Dendrobine Alleviates Cellular Senescence and Osteoarthritis via the ROS/NF-κB Axis
Abstract
:1. Introduction
2. Results
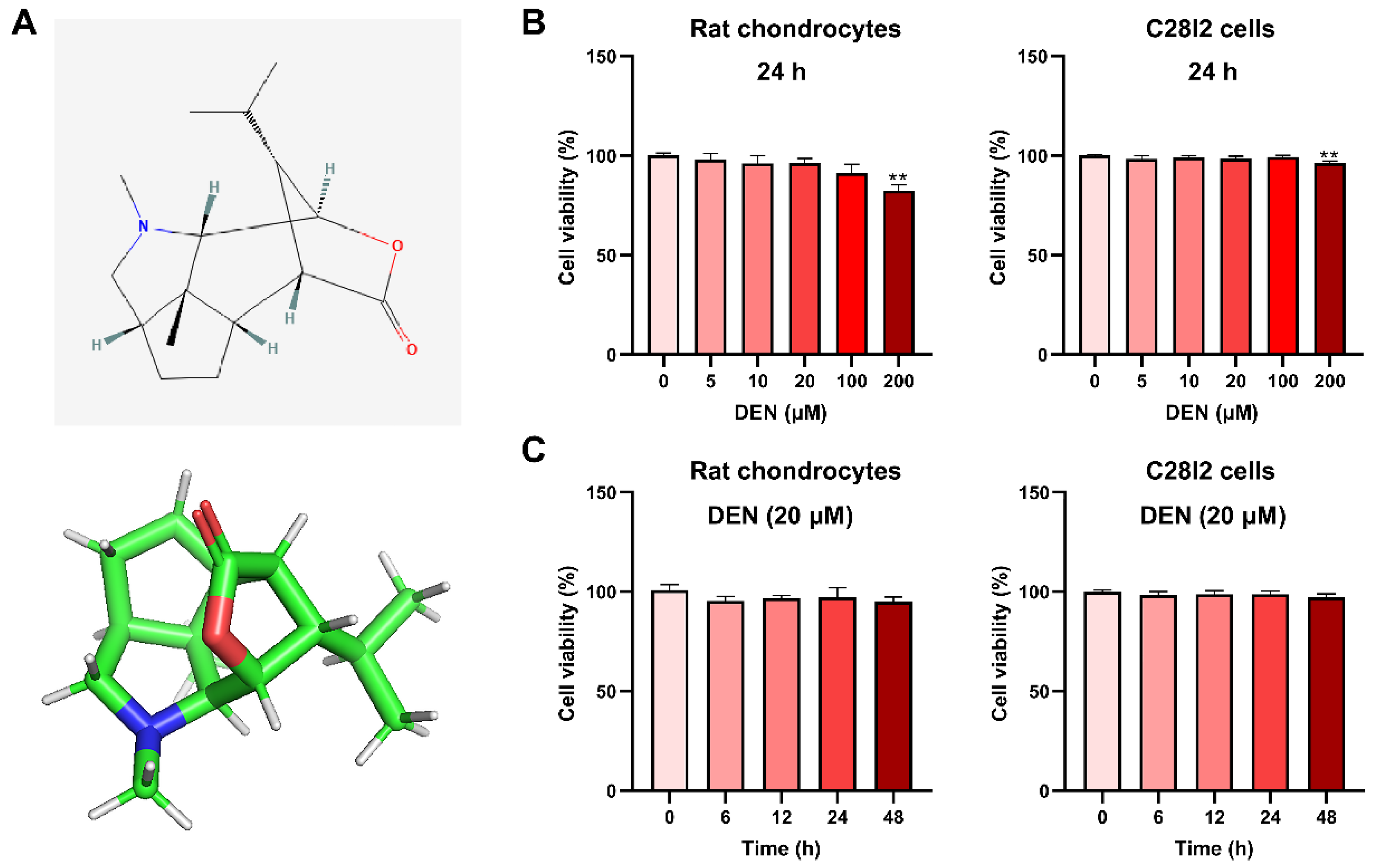
2.1. The Effect of DEN on the Viability of Chondrocytes
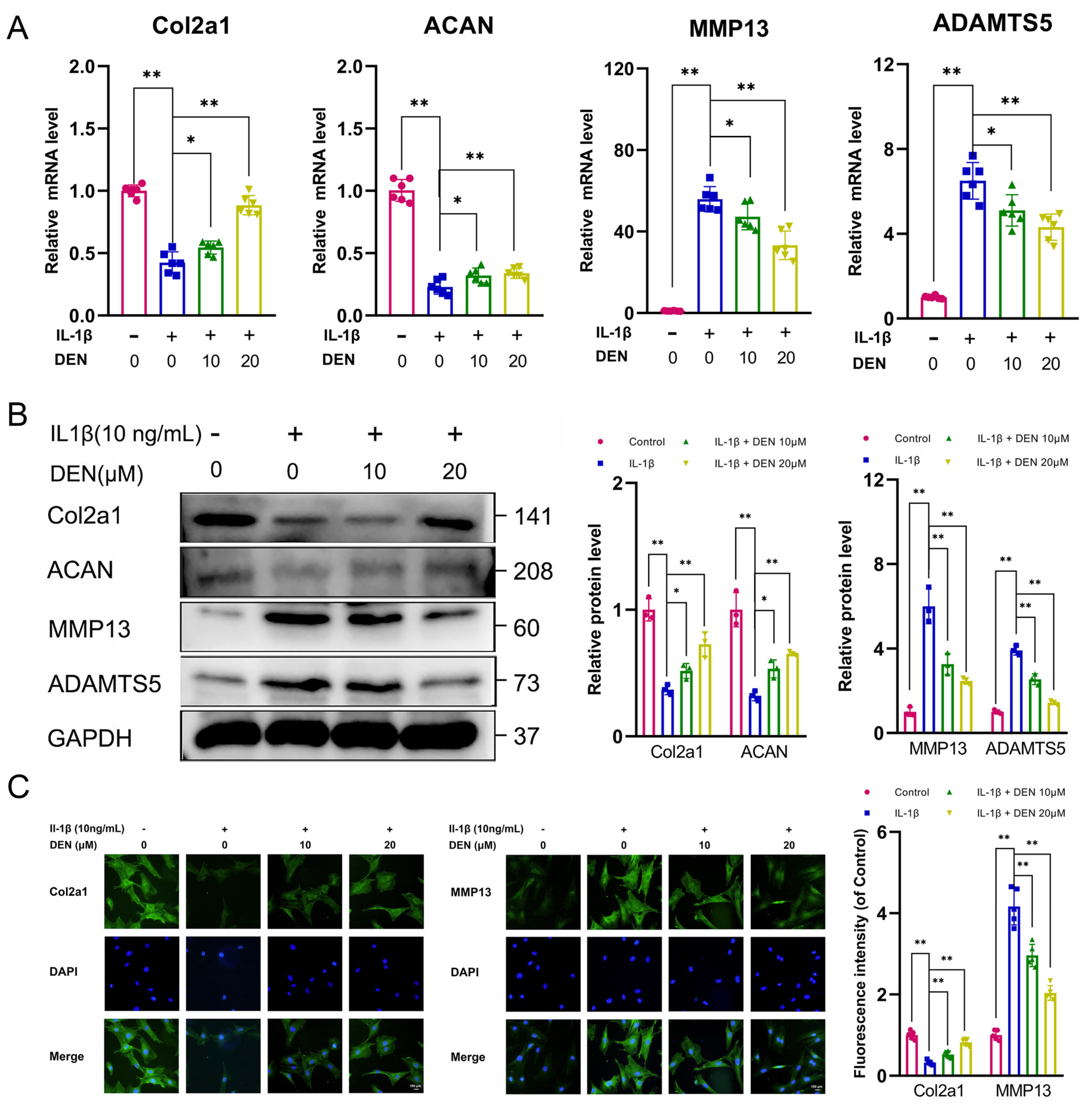
2.2. DEN Inhibited Extracellular Matrix (ECM) Degradation and Increased ECM Synthesis in IL-1β-Treated Chondrocytes
2.3. DEN Inhibited the SASP Factors Expression and Senescence Phenotype in IL-1β-Treated Chondrocytes
2.4. DEN Improved Mitochondrial Function and Reduced the Production of Intracellular ROS in IL-1β-Treated Chondrocytes
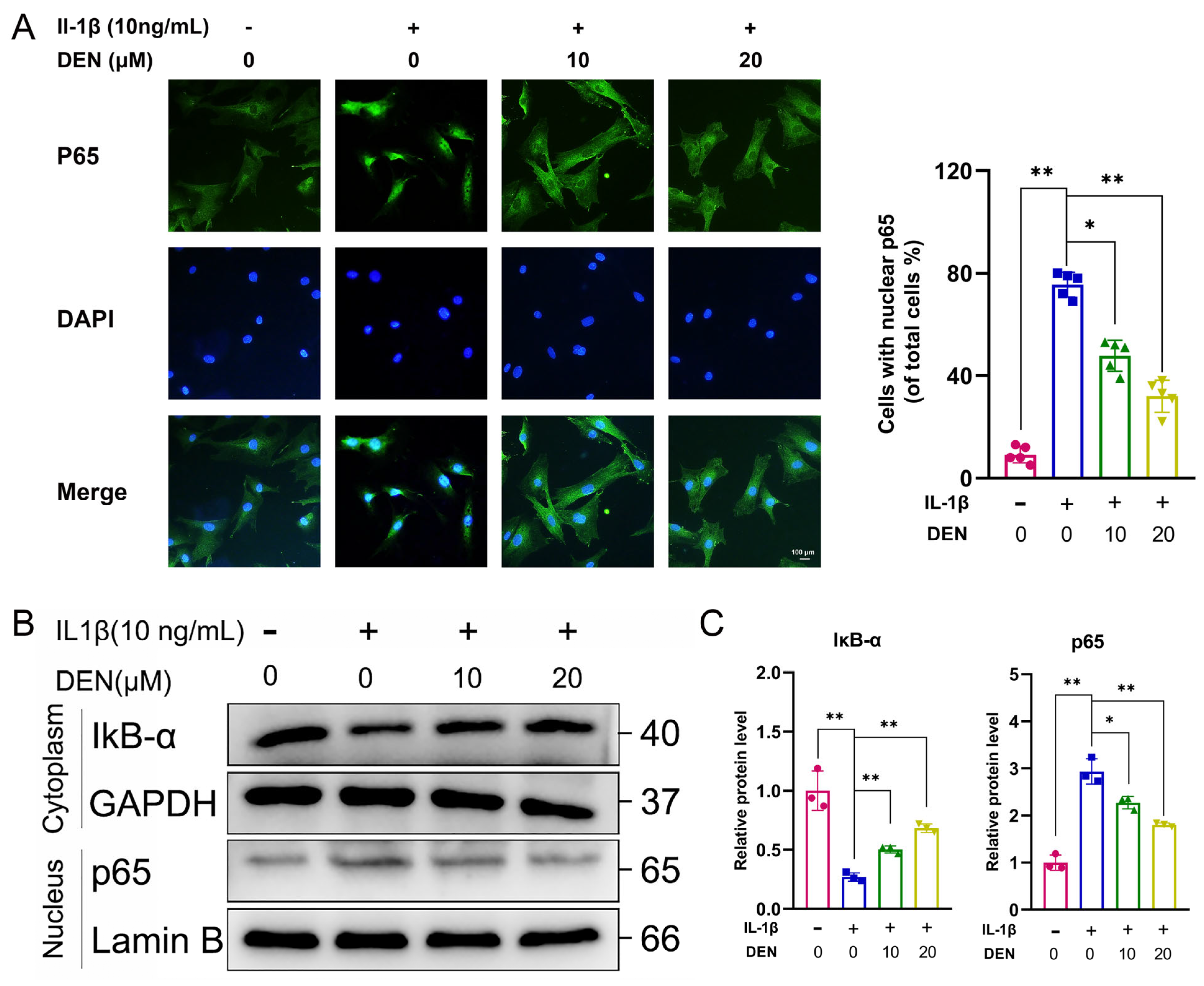
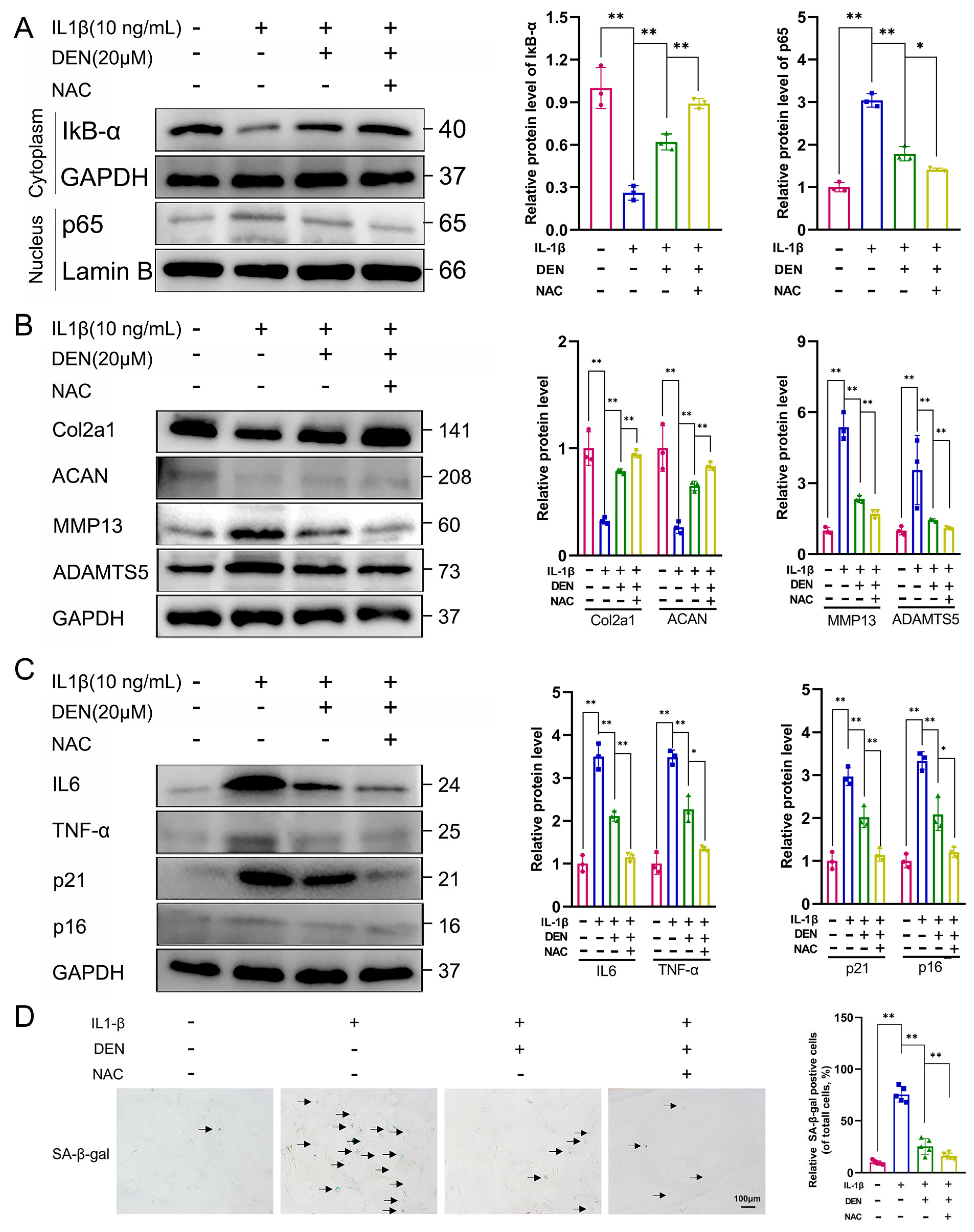
2.5. DEN Inhibited the ROS/NF-κB Pathway in IL-1β-Treated Chondrocytes
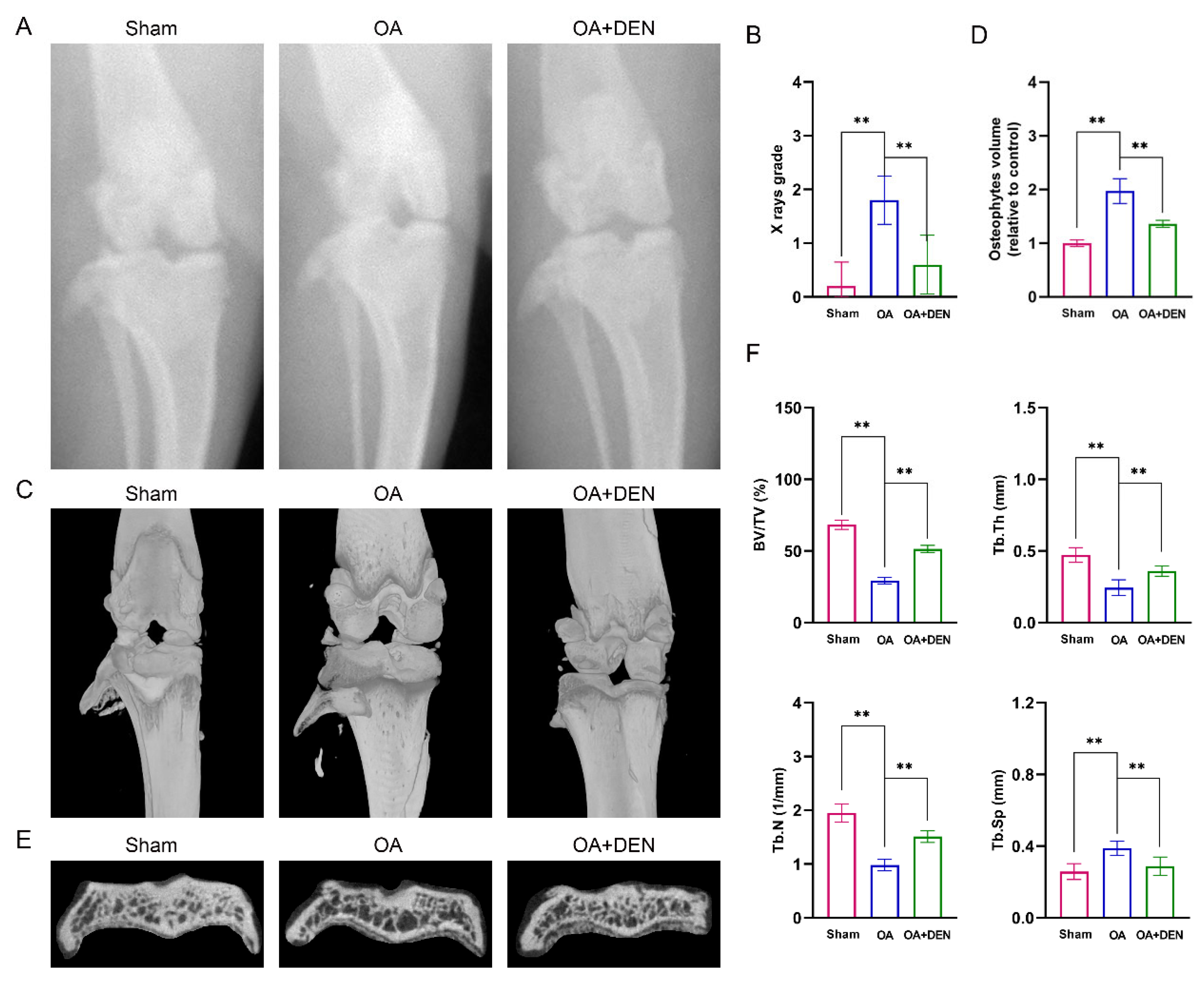
2.6. DEN Alleviates the Progression of OA in an Anterior Cruciate Ligament Transection (ACLT) Rat Model
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Extraction and Culture of Chondrocytes and Cell Viability Assay
4.3. Total RNA Extraction and RT-qPCR
4.4. WB
4.5. SA-β-Gal Staining
4.6. Cellular Immunofluorescence Staining
4.7. Intracellular ROS
4.8. Mitochondrial Membrane Potential
4.9. Rat Model of OA
4.10. X-ray Images
4.11. Micro-CT
4.12. Histopathological Analysis
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 386, 743–800. [Google Scholar] [CrossRef] [Green Version]
