Antimicrobial Compounds in Food Packaging
Abstract
:1. Introduction
2. Food Packaging
2.1. Smart Packaging
2.2. Active Packaging
3. Antimicrobial Agents and Their Application in Food Industry
3.1. Metal-Based Nanoparticles as Antimicrobial Agents
3.1.1. Metal Nanoparticles
3.1.2. Metal Oxide Nanoparticles
3.2. Organic Acids
3.3. Antimicrobial Peptides and Bacteriocins
3.4. Natural Antimicrobial Agents of Plant Origin
3.5. Enzymes
3.6. Lactoferrin
3.7. Chitosan
3.8. Allyl Isothiocyanate
3.9. The Reuterin System (3-Hydroxypropionaldehyde/3-HPA/, 3-HPA Dimer, Acrolein, HPA Hydrate, and 3-Hydroxypropionic Acid)
3.10. Bacteriophages
4. Challenges and Possible Directions of Development in Food Packaging Industry
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.; Winters, C.; Zamboni, F.; Collins, M.N. Biomaterials: Antimicrobial surfaces in biomedical engineering and healthcare. Curr. Opin. Biomed. Eng. 2022, 22, 100373. [Google Scholar] [CrossRef]
- Agnihotri, S.; Dhiman, N.K. Development of nano-antimicrobial biomaterials for biomedical applications. In Advances in Biomaterials for Biomedical Applications; Tripathi, A., Melo, J.S., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 479–544. [Google Scholar]
- Alves, D.; Pereira, M.O. Mini-review: Antimicrobial peptides and enzymes as promising candidates to functionalize biomaterial surfaces. Biofouling 2014, 30, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Valero, A.; Rodríguez, M.-Y.; Posada-Izquierdo, G.D.; Pérez-Rodríguez, F.; Carrasco, E.; García-Gimeno, R.M. Risk Factors influencing microbial contamination in food service centers significance. In Prevention and Control of Food Related Diseases; Makun, H.A., Ed.; InTechOpen: London, UK, 2016; pp. 27–58. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main groups of microorganisms of relevance for food safety and stability: General aspects and overall description. In Innovative Technologies for Food Preservation: Inactivation of Spoilage and Pathogenic Microorganisms; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–107. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the united states—Major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Burden of Foodborne Diseases in the WHO European Region; Publications WHO Regional Office for Europe: Copenhagen, Denmark, 2017; pp. 1–26.
- Marsh, K.; Bugusu, A. Food packaging—Roles, materials, and environmental Issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel bio-based materials and applications in antimicrobial food packaging: Recent advances and future trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef]
- Agarwal, A.; Shaida, B.; Rastogi, M.; Singh, N.B. food packaging materials with special reference to biopolymers-properties and applications. Chem. Afr. 2022, 5, 1–28. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and challenges in the development of bio-based barrier coating materials for paper/cardboard food packaging; A review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Wang, J.; Euring, M.; Ostendorf, K.; Zhang, K. Biobased materials for food packaging. J. Bioresour. Bioprod. 2022, 7, 1–13. [Google Scholar] [CrossRef]
- Baghi, F.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S. Advancements in biodegradable active films for food packaging: Effects of nano/microcapsule incorporation. Foods 2022, 11, 760. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhayagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Suvarna, V.; Nair, A.; Mallya, R.; Khan, T.; Omri, A. Antimicrobial nanomaterials for food packaging. Antibiotics 2022, 11, 729. [Google Scholar] [CrossRef] [PubMed]
- Drago, E.; Campardelli, R.; Pettinato, M.; Perego, P. Innovations in smart packaging concepts for food: An extensive review. Foods 2020, 9, 1628. [Google Scholar] [CrossRef] [PubMed]
