SARS-CoV-2 as an Oncolytic Virus Following Reactivation of the Immune System: A Review
Abstract
:1. Introduction
2. Discussion
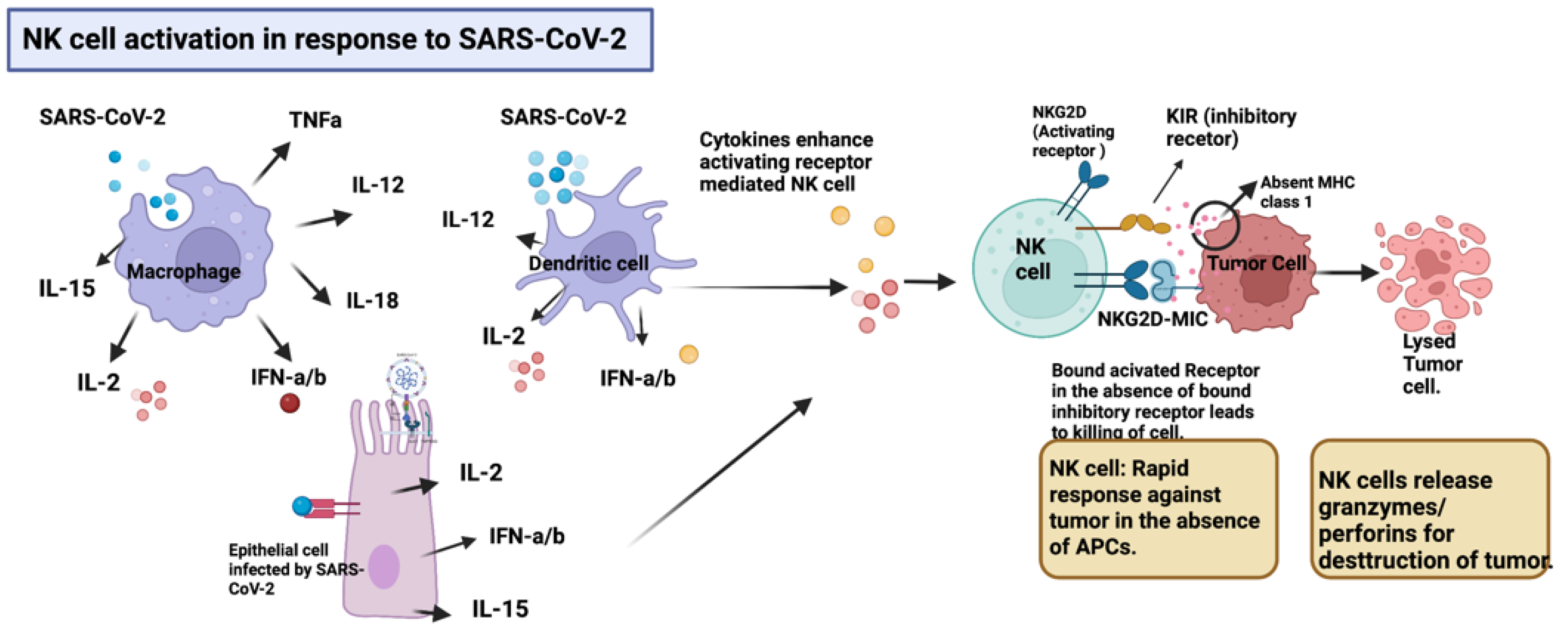
2.1. NK Cell Activation in Response to SARS-CoV-2
2.2. Molecular Mimicry or Cross-Reactivity
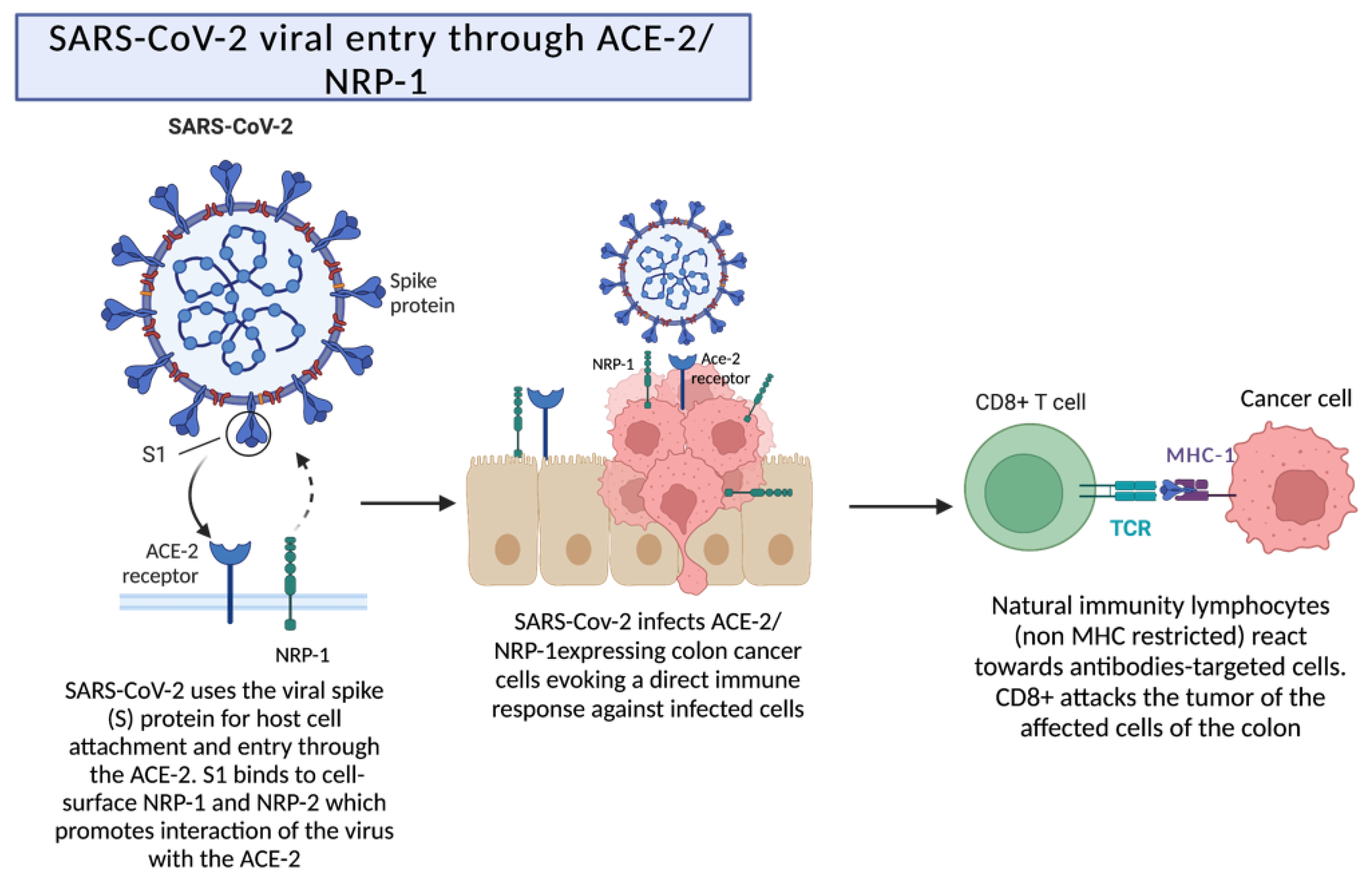
2.3. SARS-CoV-2 Viral Entry through ACE-2/NRP-1 following Destruction by Cytotoxic T Cells
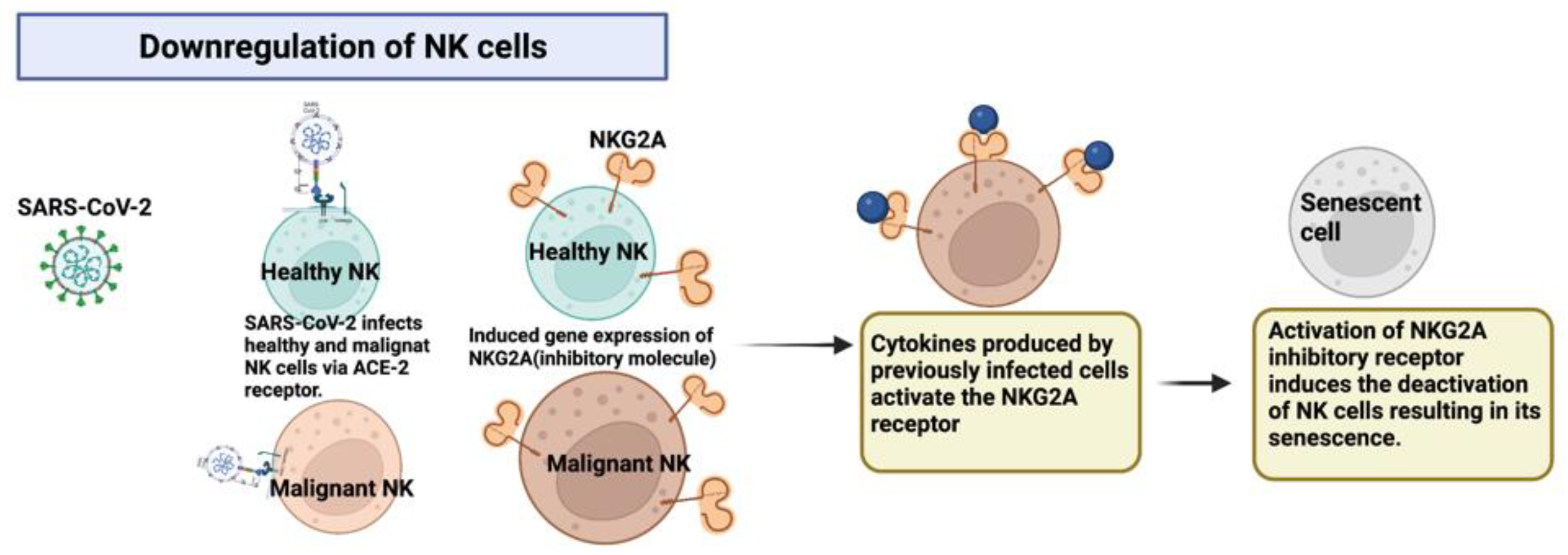
2.4. Downregulation of NK Cells via the Expression of Inhibitory Molecules after Infection by SARS-CoV-2 through the ACE2 Receptor
2.5. Proposed Mechanisms for SARS-CoV-2 as an Oncolytic Virus
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gandhi, R.T.; Lynch, J.B.; Del Rio, C. Mild or Moderate COVID-19. N. Engl. J. Med. 2020, 383, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Carballa, A.; Martinon-Torres, F.; Salas, A. Is SARS-CoV-2 an oncogenic virus? J. Infect. 2022, 85, 573–607. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Gillard, A.; Van Wieren, A.; Gomez-Manzano, C.; Fueyo, J. Remission of liquid tumors and SARS-CoV-2 infection: A literature review. Mol. Ther. Oncolytics 2022, 26, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Mantwill, K.; Klein, F.G.; Wang, D.; Hindupur, S.V.; Ehrenfeld, M.; Holm, P.S.; Nawroth, R. Concepts in Oncolytic Adenovirus Therapy. Int. J. Mol. Sci. 2021, 22, 10522. [Google Scholar] [CrossRef] [PubMed]
- Barkhordar, M.; Rostami, F.T.; Yaghmaie, M.; Abbaszadeh, M.; Chahardouli, B.; Mousavi, S.A. Spontaneous Complete Remission of Acute Myeloid Leukemia in the Absence of Disease-Modifying Therapy following Severe Pulmonary Involvement by Coronavirus Infectious Disease-19. Case Rep. Hematol. 2022, 2022, 2603607. [Google Scholar] [CrossRef] [PubMed]
- Pasin, F.; Mascalchi Calveri, M.; Calabrese, A.; Pizzarelli, G.; Bongiovanni, I.; Andreoli, M.; Cattaneo, C.; Rignanese, G. Oncolytic effect of SARS-CoV2 in a patient with NK lymphoma. Acta Biomed. 2020, 91, e2020047. [Google Scholar] [CrossRef]