- Emery, C.A.; Whittaker, J.L.; Mahmoudian, A.; Lohmander, L.S.; Roos, E.M.; Bennell, K.L.; Toomey, C.M.; Reimer, R.A.; Thompson, D.; Ronsky, J.L.; et al. Establishing outcome measures in early knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 438–448. [Google Scholar] [CrossRef]
- Nagata, K.; Hojo, H.; Chang, S.H.; Okada, H.; Yano, F.; Chijimatsu, R.; Omata, Y.; Mori, D.; Makii, Y.; Kawata, M.; et al. Runx2 and Runx3 differentially regulate articular chondrocytes during surgically induced osteoarthritis development. Nat. Commun. 2022, 13, 6187. [Google Scholar] [CrossRef]
- Heijink, A.; Gomoll, A.H.; Madry, H.; Drobnič, M.; Filardo, G.; Espregueira-Mendes, J.; Van Dijk, C.N. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg. Sport. Traumatol. Arthrosc. 2012, 20, 423–435. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Ni, Q.; Li, B.; Chen, L. Identification of differentially expressed genes in synovial tissue of osteoarthritis based on a more robust integrative analysis method. Clin. Rheumatol. 2021, 40, 3745–3754. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The role of oxidative stress in the development of knee osteoarthritis: A comprehensive research review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef]
- Coryell, P.R.; Diekman, B.O.; Loeser, R.F. Mechanisms and therapeutic implications of cellular senescence in osteoarthritis. Nat. Rev. Rheumatol. 2021, 17, 47–57. [Google Scholar] [CrossRef]