- Biji, K.B.; Ravishankar, C.N.; Mohan, C.O.; Srinivasa Gopal, T.K. Smart packaging systems for food applications: A review. J. Food Sci. Technol. 2015, 52, 6125–6135. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Brahma, S.; Mackay, J.; Cao, C.; Aliakbarian, B. The role of smart packaging system in food supply chain. J. Food Sci. 2020, 85, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röckerd, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, S.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of nanofillers on the functional properties of biopolymer-based films: A review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef] [Green Version]
- Guerrero Correa, M.; Martínez, F.B.; Vidal, C.P.; Streitt, C.; Escrig, J.; de Dicastillo, C.L.; Beilstein, J. Antimicrobial metal-based nanoparticles: A review on their synthesis, types and antimicrobial action. Beilstein J. Nanotechnol. 2020, 11, 1450–1469. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Ashkarran, A.A.; Jimenez de Aberasturi, D.; Ruiz de Larramendi, I.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [Green Version]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Franco, D.; Calabrese, G.; Guglielmino, S.P.P.; Conoci, S. Metal-Based Nanoparticles: Antibacterial mechanisms and biomedical application. Microorganisms 2022, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Ghahramani, Y.; Gholami, A.; Hemmateenejad, B.; Dorostkar, S.; Nabavizadeh, M.; Sharghi, H. The effect of charge at the surface of silver nanoparticles on antimicrobial activity against Gram-positive and Gram-negative bacteria: A preliminary study. J. Nanomater. 2015, 16, 55. [Google Scholar] [CrossRef] [Green Version]
- Carbone, M.; Donia, D.T.; Sabbatella, G.; Antiochia, R. Silver nanoparticles in polymeric matrices for fresh food packaging. J. King Saud Univ. Sci. 2016, 28, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Ahari, H.; Soufiani, S.P. Smart and active food packaging: Insights in novel food packaging. Front. Microbiol. 2021, 12, 657233. [Google Scholar] [CrossRef] [PubMed]
- Ahari, H.; Fakhrabadipour, M.; Paidari, S.; Goksen, G.; Xu, B. Role of AuNPs in active food packaging improvement: A review. Molecules 2022, 27, 8027. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial food packaging based on sustainable Bio-based materials for reducing foodborne Pathogens: A review. Food Chem. 2020, 310, 125915. [Google Scholar] [CrossRef]
- Sengan, M.; Subramaniyan, S.B.; Prakash, S.A.; Kamlekar, R.; Veerappan, A. Effective elimination of biofilm formed with waterborne pathogens using copper nanoparticles. Microb. Pathog. 2019, 127, 341–346. [Google Scholar] [CrossRef]
- Gowramma, B.; Keerthi, U.; Mokula Rafi, M.; Muralidhara Rao, D. Biogenic silver nanoparticles production and characterization from native stain of Corynebacterium species and its antimicrobial activity. 3 Biotech. 2015, 5, 195–201. [Google Scholar] [CrossRef] [Green Version]
- Hatipoğlu, A. Green synthesis of gold nanoparticles from Prunus cerasifera pissardii nigra leaf and their antimicrobial activities on some food pathogens. Prog. Nutr. 2021, 23, e2021241. [Google Scholar] [CrossRef]
- Qian, J.; Pan, C.; Liang, C. Antimicrobial activity of Fe-loaded chitosan nanoparticles. Eng. Life Sci. 2017, 17, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valipoor Motlagh, N.; Hamed Mosavian, M.T.; Mortazavi, S.A. Effect of polyethylene packaging modified with silver particles on the microbial, sensory and appearance of dried barberry. Packag. Technol. Sci. 2012, 26, 39–49. [Google Scholar] [CrossRef]