- Sousa, L.G.; McGrail, D.J.; Li, K.; Marques-Piubelli, M.L.; Gonzalez, C.; Dai, H.; Ferri-Borgogno, S.; Godoy, M.; Burks, J.; Lin, S.Y.; et al. Spontaneous tumor regression following COVID-19 vaccination. J. Immunother. Cancer 2022, 10, e004371. [Google Scholar] [CrossRef]
- Raishan, S.; Alsabri, M.; Hanna, A.M.; Brett, M. Resolution of pituitary microadenoma after coronavirus disease 2019: A case report. J. Med. Case Rep. 2021, 15, 544. [Google Scholar] [CrossRef]
- Challenor, S.; Tucker, D. SARS-CoV-2-induced remission of Hodgkin lymphoma. Br. J. Haematol. 2021, 192, 415. [Google Scholar] [CrossRef]
- Ottaiano, A.; Scala, S.; D’Alterio, C.; Trotta, A.; Bello, A.; Rea, G.; Picone, C.; Santorsola, M.; Petrillo, A.; Nasti, G. Unexpected tumor reduction in metastatic colorectal cancer patients during SARS-CoV-2 infection. Ther. Adv. Med. Oncol. 2021, 13, 17588359211011455. [Google Scholar] [CrossRef]
- Sollini, M.; Gelardi, F.; Carlo-Stella, C.; Chiti, A. Complete remission of follicular lymphoma after SARS-CoV-2 infection: From the “flare phenomenon” to the “abscopal effect”. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2652–2654. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, E.Z.; Refaat, L.; Abdel-Fatah, R.; Samra, M.; Bayoumi, A.; Abdellateif, M.S.; Abdel-Hady, H.; Ali, M.; Khafagy, M. Could COVID-19 induce remission of acute leukemia? Hematology 2021, 26, 870–873. [Google Scholar] [CrossRef] [PubMed]
- Buckner, T.W.; Dunphy, C.; Fedoriw, Y.D.; van Deventer, H.W.; Foster, M.C.; Richards, K.L.; Park, S.I. Complete spontaneous remission of diffuse large B-cell lymphoma of the maxillary sinus after concurrent infections. Clin. Lymphoma Myeloma Leuk. 2012, 12, 455–458. [Google Scholar] [CrossRef]
- Burgio, S.; Conway de Macario, E.; Macario, A.J.; Cappello, F. SARS-CoV-2 in patients with cancer: Possible role of mimicry of human molecules by viral proteins and the resulting anti-cancer immunity. Cell Stress Chaperones 2021, 26, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Cerna, M.G.; Martinez-Garcia, E.A.; Torres-Bugarin, O.; Rubio-Jurado, B.; Riebeling, C.; Nava, A. The clinical significance of posttranslational modification of autoantigens. Clin. Rev. Allergy Immunol. 2014, 47, 73–90. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef] [PubMed]