- Jeon, O.H.; David, N.; Campisi, J.; Elisseeff, J.H. Senescent cells and osteoarthritis: A painful connection. J. Clin. Investig. 2018, 128, 1229–1237. [Google Scholar] [CrossRef]
- Wan, M.; Gray-Gaillard, E.F.; Elisseeff, J.H. Cellular senescence in musculoskeletal homeostasis, diseases, and regeneration. Bone Res. 2021, 9, 41. [Google Scholar] [CrossRef]
- Xie, J.; Wang, Y.; Lu, L.; Liu, L.; Yu, X.; Pei, F. Cellular senescence in knee osteoarthritis: Molecular mechanisms and therapeutic implications. Ageing Res. Rev. 2021, 70, 101413. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qin, J.; Shi, H.; Li, Q.; Zhou, S.; Chen, L. Rhoifolin ameliorates osteoarthritis via the Nrf2/NF-κB axis: In vitro and in vivo experiments. Osteoarthr. Cartil. 2022, 30, 735–745. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ding, Q.; Chen, W.; Lin, C.; Ge, L.; Ying, C.; Xu, K.; Wu, Z.; Xu, L.; Ran, J.; et al. LONP1 downregulation with ageing contributes to osteoarthritis via mitochondrial dysfunction. Free Radic. Biol. Med. 2022, 191, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Ding, Z.; Wang, Y.; Zou, B.; Zheng, J.; Tan, Y.; Yang, Q.; Ke, M.; Chen, Y.; Wang, S.; et al. Dendrobine attenuates osteoclast differentiation through modulating ROS/NFATc1/ MMP9 pathway and prevents inflammatory bone destruction. Phytomedicine 2022, 96, 153838. [Google Scholar] [CrossRef]