- Incoronato, A.L.; Conte, A.; Buonocore, G.G.; Del Nobile, M.A. Agar hydrogel with silver nanoparticles to prolong the shelf life of Fior di Latte cheese. J. Dairy Sci. 2011, 94, 1697–1704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastromatteo, M.; Conte, A.; Lucera, A.; Saccotelli, M.A.; Buonocore, G.G.; Zambrini, A.V.; Del Nobile, M.A. Packaging solutions to prolong the shelf life of Fior di Latte cheese: Bio-based nanocomposite coating and modified atmosphere packaging. LWT 2015, 60, 230–237. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abdel-Aziz, M.S. preparation of polystyrene nanocomposites based on silver nanoparticles using marine bacterium for packaging. Polym. Plast. Technol. Eng. 2013, 52, 607–613. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, S.; Jia, W.; Wang, F.; Wang, Z.; Cao, X.; Shen, X.; Yao, Z. Novel silver-modified carboxymethyl chitosan antibacterial membranes using environment-friendly polymers. Chemosphere 2022, 307, 136059. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, J.S.; Gonzaga, V.A.M.; Poli, A.L.; Schmitt, C.C. Photochemical synthesis of silver nanoparticles on chitosans/montmorillonite nanocomposite films and antibacterial activity. Carbohydr. Polym. 2017, 171, 202–210. [Google Scholar] [CrossRef]
- Youssef, A.M.; Abdel-Aziz, M.S.; El-Sayed, S.M. Chitosan nanocomposite films based on Ag-NP and Au-NP biosynthesis by Bacillus subtilis as packaging materials. Int. J. Biol. Macromol. 2014, 69, 185–191. [Google Scholar] [CrossRef]
- Chowdhury, S.; Teoh, Y.L.; Ong, K.M.; Rafflisman Zaidi, N.S.; Mah, S.-K. Poly(vinyl) alcohol crosslinked composite packaging film containing gold nanoparticles on shelf life extension of banana. Food Packag. Shelf Life 2020, 24, 100463. [Google Scholar] [CrossRef]
- Vera, P.; Canellas, E.; Nerín, C. New Antioxidant Multilayer Packaging with Nanoselenium to Enhance the Shelf-Life of Market Food Products. Nanomaterials 2018, 8, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Selenium nanoparticles–enhanced potato starch film for active food packaging application. Int. J. Food Sci. 2022, 57, 6512–6521. [Google Scholar] [CrossRef]
- Bhawna; Choudhary, A.K.; Gupta, A.; Kumar, S.; Kumar, P.; Singh, R.P.; Singh, P.; Kumar, V. Synthesis, antimicrobial activity, and photocatalytic performance of Ce doped SnO2 nanoparticles. Front. Nanotechnol. 2020, 2, 595352. [Google Scholar] [CrossRef]
- Amininezhad, S.M.; Rezvani, A.; Amouheidari, M.; Amininejad, S.M.; Rakhshani, S. The Antibacterial activity of SnO2 nanoparticles against Escherichia coli and Staphylococcus aureus. Zahedan J. Res. Med. Sci. 2015, 17, e1053. [Google Scholar] [CrossRef] [Green Version]
- Marra, A.; Silvestre, C.; Duraccio, D.; Cimmino, S. Polylactic acid/zinc oxide biocomposite films for food packaging application. Int. J. Biol. Macromol. 2016, 88, 254–262. [Google Scholar] [CrossRef]
- Khashan, K.S.; Sulaiman, G.M.; Hamad, A.H.; Abdulameer, F.A.; Hadi, A. Generation of NiO nanoparticles via pulsed laser ablation in eionized water and their antibacterial activity. Appl. Phys. A 2017, 123, 190. [Google Scholar] [CrossRef]
- Ardebilchi Marand, S.; Almasi, H.; Ardebilchi Marand, N. Chitosan-based nanocomposite films incorporated with NiO nanoparticles: Physicochemical, photocatalytic and antimicrobial properties. Int. J. Biol. Macromol. 2021, 190, 667–678. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Błaszczyk, U. The impact of nickel on human health. J. Elementol. 2008, 13, 685–696. [Google Scholar]
- Xing, Y.; Li, X.; Guo, X.; Li, W.; Chen, J.; Liu, Q.; Xu, Q.; Wang, Q.; Yang, H.; Shui, Y.; et al. Effects of different TiO2 nanoparticles concentrations on the physical and antibacterial activities of chitosan-based coating film. Nanomaterials 2020, 10, 1365. [Google Scholar] [CrossRef]
- Othman, S.H.; Salam, N.R.A.; Zainal, N.; Basha, R.K.; Talib, R.A. Antimicrobial activity of TiO2 nanoparticle-coated film for potential food packaging applications. Int. J. Photoenergy 2014, 2014, 945930. [Google Scholar] [CrossRef] [Green Version]