- Hamming, I.; Timens, W.; Bulthuis, M.L.; Lely, A.T.; Navis, G.; van Goor, H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Klein, K.; Chen, K.E.; Williamson, M.K.; Anton-Plagaro, C.; Shoemark, D.K.; Simon-Gracia, L.; Bauer, M.; Hollandi, R.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 2020, 370, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Byun, J.E.; Yoon, S.R.; Koohy, H.; Jung, H.; Choi, I. SARS-CoV-2 peptides bind to NKG2D and increase NK cell activity. Cell. Immunol. 2022, 371, 104454. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, J.; Huang, J. CD8(+) T Cells and NK Cells: Parallel and Complementary Soldiers of Immunotherapy. Curr. Opin. Chem. Eng. 2018, 19, 9–20. [Google Scholar] [CrossRef]
- Rha, M.S.; Shin, E.C. Activation or exhaustion of CD8(+) T cells in patients with COVID-19. Cell. Mol. Immunol. 2021, 18, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 cytokine: An overview of the immune regulation, immune dysregulation, and therapeutic approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef] [PubMed]
- Meanwatthana, J.; Majam, T. Interleukin-6 Antagonists: Lessons From Cytokine Release Syndrome to the Therapeutic Application in Severe COVID-19 Infection. J. Pharm. Pract. 2022, 35, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Layfield, L.J.; Zhang, T.; Esebua, M. PD-L1 immunohistochemical testing: A review with reference to cytology specimens. Diagn. Cytopathol. 2022, 51, 51–58. [Google Scholar] [CrossRef] [PubMed]
| Reference | Diagnosis before COVID-19 | COVID-19 Clinical Course | Clinical Evaluation after COVID-19 |
|---|---|---|---|
| [5] | 57-year-old woman with acute myeloid leukemia M2 with 11q23/KMT2A abnormality | Before initiation of chemotherapy, the patient reported fever, pulmonary symptoms, and progressively worse hypoxemia, with a positive PCR for SARS-CoV-2. Treated in the ICU with remdesivir and dexamethasone IV for 10 days, then treated for COVID-19-related pulmonary fibrosis | Two months after the