- Lou, D.; Xing, X.; Liang, Y. Dendrobine modulates autophagy to alleviate ox-LDL-induced oxidative stress and senescence in HUVECs. Drug Dev. Res. 2022, 83, 1125–1137. [Google Scholar] [CrossRef]
- Nie, J.; Tian, Y.; Zhang, Y.; Lu, Y.L.; Li, L.S.; Shi, J.S. Dendrobium alkaloids prevent Aβ(25-35)-induced neuronal and synaptic loss via promoting neurotrophic factors expression in mice. PeerJ 2016, 4, e2739. [Google Scholar] [CrossRef] [Green Version]
- Yue, Q.; Chen, X.; Gao, J.; Gong, Q.; Shi, J.; Li, F. Dendrobine protects HACAT cells from H(2)O(2)-induced oxidative stress and apoptosis damage via Nrf2/Keap1/ARE signaling pathway. Toxicol. Appl. Pharmacol. 2022, 454, 116217. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.H.; Chung, C.P.; Wang, Y.Y.; Kuo, Y.H.; Yeh, C.H.; Lee, I.J.; Lin, Y.L. Dendrobium nobile protects retinal cells from UV-induced oxidative stress damage via Nrf2/HO-1 and MAPK pathways. J. Ethnopharmacol. 2022, 288, 114886. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, Z.; Chen, L.; Yang, T.; Liang, B.; Zhang, Z.; Shao, J.; Xu, X.; Yin, G.; Wang, S.; et al. Inhibition of ASCT2 induces hepatic stellate cell senescence with modified proinflammatory secretome through an IL-1α/NF-κB feedback pathway to inhibit liver fibrosis. Acta Pharm. Sin. B 2022, 12, 3618–3638. [Google Scholar] [CrossRef] [PubMed]
