Characterization of the 1-Deoxy-D-xylulose 5-Phosphate synthase Genes in Toona ciliata Suggests Their Role in Insect Defense
Abstract
1. Introduction
2. Results
2.1. Identification and Characterization
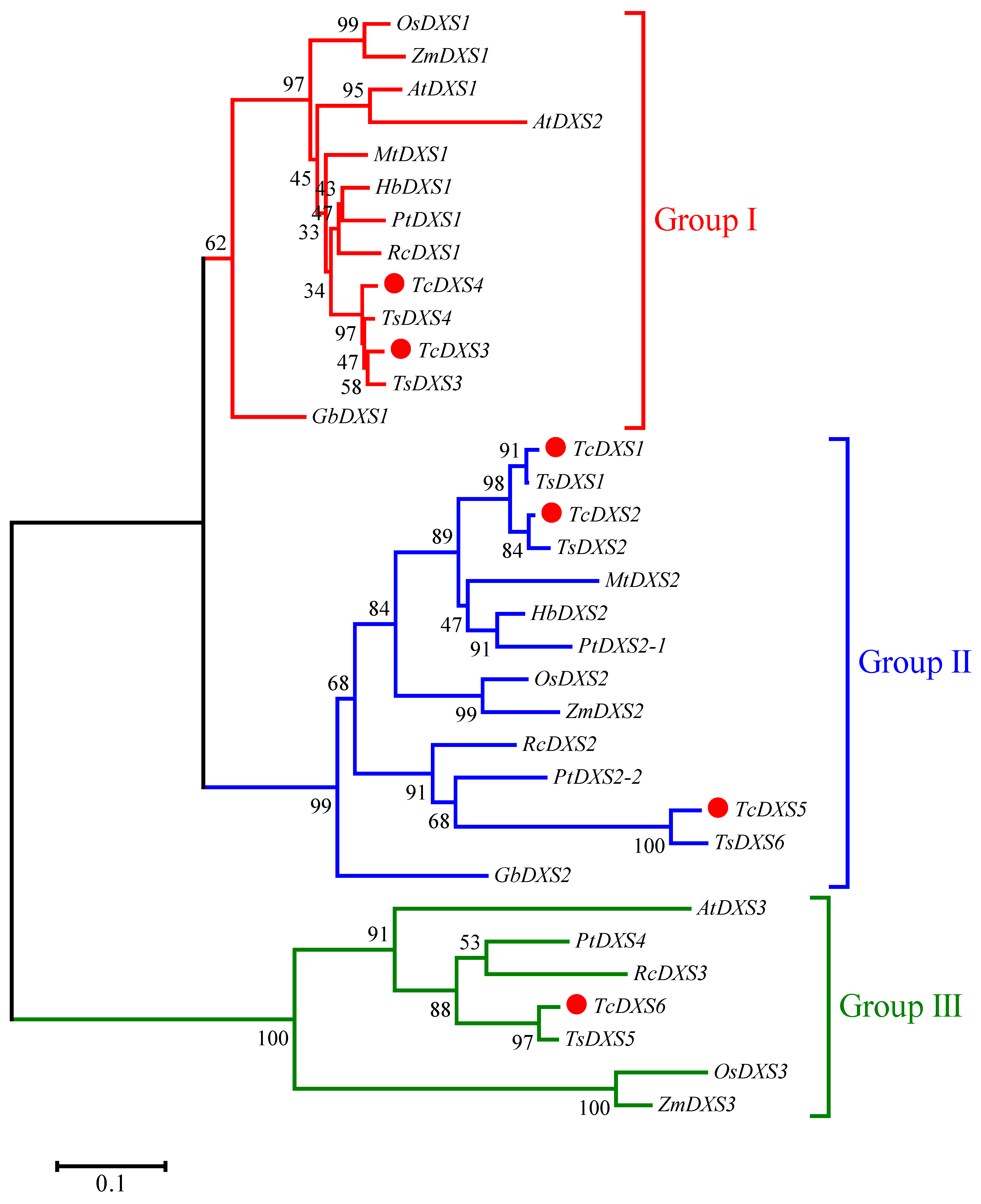
2.2. Multiple Sequence Alignment and Phylogenetic Analysis
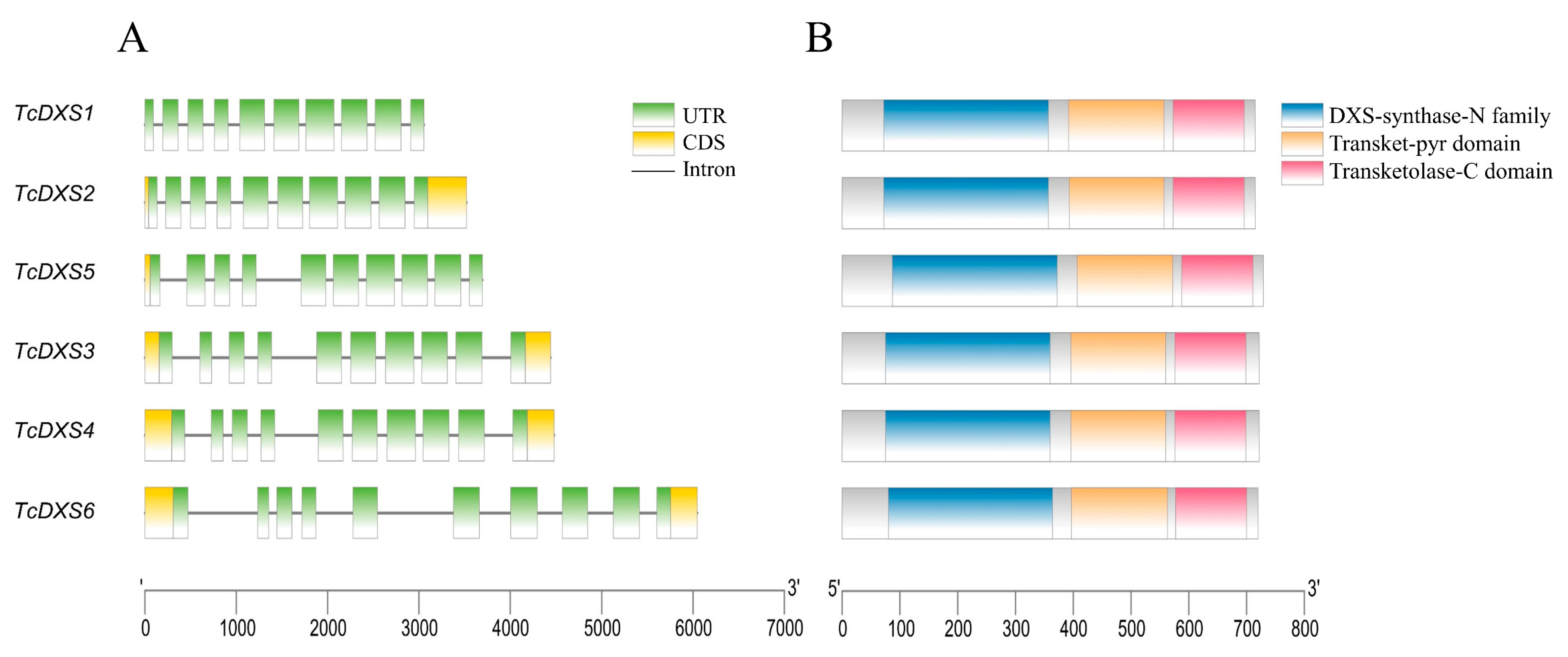
2.3. Chromosome Location, Gene Structure, and Conserved Domain Analysis
2.4. TcDXSs Promoter cis-Acting Element Prediction
2.5. Expression Patterns under H. robusta Stress
2.6. Expression Patterns of Different Tissues and Provenances
2.7. Subcellular Location of TcDXS Proteins
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatments
4.2. TcDXS Gene Family of T. ciliata Identification and Analysis
4.3. Multiple Sequence Alignment and Phylogenetic Analysis
4.4. Chromosome Location, Gene Structure, and Conserved Domain Analysis
4.5. Promoter cis-Acting Element Prediction
4.6. Gene Cloning
4.7. RNA Extraction and Reverse Transcription-Quantitative PCR (RT-qPCR) Analysis
4.8. Vector Construction and Subcellular Localization Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhan, X.; Li, P.; Hui, W.; Deng, Y.; Gan, S.; Sun, Y.; Zhao, X.; Chen, X.; Deng, X. Genetic diversity and population structure of Toona ciliata revealed by simple sequence repeat markers. Biotechnol. Biotechnol. Equip. 2019, 33, 214–222. [Google Scholar] [CrossRef]
- Li, P.; Shang, Y.; Zhou, W.; Hu, X.; Mao, W.; Li, J.; Li, J.; Chen, X. Development of an efficient regeneration system for the precious and fast-growing timber tree Toona ciliata. Plant Biotechnol. 2018, 35, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Kaewmano, A.; Fu, P.; Fan, Z.; Pumijumnong, N.; Zuidema, P.A.; Bräuning, A. Climatic influences on intra-annual stem radial variations and xylem formation of Toona ciliata at two Asian tropical forest sites with contrasting soil water availability. Agric. For. Meteorol. 2022, 318, 108906. [Google Scholar] [CrossRef]
- Mao, W.; Song, H.; Li, Y.; Wang, Y.; Lin, H.; Yao, C.; Zhou, W.; Yang, B.; Chen, X.; Li, P. Efficient plant regeneration and genetic transformation system of the precious fast-growing tree Toona ciliata. Ind. Crops Prod. 2021, 172, 114015. [Google Scholar] [CrossRef]
- Song, H.; Duan, Z.; Wang, Z.; Li, Y.; Wang, Y.; Li, C.; Mao, W.; Que, Q.; Chen, X.; Li, P. Genome-wide identification, expression pattern and subcellular localization analysis of the JAZ gene family in Toona ciliata. Ind. Crops Prod. 2022, 178, 114582. [Google Scholar] [CrossRef]