- Yemmireddy, V.K.; Farrell, G.D.; Hung, Y.-C. Development of titanium dioxide (TiO2) nanocoatings on food contact surfaces and method to evaluate their durability and photocatalytic bactericidal property. J. Food Sci. 2015, 80, N1903–N1911. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Partovi, R.; Talebi, F.; Babaei, A. Chitosan/TiO2 nanoparticle/Cymbopogon citratus essential oil film as food packaging material: Physico-mechanical properties and its effects on microbial, chemical, and organoleptic quality of minced meat during refrigeration. J. Food Process. Preserv. 2020, 44, e14536. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Morsy, M.K.; Khalaf, H.H.; Sharoba, A.M.; El-Tanahi, H.H.; Cutter, C.N. Incorporation of essential oils and nanoparticles in pullulan films to control foodborne pathogens on meat and poultry products. J. Food Sci. 2014, 79, M675–M684. [Google Scholar] [CrossRef]
- Zare, M.; Namratha, K.; Ilyas, S.; Hezam, A.; Mathur, S.; Byrappa, K. Smart fortified PHB-CS biopolymer with ZnO-Ag nanocomposites for enhanced shelf life of food packaging. ACS Appl. Mater. Interfaces 2019, 11, 48309–48320. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.I.; Alsafadi, D.; Alamry, K.A.; Oves, M.; Alosaimi, A.M.; Hussein, M.A. A promising antimicrobial bionanocomposite based poly(3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced silver doped zinc oxide nanoparticles. Nat. Portfolio 2022, 12, 14299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hortal, M.; Jordá-Beneyto, M.; Rosa, E.; Lara-Lledo, M.; Lorente, I. ZnO-PLA nanocomposite coated paper for antimicrobial packaging application. LWT 2017, 78, 250–257. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Antimicrobial packaging efficiency of ZnO-SiO2 nanocomposites infused into PVA/CS film for enhancing the shelf life of food products. Food Packag. Shelf Life 2020, 25, 100523. [Google Scholar] [CrossRef]
- Ojha, N.; Das, N. Fabrication and characterization of biodegradable PHBV/SiO2 nanocomposite for thermo-mechanical and antibacterial applications in food packaging. IET Nanobiotechnol. 2020, 14, 785–795. [Google Scholar] [CrossRef]
- Wang, Y.; Cen, C.; Chen, J.; Fu, L. MgO/Carboxymethyl chitosan nanocomposite improves thermal stability, waterproof and antibacterial performance for food packaging. Carbohydr. Polym. 2020, 236, 116078. [Google Scholar] [CrossRef]
- Swaroop, C.; Shukla, M. Nano-magnesium oxide reinforced polylactic acid biofilms for food packaging applications. Int. J. Biol. Macromol. 2018, 113, 729–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valerini, D.; Tammaro, L.; Di Benedetto, F.; Vigliotta, G.; Capodieci, L.; Terzi, R.; Rizzo, A. Aluminum-doped zinc oxide coatings on polylactic acid films for antimicrobial food packaging. Thin Solid Film. 2018, 645, 187–192. [Google Scholar] [CrossRef]
- Bala, T.; Armstrong, G.; Laffir, F.; Thornton, R. Titania–silver and alumina–silver composite nanoparticles: Novel, versatile synthesis, reaction mechanism and potential antimicrobial application. J. Colloid Interface Sci. 2011, 356, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Russel, J.B. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Bacteriol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Salmond, C.V.; Kroll, R.G.; Booth, I.R. The Effect of food preservatives on pH homeostasis in Escherichia coli. Microbiology 1984, 130, 2845–2850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Fernández, E.N.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding how microorganisms respond to acid ph is central to their control and successful exploitation. Front. Microbiol. 2020, 24, 11. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef] [Green Version]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morey, A.; Bowers, J.W.J.; Bauermeister, L.J.; Singh, M.; Huang, T.-S.; McKee, S.R. Effect of Salts of Organic Acids on Listeria monocytogenes, Shelf Life, Meat Quality, and Consumer Acceptability of Beef Frankfurters. J. Food Sci. 2014, 79, M54–M60. [Google Scholar] [CrossRef]