- Songkiatisak, P.; Rahman, S.M.T.; Aqdas, M.; Sung, M.H. NF-κB, a culprit of both inflamm-ageing and declining immunity? Immun. Ageing 2022, 19, 20. [Google Scholar] [CrossRef]
- Li, S.; Shi, Y.; Zhang, S.; Li, H.; Ye, Z.; Kong, J.; Hong, W.; Tu, Y.; Ren, J.; Meftah, Z.; et al. Corynoline Alleviates Osteoarthritis Development via the Nrf2/NF-κB Pathway. Oxidative Med. Cell. Longev. 2022, 2022, 2188145. [Google Scholar] [CrossRef]
- Su, W.; Liu, G.; Mohajer, B.; Wang, J.; Shen, A.; Zhang, W.; Liu, B.; Guermazi, A.; Gao, P.; Cao, X.; et al. Senescent preosteoclast secretome promotes metabolic syndrome associated osteoarthritis through cyclooxygenase 2. eLife 2022, 11, e79773. [Google Scholar] [CrossRef] [PubMed]
- Astrike-Davis, E.M.; Coryell, P.; Loeser, R.F. Targeting cellular senescence as a novel treatment for osteoarthritis. Curr. Opin. Pharmacol. 2022, 64, 102213. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.B.; Tuan, R.S. Anabolic/Catabolic balance in pathogenesis of osteoarthritis: Identifying molecular targets. PM&R 2011, 3 (Suppl. 1), S3–S11. [Google Scholar]
- Chen, Y.; Wu, Y.Y.; Si, H.B.; Lu, Y.R.; Shen, B. Mechanistic insights into AMPK-SIRT3 positive feedback loop-mediated chondrocyte mitochondrial quality control in osteoarthritis pathogenesis. Pharmacol. Res. 2021, 166, 105497. [Google Scholar] [CrossRef] [PubMed]
- Nakhle, J.; Rodriguez, A.M.; Vignais, M.L. Multifaceted Roles of Mitochondrial Components and Metabolites in Metabolic Diseases and Cancer. Int. J. Mol. Sci. 2020, 21, 4405. [Google Scholar] [CrossRef] [PubMed]