- Song, H.; Mao, W.; Duan, Z.; Que, Q.; Zhou, W.; Chen, X.; Li, P. Selection and validation of reference genes for measuring gene expression in Toona ciliata under different experimental conditions by quantitative real-time PCR analysis. BMC Plant Biol. 2020, 20, 450. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Nagegowda, D.A.; Gupta, P. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci. 2020, 294, 110457. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Tang, Q.; Kong, W.; Chung, W.J.; Lu, T. MEP Pathway-mediated isopentenol production in metabolically engineered Escherichia coli. Microb. Cell Fact. 2014, 13, 135. [Google Scholar] [CrossRef]
- Martin, V.J.; Pitera, D.J.; Withers, S.T.; Newman, J.D.; Keasling, J.D. Engineering a mevalonate pathway in Escherichia coli for production of terpenoids. Nat. Biotechnol. 2003, 21, 796–802. [Google Scholar] [CrossRef]
- Liu, C.; Bi, H.; Bai, Z.; Fan, L.; Tan, T. Engineering and manipulation of a mevalonate pathway in Escherichia coli for isoprene production. Appl. Microbiol. Biotechnol. 2019, 103, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Bergman, M.E.; Davis, B.; Phillips, M.A. Medically Useful Plant Terpenoids: Biosynthesis, Occurrence, and Mechanism of Action. Molecules 2019, 24, 3961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, M.; Wen, H.; Wang, Z.; Chen, J.; Fang, L.; Zhang, H.; Xie, Z.; Jiang, D.; Cheng, Y.; et al. Transcriptomic and metabolomic analyses provide insight into the volatile compounds of citrus leaves and flowers. BMC Plant Biol. 2020, 20, 7. [Google Scholar] [CrossRef]
- Du, Y.; Poppy, G.M.; Powell, W.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Identification of Semiochemicals Released during Aphid Feeding That Attract Parasitoid Aphidius ervi. J. Chem. Ecol. 1998, 24, 1355–1368. [Google Scholar] [CrossRef]
- Song, H.; Li, Y.; Wang, Z.; Duan, Z.; Wang, Y.; Yang, E.; Que, Q.; Chen, X.; Li, P. Transcriptome profiling of Toona ciliata young stems in response to Hypsipyla robusta Moore. Front. Plant Sci. 2022, 13, 950945. [Google Scholar] [CrossRef]
- Dabiri, M.; Majdi, M.; Bahramnejad, B. Partial sequence isolation of DXS and AOS genes and gene expression analysis of terpenoids and pyrethrin biosynthetic pathway of Chrysanthemum cinerariaefolium under abiotic elicitation. Acta Physiol. Plant 2020, 42, 30. [Google Scholar] [CrossRef]
- Zhang, S.; Ding, G.; He, W.; Liu, K.; Luo, Y.; Tang, J.; He, N. Functional Characterization of the 1-Deoxy-D-Xylulose 5-Phosphate Synthase Genes in Morus notabilis. Front. Plant Sci. 2020, 11, 1142. [Google Scholar] [CrossRef]