- Sommers, C.H.; Cooke, P.H.; Fan, X.; Sites, J.E. Ultraviolet Light (254 nm) Inactivation of Listeria monocytogeneson Frankfurters That Contain Potassium Lactate and Sodium Diacetate. J. Food Sci. 2009, 74, M114–M119. [Google Scholar] [CrossRef]
- Sallam, K.I. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilinc, B.; Cakli, S.; Dincer, T.; Tolasa, S. Microbiological, chemical, sensory, color, and textural changes of rainbow trout fillets treated with sodium acetate, sodium lactate, sodium citrate, and stored at 4 °C. J. Aquat. Food Prod. Technol. 2009, 18, 3–17. [Google Scholar] [CrossRef]
- EFSA. Safety of the extension of use of sodium propionate (E 281) as a food additive. EFSA J. 2016, 14, 4546. [Google Scholar] [CrossRef]
- Adler, G.K.; Hornik, E.S.; Murray, G.; Bhandari, S.; Yadav, Y.; Heydarpour, M.; Basu, R.; Garg, R.; Tirosh, A. Acute effects of the food preservative propionic acid on glucose metabolism in humans. BMJ Open Diabetes Res. Care 2021, 9, e002336. [Google Scholar] [CrossRef]
- Wind, C.E.; Restain, L. Antimicrobial effectiveness of potassium sorbate and sodium benzoate against Zygosaccharomyces bailii in a salsa mayonnaise. J. Food Prot. 1995, 58, 1257–1259. [Google Scholar] [CrossRef]
- Hu, S.; Yu, J.; Wang, Z.; Li, L.; Du, Y.; Wang, L.; Liu, Y. Effects of sorbic acid-chitosan microcapsules as antimicrobial agent on the properties of ethylene vinyl alcohol copolymer film for food packaging. J. Food Sci. 2017, 82, 1451–1460. [Google Scholar] [CrossRef]
- Stanojevic, D.; Comic, L.; Stefanovic, O.; Solujic-Sukdolak, S. Antimicrobial effects of sodium benzoate, sodium nitrite and potassium sorbate and their synergistic action in vitro. Bulg. J. Agric. Sci. 2009, 15, 307–311. [Google Scholar]
- Turantas, F.; Goksungur, Y.; Dincer, A.H.; Unluturk, A.; Guvenc, U.; Zorlu, N. Effect of potassium sorbate and sodium benzoate on microbial population and fermentation of black olives. J. Sci. Food Agric. 1999, 79, 1197–1202. [Google Scholar] [CrossRef]
- López, O.V.; Giannuzzi, L.; Zaritzky, N.E.; García, M.A. Potassium sorbate controlled release from corn starch films. Mater. Sci. Eng. 2013, 33, 1583–1591. [Google Scholar] [CrossRef]
- Fan, F.; Lu, N.; Pu, S.; Yang, F. Preparation and properties of antibacterial sodium dehydroacetate/modified film. Food Sci. Technol. 2022, 42, e97521. [Google Scholar] [CrossRef]
- Hoffman, K.L.; Han, I.Y.; Dawson, P.L. Antimicrobial effects of corn zein films impregnated with nisin, lauric acid, and EDTA. J. Food Prot. 2001, 64, 885–889. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.L.; Carl, G.D.; Acton, J.C.; Han, I.Y. Effect of lauric acid and nisin-impregnated soy-based films on the growth of Listeria monocytogenes on Turkey Bologna. Poult. Sci. 2002, 81, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Rydlo, T.; Miltz, J.; Mor, A. eukaryotic antimicrobial peptides: Promises and premises in food safety. J. Food Sci. 2006, 71, R125–R135. [Google Scholar] [CrossRef]
- Santos, J.C.P.; Sousa, R.C.S.; Otoni, C.G.; Moraes, A.R.F.; Souza, W.G.L.; Medeiros, E.A.A.; Espitia, P.J.P.; Pires, A.C.S.; Coimbra, J.S.R.; Soares, N.F.F. Nisin and other antimicrobial peptides: Production, mechanisms of action, and application in active food packaging. Innov. Food Sci. Emerg. Technol. 2018, 48, 179–194. [Google Scholar] [CrossRef]
- Liu, Y.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W. Antimicrobial peptides and their application in food packaging. Trends Food Sci. Technol. 2021, 112, 471–483. [Google Scholar] [CrossRef]
- Kościuczuk, E.M.; Lisowski, P.; Jarczak, J.; Strzałkowska, N.; Jóźwik, A.; Horbańczuk, J.; Krzyżewski, J.; Zwierzchowski, L.; Bagnicka, E. Cathelicidins: Family of antimicrobial peptides. A review. Mol. Biol. Rep. 2012, 39, 10957–10970. [Google Scholar] [CrossRef] [Green Version]