initial diagnosis, blood cell counts improved, returning to normal. Bone marrow sampling to reevaluate leukemia revealed a normocellular marrow with 55% cellularity and <5% of blasts. Final diagnosis: spontaneous morphologic remission in the absence of disease-modifying therapy for acute leukemia. One month later, the FISH analysis revealed a normal result and proved the molecular cytogenetic remission. Eight months later, the patient had hematological recurrence of the primary disease. |
| [6] | 20-year-old male with relapsed/refractory NK/T cell lymphoma associated with Epstein–Barr virus and autoimmune hemolytic anemia | The patient presented with a 5-day history of fatigue, fever, cough, and dyspnea. O2 saturation was 93%. Chest tomography showed diffuse bilateral ground-glass opacities, and lab studies indicated thrombocytopenia, leukocytosis, severe anemia, elevated CRP, and a positive test for COVID-19. Treated with methylprednisolone and O2 | Eleven days after the onset of COVID-19, the patient presented with a spontaneous steady clinical improvement, with hemolytic markers and platelets count normalization. Reduction in the number of both healthy and malignant NK clonal cells and increase in CD8+ T cells. Dropped values in plasma EVB-DNA, from 229,876 copies/mL to 495 copies/mL. Final diagnosis: remission of NK lymphoma during the COVID-19 infection. Two months after the COVID-19 infection, a recurrence was observed. |
| [7] | 61-year-old woman with T2NOMX metastatic myoepithelial carcinoma of the left parotid gland. | Six months after the diagnosis, the patient received the 2nd booster of the mRNA-1273 COVID vaccine, with severe side effects (fever, chills, fatigue, myalgias, muscular weakness, headache, and mental fogginess for 7 days). | Evidence of persistent tumor shrinkage on CT scans: 50%, 67%, and 73% reduction at 3, 6, and 9 months, respectively, after the second dose of the vaccine. |