- Suomalainen, A.; Battersby, B.J. Mitochondrial diseases: The contribution of organelle stress responses to pathology. Nat. Rev. Mol. Cell Biol. 2018, 19, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- He, Y.; Li, Z.; Xu, T.; Luo, D.; Chi, Q.; Zhang, Y.; Li, S. Polystyrene nanoplastics deteriorate LPS-modulated duodenal permeability and inflammation in mice via ROS drived-NF-κB/NLRP3 pathway. Chemosphere 2022, 307 Pt 1, 135662. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Muriel, P. Molecular Mechanisms That Link Oxidative Stress, Inflammation, and Fibrosis in the Liver. Antioxidants 2020, 9, 1279. [Google Scholar] [CrossRef]
- Sun, X.; Ma, L.; Li, X.; Wang, J.; Li, Y.; Huang, Z. Ferulic acid alleviates retinal neovascularization by modulating microglia/macrophage polarization through the ROS/NF-κB axis. Front. Immunol. 2022, 13, 976729. [Google Scholar] [CrossRef]
- Arra, M.; Swarnkar, G.; Ke, K.; Otero, J.E.; Ying, J.; Duan, X.; Maruyama, T.; Rai, M.F.; O’Keefe, R.J.; Mbalaviele, G.; et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat. Commun. 2020, 11, 3427. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Emerging role of NF-κB signaling in the induction of senescence-associated secretory phenotype (SASP). Cell. Signal. 2012, 24, 835–845. