with studies of cardiovascular risk assessment |
| Traditional Lipid Biomarkers | Newer Lipid Biomarkers | Research Lipid Biomarkers |
|---|---|---|
| total cholesterol (P) | lipoprotein(a) (P) | oxidised lipids (lipid peroxidation) (P) |
| low-density lipoprotein cholesterol (P) | apolipoproteins A: AI (A), AII (U), AIV (U), AV (U) | lipoprotein X (P) |
| high-density lipoprotein cholesterol (A) | apolipoproteins B: B48 (P), B100 (P) | lipoprotein-associated phospholipase A2 (P) |
| Triglycerides (P) | apolipoproteins C: CI (P), CII (P), CIII (P) | Ceramides (P) |
| apolipoproteins E (P) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Močnik, M.; Marčun Varda, N. Lipid Biomarkers and Atherosclerosis—Old and New in Cardiovascular Risk in Childhood. Int. J. Mol. Sci. 2023, 24, 2237. https://doi.org/10.3390/ijms24032237
Močnik M, Marčun Varda N. Lipid Biomarkers and Atherosclerosis—Old and New in Cardiovascular Risk in Childhood. International Journal of Molecular Sciences. 2023; 24(3):2237. https://doi.org/10.3390/ijms24032237
Chicago/Turabian StyleMočnik, Mirjam, and Nataša Marčun Varda. 2023. "Lipid Biomarkers and Atherosclerosis—Old and New in Cardiovascular Risk in Childhood" International Journal of Molecular Sciences 24, no. 3: 2237. https://doi.org/10.3390/ijms24032237