| [8] | 32-year-old man with a pituitary microadenoma and secondary adrenal insufficiency and scotoma, managed with steroid therapy for 2 years | Febrile with shortness of breath, pulmonary rhonchi, and crepitation, increase in blurry vision and scotoma. O2 saturation was 80%. Patient’s white blood cell count, CRP levels, D-dimer, lactate dehydrogenase increased. Treated with methylprednisolone. After 10 days, the patient presented improvement but remained febrile for 1.5 months | Three months after the resolution of the COVID-19 infection, the patient underwent a control MRI which showed improvement in the pituitary microadenoma. The changes included disappearance of the hypointense lesion and hyperintense enhancement that were seen in the previous MRI (6 months before). Clinically, the patients’ blurry vision improved, as well as the headaches. |
| [9] | 61-year-old male with stage III Hodgkin lymphoma caused by Epstein–Barr virus | SARS-CoV-2 pneumonia with a course of 11 days of supportive ward-based care without any treatments | Remission of Hodgkin lymphoma in four months, with widespread resolution of lymphadenopathy and reduction of metabolic uptake. PCR showed EBV viral reduction to 413 copies/mL (log10 2⋅62) |
| [10] | Patient 1, a 65-year-old man with a pT4apN1b metastatic colorectal adenocarcinoma. Patient 2, a 58-year-old man with a pT3pN0 RAS-mutated, metastatic colorectal adenocarcinoma Patient 3, a 60-year-old woman with a pT3pN2A metastatic colorectal adenocarcinoma | Patient 1 developed severe COVID-19 symptoms and Patients 2 and 3 developed mild symptomatic COVID-19 | Patients 1 and 2: CT scan results showed regression of metastatic liver lesions secondary to the immune response induced by COVID-19. Patient 3 experienced a reduction in peritoneal and lung disease by CT scan after recovery from COVID-19. |
| [11] | 61-year-old patient with follicular lymphoma treated with R-bentdamustine | Patient with COVID-19 bilateral pneumonia and partial response to R-bendamustine. | After COVID-19 recovery, a complete remission was observed with a CT-guided biopsy performed twice and by a second follow-up scan. |