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Ma, L.; Ding, Y.; He, L.; Chang, M.; Shan, Y.; Siwko, S.; Chen, G.; Liu, Y.; Jin, Y.; et al. Fatty acid sensing GPCR (GPR84) signaling safeguards cartilage homeostasis and protects against osteoarthritis. Pharmacol. Res. 2021, 164, 105406. [Google Scholar] [CrossRef] [PubMed]
- Fukui, T.; Yik, J.H.N.; Doyran, B.; Davis, J.; Haudenschild, A.K.; Adamopoulos, I.E.; Han, L.; Haudenschild, D.R. Bromodomain-containing-protein-4 and cyclin-dependent-kinase-9 inhibitors interact synergistically in vitro and combined treatment reduces post-traumatic osteoarthritis severity in mice. Osteoarthr. Cartil. 2021, 29, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.M.; Cohen, D.J.; Hays, M.; Nielson, D.W.; Grinstaff, M.W.; Lawson, T.B.; Snyder, B.D.; Boyan, B.D.; Schwartz, Z. Regulation of inflammatory and catabolic responses to IL-1β in rat articular chondrocytes by microRNAs miR-122 and miR-451. Osteoarthr. Cartil. 2021, 29, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Stoop, R.; Buma, P.; van der Kraan, P.M.; Hollander, A.P.; Billinghurst, R.C.; Meijers, T.H.; Poole, A.R.; van den Berg, W.B. Type II collagen degradation in articular cartilage fibrillation after anterior cruciate ligament transection in rats. Osteoarthr. Cartil. 2001, 9, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Gerwin, N.; Bendele, A.M.; Glasson, S.; Carlson, C.S. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the rat. Osteoarthr. Cartil. 2010, 18 (Suppl. 3), S24–S34. [Google Scholar] [CrossRef] [Green Version]