- You, M.K.; Lee, Y.J.; Kim, J.K.; Baek, S.A.; Jeon, Y.A.; Lim, S.H.; Ha, S.H. The organ-specific differential roles of rice DXS and DXR, the first two enzymes of the MEP pathway, in carotenoid metabolism in Oryza sativa leaves and seeds. BMC Plant Biol. 2020, 20, 167. [Google Scholar] [CrossRef]
- Cordoba, E.; Porta, H.; Arroyo, A.; San, R.C.; Medina, L.; Rodriguez-Concepcion, M.; Leon, P. Functional characterization of the three genes encoding 1-deoxy-D-xylulose 5-phosphate synthase in maize. J. Exp. Bot. 2011, 62, 2023–2038. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, W.; Xia, J.; Zeng, J.; Xiang, L.; Zhu, S.; Zheng, Q.; Xie, H.; Yang, C.; Chen, M.; et al. Molecular Characterization of the 1-Deoxy-D-Xylulose 5-Phosphate Synthase Gene Family in Artemisia annua. Front. Plant Sci. 2018, 9, 952. [Google Scholar] [CrossRef]
- Pan, X.; Li, Y.; Pan, G.; Yang, A. Bioinformatics study of 1-deoxy-D-xylulose-5-phosphate synthase (DXS) genes in Solanaceae. Mol. Biol. Rep. 2019, 46, 5175–5184. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wei, H.; Movahedi, A.; Sun, W.; Ma, X.; Li, D.; Yin, T.; Zhuge, Q. Evaluation, characterization, expression profiling, and functional analysis of DXS and DXR genes of Populus trichocarpa. Plant Physiol. Biochem. 2019, 142, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Jadaun, J.S.; Sangwan, N.S.; Narnoliya, L.K.; Singh, N.; Bansal, S.; Mishra, B.; Sangwan, R.S. Over-expression of DXS gene enhances terpenoidal secondary metabolite accumulation in rose-scented geranium and Withania somnifera: Active involvement of plastid isoprenogenic pathway in their biosynthesis. Physiol. Plant. 2017, 159, 381–400. [Google Scholar] [CrossRef]
- Li, R.; Chen, P.; Zhu, L.; Wu, F.; Chen, Y.; Zhu, P.; Ji, K. Characterization and Function of the 1-Deoxy-D-xylose-5-Phosphate Synthase (DXS) Gene Related to Terpenoid Synthesis in Pinus massoniana. Int. J. Mol. Sci. 2021, 22, 848. [Google Scholar] [CrossRef]
- Fan, H.; Wu, Q.; Wang, X.; Wu, L.; Cai, Y.; Lin, Y. Molecular cloning and expression of 1-deoxy-d-xylulose-5-phosphate synthase and 1-deoxy-d-xylulose-5-phosphate reductoisomerase in Dendrobium officinale. Plant Cell Tissue Organ Cult. 2016, 125, 381–385. [Google Scholar] [CrossRef]
- Zolfaghari, F.; Monfared, S.R.; Moeini, A.; Abedini, D.; Ebrahimi, A. Improving diosgenin production and its biosynthesis in Trigonella foenum-graecum L. hairy root cultures. Ind. Crops Prod. 2020, 145, 112075. [Google Scholar] [CrossRef]
- Sharma, E.; Pandey, S.; Gaur, A.K. Identification and expression analysis of DXS1 gene isolated from Aconitum balfourii Stapf. Acta Physiol. Plant. 2016, 38, 233. [Google Scholar] [CrossRef]