| [12] | Patient 1, a 63-year-old female with acute myeloid leukemia Patient 2, a 28-year-old previously treated for a diagnosis of T acute lymphocytic leukemia | Patient 1 presented with fever, dyspnea, and wheezy chest and was diagnosed with moderate COVID-19 by PCR. Her treatment for AML was postponed until she recovered from COVID-19; she received azithromycin and prednisone for five days. Patient 2 presented with fever, headache, malaise, sore throat, dry cough, loss of smell and taste, and multiple cervical lymphadenopathies. A nasopharyngeal swab confirmed COVID-19 infection. The patient was treated with azithromycin and prednisone for five days. | Patient 1 showed an improvement after 5 weeks of recovery from COVID-19. Her CBC showed a hemoglobin level of 8.7 g/dL, TLC was 1.6 × 100,000, with no blast cells. BMA: blast count was reduced to 3% with explicit dysplasia in the trilineage and normal karyotype. After 3 rounds of repeated investigations, the final diagnosis was a reduction of blast cells after COVID-19, with no relapse until last visited. Patient 2 showed improvement after 6 weeks of recovering from COVID-19. The patient’s cervical lymphadenopathy disappeared, and the total lymphocyte count decreased from 28 × 103/UL and 30% of blast cells to 6.5 × 103/UL, with no evidence of atypical cells or relapses. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bounassar-Filho, J.P.; Boeckler-Troncoso, L.; Cajigas-Gonzalez, J.; Zavala-Cerna, M.G. SARS-CoV-2 as an Oncolytic Virus Following Reactivation of the Immune System: A Review. Int. J. Mol. Sci. 2023, 24, 2326. https://doi.org/10.3390/ijms24032326
Bounassar-Filho JP, Boeckler-Troncoso L, Cajigas-Gonzalez J, Zavala-Cerna MG. SARS-CoV-2 as an Oncolytic Virus Following Reactivation of the Immune System: A Review. International Journal of Molecular Sciences. 2023; 24(3):2326. https://doi.org/10.3390/ijms24032326
Chicago/Turabian StyleBounassar-Filho, Joao P., Laura Boeckler-Troncoso, Jocelyne Cajigas-Gonzalez, and Maria G. Zavala-Cerna. 2023. "SARS-CoV-2 as an Oncolytic Virus Following Reactivation of the Immune System: A Review" International Journal of Molecular Sciences 24, no. 3: 2326. https://doi.org/10.3390/ijms24032326