| Gene | Forward Primer | Reverse Primer | Annealing |
|---|---|---|---|
| Col2a1 | GAGTGGAAGAGCGGAGACTACTG | CTCCATGTTGCAGAAGACTTTCA | 60 °C |
| Aggrecan | CTAGCTGCTTAGCAGGGATAACG | TGACCCGCAGAGTCACAAAG | 58 °C |
| IL-6 | GCCAGAGTCATTCAGAGCAAT | CTTGGTCCTTAGCCACTCCT | 60 °C |
| TNF-α | ACCTTATCTACTCCCAGGTTCT | GGCTGACTTTCTCCTGGTATG | 60 °C |
| ADAMTS5 | GCAACAAAGTGGGACTACA | GAGAGAATGCATCCCTTAGC | 60 °C |
| MMP-13 | GCAGCTCCAAAGGCTACAACTT | GTAATGGCATCAAGGGATAGGG | 60 °C |
| GAPDH | GCAAGTTCAACGGCACAG | GCCAGTAGACTCCACGACA | 60 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Tu, M.; Liu, S.; Wen, Y.; Chen, L. Dendrobine Alleviates Cellular Senescence and Osteoarthritis via the ROS/NF-κB Axis. Int. J. Mol. Sci. 2023, 24, 2365. https://doi.org/10.3390/ijms24032365
Chen H, Tu M, Liu S, Wen Y, Chen L. Dendrobine Alleviates Cellular Senescence and Osteoarthritis via the ROS/NF-κB Axis. International Journal of Molecular Sciences. 2023; 24(3):2365. https://doi.org/10.3390/ijms24032365
Chicago/Turabian StyleChen, Haitao, Ming Tu, Siyi Liu, Yinxian Wen, and Liaobin Chen. 2023. "Dendrobine Alleviates Cellular Senescence and Osteoarthritis via the ROS/NF-κB Axis" International Journal of Molecular Sciences 24, no. 3: 2365. https://doi.org/10.3390/ijms24032365