- de Luna-Valdez, L.; Chenge-Espinosa, M.; Hernández-Muñoz, A.; Cordoba, E.; López-Leal, G.; Castillo-Ramírez, S.; León, P. Reassessing the evolution of the 1-deoxy-D-xylulose 5-phosphate synthase family suggests a possible novel function for the DXS class 3 proteins. Plant Sci. 2021, 310, 110960. [Google Scholar] [CrossRef]
- Sitthithaworn, W.; Wungsintaweekul, J.; Sirisuntipong, T.; Charoonratana, T.; Ebizuka, Y.; De-Eknamkul, W. Cloning and expression of 1-deoxy-d-xylulose 5-phosphate synthase cDNA from Croton stellatopilosus and expression of 2C-methyl-d-erythritol 4-phosphate synthase and geranylgeranyl diphosphate synthase, key enzymes of plaunotol biosynthesis. J. Plant Physiol. 2010, 167, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Zhou, P.; Yin, M.; Liu, L.; Dai, S.; Liu, C.; Wu, Q. Cloning and bioinformatics analysis of 1-deoxy-D-xylulose 5-phosphate synthase DXS gene from Schizonepeta tenuifolia. Chin. Tradit. Herbal. Drugs (Chin.) 2021, 52, 527–537. [Google Scholar]
- Jin, R.; Zhu, C.; Xu, C. 1-Deoxy-D-Xylulose 5-Phosphate Synthase (DXS) and Its Encoding Genes. Chin. J. Cell Biol. (Chin.) 2007, 29, 706–712. [Google Scholar]
- Wang, C.; Zhou, Z.; Zhang, Q.; Li, Y. Risk Analysis of Hypsipyla robusta Moore in China. Hebei For. Sci. Technol. (Chin.) 2017, 01, 47–50. [Google Scholar]
- Yang, J.; Xiao, G. The Insect-resistance Physiology of Plants: A Review. Chin. Agric. Sci. Bull. (Chin.) 2021, 37, 130–136. [Google Scholar]
- Wang, J.; Wu, Q.; Lu, Z. Advances in research on plant secondary metabolites and insect resistance in trees. Jiangsu Agric. Sci. (Chin.) 2015, 43, 4–7. [Google Scholar]
- Gershenzon, J.; Dudareva, N. The function of terpene natural products in the natural world. Nat. Chem. Biol. 2007, 3, 408–414. [Google Scholar] [CrossRef]
- Jia, H.; Huang, D.; Cao, Y.; Kong, X.; Zhang, Z. Relationship between Resin Monoterpenes and Resistance of Chinese Pine to Red Turpentine Beetle. J. Northeast For. Univ. (Chin.) 2008, 36, 48–50. [Google Scholar]
- Lu, J.; Liu, Z.; Li, L.; Wen, X.; Li, T. Constituent Analysis of Volatile Organic Compounds in Three Meliaceae. Hubei Agric. Sci. (Chin.) 2016, 55, 461–464. [Google Scholar]
- Wang, C.; Zhou, Z.; Kong, D.; Li, Y. Constituent Analysis of Volatile Organic Compounds in leaves of Toona sisnensis Roem and Toona ciliata Roem. Hebei For. Sci. Technol. (Chin.) 2017, 02, 44–47. [Google Scholar]
- Chen, X.; Zhang, Y.; Yan, H.; Niu, M.; Xiong, Y.; Zhang, X.; Li, Y.; Teixeira Da Silva, J.A.; Ma, G. Cloning and functional analysis of 1-deoxy-d-xylulose-5-phosphate synthase (DXS) in Santalum album L. Gene 2023, 851, 146762. [Google Scholar] [CrossRef]