- Humblot, V.; Yala, J.-F.; Thebault, P.; Boukerma, K.; Hequet, A.; Berjeaud, J.-M.; Pradier, C.-M. The antibacterial activity of magainin I immobilized onto mixed thiols self-assembled monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with dual antimicrobial and anticancer activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Espitia, P.J.P.; Soares, N.F.F.; Coimbra, J.S.R.; de Andrade, N.J.; Cruz, R.S.; Medeiros, E.A.A. Bioactive peptides: Synthesis, properties, and applications in the packaging and preservation of food. Innov. Food Sci. Emerg. Technol. 2012, 11, 187–204. [Google Scholar]
- Jin, T.; Zhang, H. Biodegradable polylactic acid polymer with nisin for use in antimicrobial food packaging. J. Food Sci. 2008, 73, M127–M134. [Google Scholar] [CrossRef]
- Jin, T. Inactivation of Listeria monocytogenes in skim milk and liquid egg white by antimicrobial bottle coating with polylactic acid and nisin. J. Food Sci. 2010, 75, M83–M88. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, H.; Boyd, A. Incorporation of preservatives in polylactic acid films for inactivating Escherichia coli O157:H7 and extending microbiological shelf life of strawberry puree. J. Food Prot. 2010, 73, 812–818. [Google Scholar] [CrossRef]
- Meira, S.M.M.; Zehetmeyer, G.; Scheibel, J.M.; Werner, J.O.; Brandelli, A. Starch-halloysite nanocomposites containing nisin: Characterization and inhibition of Listeria monocytogenes in soft cheese. LWT 2016, 68, 226–234. [Google Scholar] [CrossRef]
- Barbosa, A.A.T.; de Araujo, H.G.S.; Matos, P.N.; Carnelossi, M.A.G.; de Castro, A.A. Effects of nisin-incorporated films on the microbiological and physicochemical quality of minimally processed mangoes. Int. J. Food Microbiol. 2013, 164, 135–140. [Google Scholar] [CrossRef]
- Pires, A.C.S.; Soares, N.F.F.; de Andrade, N.J.; da Silva, L.H.M.; Camilloto, G.P.; Bernardes, P.C. Development and evaluation of active packaging for sliced mozzarella preservation. Packag. Technol. Sci. 2008, 21, 375–383. [Google Scholar] [CrossRef]
- Neetoo, H.; Ye, M.; Chen, H.; Joerger, R.D.; Hicks, D.T.; Hoover, D.G. Use of nisin-coated plastic films to control Listeria monocytogenes on vacuum-packaged cold-smoked salmon. Int. J. Food Microbiol. 2008, 122, 8–15. [Google Scholar] [CrossRef]
- Ko, S.; Janes, M.E.; Hettiarachchy, N.S.; Johnson, M.G. Physical and chemical properties of edible films containing nisin and their action against Listeria monocytogenes. J. Food Sci. 2001, 66, 1006–1011. [Google Scholar] [CrossRef]
- Song, Z.; Li, F.; Guan, H.; Xu, Y.; Fu, Q.; Li, D. Combination of nisin and ε-polylysine with chitosan coating inhibits the white blush of fresh-cut carrots. Food Control. 2017, 74, 34–44. [Google Scholar] [CrossRef]
- Janes, M.E.; Kooshesh, S.; Johnson, M.G. Control of Listeria monocytogenes on the surface of refrigerated, ready-to-eat chicken coated with edible zein film coatings containing nisin and/or calcium propionate. J. Food Sci. 2002, 67, 2754–2757. [Google Scholar] [CrossRef]
- Malheiros, P.S.; Sant’Anna, V.; Barbosa, M.S.; Brandelli, A.; Franco, B.D.G.M. Effect of liposome-encapsulated nisin and bacteriocin-like substance P34 on Listeria monocytogenes growth in Minas frescal cheese. Int. J. Food Microbiol. 2012, 156, 272–277. [Google Scholar] [CrossRef]
- Theivendran, S.; Hettiarachchy, N.S.; Johnson, M.G. Inhibition of Listeria monocytogenes by nisin combined with grape seed extract or green tea extract in soy protein film coated on turkey frankfurters. J. Food Sci. 2006, 71, M39–M44. [Google Scholar] [CrossRef]
- Lee, N.-K.; Han, E.J.; Han, K.J.; Paik, H.-D. Antimicrobial effect of Bacteriocin KU24 produced by Lactococcus lactis KU24 against Methicillin-Resistant Staphylococcus aureus. J. Food. Sci. 2013, 78, M465–M469. [Google Scholar] [CrossRef]
- Iseppi, A.; Pilati, F.; Marini, M.; Toselli, M.; de Niederhäusern, S.; Guerrieri, E.; Messi, P.; Sabia, C.; Manicardi, G.; Anacarso, I.; et al. Anti-listerial activity of a polymeric film coated with hybrid coatings doped with Enterocin 416K1 for use as bioactive food packaging. Int. J. Food Microbiol. 2008, 123, 281–287. [Google Scholar] [CrossRef]