- Lang, C. The Regulation of Isoprenoid Biosynthesis by a Key Enzyme and Intermediates from the 2-C-methyl-D-erythritol-4-phosphate (MEP) Pathway in Arabidopsis thaliana. Ph.D. Dissertation, Jilin University, Changchun, China, 2016. [Google Scholar]
- Walter, M.H.; Hans, J.; Strack, D. Two distantly related genes encoding 1-deoxy-d-xylulose 5-phosphate synthases: Differential regulation in shoots and apocarotenoid-accumulating mycorrhizal roots. Plant J. 2002, 31, 243–254. [Google Scholar] [CrossRef]
- Liu, W. Study on Functional Differentiation of AaDXS Gene Family and Molecular Mechanism of Low Temperature Improving Artemisinin Production in Artemisia annua L. Ph.D. Dissertation, Chongqing University, Chongqing, China, 2016. [Google Scholar]
- Zhang, Y.; Cao, X.; Liu, W.; Wang, Y.; Wang, J. Cloning and expression analysis of DXS gene in Cinnamomum camphora. Genom. Appl. Biol. (Chin.) 2020, 39, 3570–3577. [Google Scholar]
- Hernandez-Garcia, C.M.; Finer, J.J. Identification and validation of promoters and cis-acting regulatory elements. Plant Sci. 2014, 217–218, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Sahoo, D.K.; Dey, N.; Houtz, R.L.; Maiti, I.B. An intergenic region shared by At4g35985 and At4g35987 in Arabidopsis thaliana is a tissue specific and stress inducible bidirectional promoter analyzed in transgenic arabidopsis and tobacco plants. PLoS ONE 2013, 8, e79622. [Google Scholar] [CrossRef] [PubMed]
- Kovalchuk, N.; Jia, W.; Eini, O.; Morran, S.; Pyvovarenko, T.; Fletcher, S.; Bazanova, N.; Harris, J.; Beck-Oldach, K.; Shavrukov, Y.; et al. Optimization of TaDREB3 gene expression in transgenic barley using cold-inducible promoters. Plant Biotechnol. J. 2013, 11, 659–670. [Google Scholar] [CrossRef]
- Bang, S.W.; Park, S.; Jeong, J.S.; Kim, Y.S.; Jung, H.; Ha, S.; Kim, J. Characterization of the stress-inducible OsNCED3 promoter in different transgenic rice organs and over three homozygous generations. Planta 2013, 237, 211–224. [Google Scholar] [CrossRef]
- Luo, Q.; Li, Y.; Gu, H.; Zhao, L.; Gu, X.; Li, W. The promoter of soybean photoreceptor GmPLP1 gene enhances gene expression under plant growth regulator and light stresses. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 114, 109–119. [Google Scholar] [CrossRef]
- Srinath, M.; Shailaja, A.; Bindu, B.B.V.; Giri, C.C. Molecular Cloning and Differential Gene Expression Analysis of 1-Deoxy-D-xylulose 5-Phosphate Synthase (DXS) in Andrographis paniculata (Burm. f) Nees. Mol. Biotechnol. 2021, 63, 109–124. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H.B. Plant-mPLoc: A Top-Down Strategy to Augment the Power for Predicting Plant Protein Subcellular Localization. PLoS ONE 2010, 5, e11335. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Nicholas, K.B. GeneDoc: Analysis and visualization of genetic variation. Embnew News 1997, 4, 14. [Google Scholar]
- Sudhir, K.; Glen, S.; Koichiro, T. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar]
- Lescot, M.; Déhais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouzé, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Medrano, J.F. Real-time PCR for mRNA quantitation. Biotechniques 2005, 39, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.D.; Cho, Y.H.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed]







| Gene Name | Gene ID | Strand | CDS (bp) | Protein | Predicted Subcellular Localization | ||
|---|---|---|---|---|---|---|---|
| Length (aa) | PI | MW (kDa) | |||||
| TcDXS1 | Tci11G005020.1 | − | 2145 | 714 | 7.93 | 76.83 | Chloroplast |
| TcDXS2 | Tci12G005860.1 | + | 2145 | 714 | 6.94 | 77.02 | Chloroplast |
| TcDXS3 | Tci21G002650.1 | − | 2166 | 721 | 7.03 | 77.74 | Chloroplast |
| TcDXS4 | Tci22G009550.1 | + | 2166 | 721 | 7.15 | 77.68 | Chloroplast |
| TcDXS5 | Tci25G002430.1 | − | 2187 | 728 | 7.11 | 78.77 | Chloroplast |
| TcDXS6 | Tci25G013250.1 | + | 2160 | 719 | 5.99 | 78.39 | Chloroplast |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, Y.; Song, H.; Wang, Z.; Li, P. Characterization of the 1-Deoxy-D-xylulose 5-Phosphate synthase Genes in Toona ciliata Suggests Their Role in Insect Defense. Int. J. Mol. Sci. 2023, 24, 2339. https://doi.org/10.3390/ijms24032339
Wang Y, Li Y, Song H, Wang Z, Li P. Characterization of the 1-Deoxy-D-xylulose 5-Phosphate synthase Genes in Toona ciliata Suggests Their Role in Insect Defense. International Journal of Molecular Sciences. 2023; 24(3):2339. https://doi.org/10.3390/ijms24032339
Chicago/Turabian StyleWang, Yueyang, Yue Li, Huiyun Song, Zhi Wang, and Pei Li. 2023. "Characterization of the 1-Deoxy-D-xylulose 5-Phosphate synthase Genes in Toona ciliata Suggests Their Role in Insect Defense" International Journal of Molecular Sciences 24, no. 3: 2339. https://doi.org/10.3390/ijms24032339
APA StyleWang, Y., Li, Y., Song, H., Wang, Z., & Li, P. (2023). Characterization of the 1-Deoxy-D-xylulose 5-Phosphate synthase Genes in Toona ciliata Suggests Their Role in Insect Defense. International Journal of Molecular Sciences, 24(3), 2339. https://doi.org/10.3390/ijms24032339