- Marcos, B.; Aymerich, T.; Monfort, J.M.; Garriga, M. High-pressure processing and antimicrobial biodegradable packaging to control Listeria monocytogenes during storage of cooked ham. Food Microbiol. 2008, 25, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Aspri, M.; O’Connor, P.M.; Field, D.; Cotter, P.D.; Ross, P.; Hill, C.; Papademas, P. Application of bacteriocin-producing Enterococcus faecium isolated from donkey milk, in the bio-control of Listeria monocytogenes in fresh whey cheese. Int. Dairy J. 2017, 73, 1–9. [Google Scholar] [CrossRef]
- Blanco Massani, M.; Fernandez, M.R.; Ariosti, A.; Eisenberg, P.; Vignolo, G. Development and characterization of an active polyethylene film containing Lactobacillus curvatus CRL705 bacteriocins. Food Addit. Contam. Part A. 2008, 25, 1424–1430. [Google Scholar] [CrossRef]
- Scannell, A.G.; Hill, C.; Ross, R.; Marx, S.; Hartmeier, W.; Arendt, E.K. Development of bioactive food packaging materials using immobilised bacteriocins Lacticin 3147 and Nisaplin®. Int. J. Food Microbiol. 2000, 60, 241–249. [Google Scholar] [CrossRef]
- Santiago-Silva, P.; Soares, N.F.F.; Nóbrega, J.E.; Júnior, M.A.W.; Barbosa, K.B.F.; Volp, A.C.P.; Zerdas, E.R.M.A.; Würlitzer, N.J. Antimicrobial efficiency of film incorporated with pediocin (ALTA® 2351) on preservation of sliced ham. Food Control 2009, 20, 85–89. [Google Scholar] [CrossRef]
- Yang, W.; Xie, Y.; Jin, J.; Liu, H.; Zhang, H. Development and application of an active plastic multilayer film by coating a plantaricin bm-1 for chilled meat preservation. J. Food Sci. 2019, 84, 1864–1870. [Google Scholar] [CrossRef] [Green Version]
- Guyomard, A.; Dé, E.; Jouenne, T.; Malandain, J.-J.; Muller, G.; Glinel, K. Incorporation of a hydrophobic antibacterial peptide into amphiphilic polyelectrolyte multilayers: A bioinspired approach to prepare biocidal thin coatings. Adv. Funct. Mater. 2008, 18, 758–765. [Google Scholar] [CrossRef]
- Gogliettino, M.; Balestrieri, M.; Ambrosio, R.L.; Anastasio, A.; Smaldone, G.; Proroga, Y.T.R.; Moretta, R.; Rea, I.; De Stefano, L.; Agrillo, B.; et al. Extending the shelf-life of meatand dairy products via pet-modified packaging activated with the antimicrobial peptide MTP1. Front. Microbiol. 2020, 10, 2963. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Edge, R.; Truscott, T.G. Singlet Oxygen and Free Radical Reactions of Retinoids and Carotenoids—A Review. Antioxidants 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Al-Sehemi, A.G.; Irfan, A. Effect of donor and acceptor groups on radical scavenging activity of phenol by density functional theory. Arab. J. Chem. 2017, 10, S1703–S1710. [Google Scholar] [CrossRef] [Green Version]
- Thbayh, D.K.; Reizer, E.; Kahaly, M.U.; Viskolcz, B.; Fiser, B. Antioxidant Potential of Santowhite as Synthetic and Ascorbic Acid as Natural Polymer Additives. Polymers 2022, 14, 3518. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RCS Adv. 2015, 35, 27986. [Google Scholar] [CrossRef] [Green Version]
- Edge, R.; Truscott, T.G. The Reactive Oxygen Species Singlet Oxygen, Hydroxy Radicals, and the Superoxide Radical Anion—Examples of Their Roles in Biology and Medicine. Oxygen 2021, 1, 77–95. [Google Scholar] [CrossRef]
- Ramel, F.; Birtic, S.; Cuiné, S.; Triantaphylidès, C.; Ravanat, J.-C.; Havaux, M. Chemical quenching of singlet oxygen by carotenoids in plants. Plant Physiol. 2012, 158, 1267–1278. [Google Scholar] [CrossRef] [Green Version]
- Eddaikra, A.; Eddaikra, N. Endogenous enzymatic antioxidant defense and pathologies. In Antioxidants: Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; InTech: Rang-Du-Fliers, France, 2021. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Wadhwani, A. Antioxidant Enzymes and Human Health. In Antioxidant Enzyme; El-Missiry, M.A., Ed.; InTech: Rang-Du-Fliers, France, 2012. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, F.E. Vitamin C: An Antioxidant Agent. In Vitamin C; Hamza, A.H., Ed.; InTech: Rang-Du-Fliers, France, 2017. [Google Scholar] [CrossRef] [Green Version]
- Akbari, A.; Jelodar, G.; Nazifi, S.; Sajedianfard, J. An overview of the characteristics and function of vitamin C in various tissues: Relying on its antioxidant function. Zahedan J. Res. Med. Sci. 2016, 18, e4037. [Google Scholar] [CrossRef] [Green Version]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and Cardiovascular Disease: An Update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Lee, M.H.; Kwon, H.A.; Kwon, D.Y.; Park, H.; Sohn, D.H.; Kim, Y.C.; Eo, S.K.; Kang, H.Y.; Kim, S.W.; Lee, J.H. Antibacterial activity of medicinal herb extracts against Salmonella. Int. J. Food Microbiol. 2006, 111, 270–275. [Google Scholar] [CrossRef]
- Yano, Y.; Satomi, M.; Oikawa, H. Antimicrobial effect of spices and herbs on Vibrio parahaemolyticus. Int. J. Food Microbiol. 2006, 111, 6–11. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.Z.; Brooks, J.D.; Corke, H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int. J. Food Microbiol. 2007, 117, 112–119. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial herb and spice compounds in food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Weerakkody, N.S.; Caffin, N.; Turner, M.S.; Dykes, G.A. In vitro antimicrobial activity of less-utilized spice and herb extracts against selected food-borne bacteria. Food Control 2010, 21, 1408–1414. [Google Scholar] [CrossRef]
- Gyawali, R.; Ibrahim, S.A. Natural products as antimicrobial agents. Food Control 2014, 46, 412–429. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The antimicrobial efficacy of plant essential oil combinations and interactions with food ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponce, A.G.; Roura, S.I.; del Valle, C.E.; Moreira, M.R. Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: In vitro and in vivo studies. Postharvest Biol. Technol. 2008, 49, 294–300. [Google Scholar] [CrossRef]
- Radha Krishnan, K.; Babuskin, S.; Azhagu Saravana Babu, P.; Sasikala, M.; Sabina, K.; Archana, G.; Sivarajan, M.; Sukumar, M. Antimicrobial and antioxidant effects of spice extracts on the shelf life extension of raw chicken meat. Int. J. Food Microbiol. 2014, 171, 32–40. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Kapsokefalou, M.; Komaitis, M. Natural antioxidant constituents from selected aromatic plants and their antimicrobial activity against selected pathogenic microorganisms. Food Technol. Biotechnol. 2008, 46, 151–156. [Google Scholar]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Combining eugenol and cinnamaldehyde to control the growth of Alicyclobacillus acidoterrestris. Food Control 2010, 21, 172–177. [Google Scholar] [CrossRef]
- Soylu, E.M.; Kurt, S.; Soylu, S. In vitro and in vivo antifungal activities of the essential oils of various plants against tomato grey mould disease agent Botrytis cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef]
- Hzounda Fokou, J.B.; Jazet Dongmo, P.M.; Fekam Boyom, F. Essential Oil’s Chemical Composition and Pharmacological Properties. In Essential Oils—Oils of Nature; El-Shemy, H.A., Ed.; InTech: Rang-Du-Fliers, France, 2020. [Google Scholar] [CrossRef] [Green Version]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The interactions between polyphenols and microorganisms, especially gut microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Fernandez, M.T.; Mira, M.L.; Florêncio, M.H.; Jennings, K.R. Iron and copper chelation by flavonoids: An electrospray mass spectrometry study. J. Inorg. Biochem. 2002, 92, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signaling molecules? Free Rad. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Galleano, M.; Verstraeten, S.V.; Oteiza, P.I.; Fraga, C.G. Antioxidant actions of flavonoids: Thermodynamic and kinetic analysis. Arch. Biochem. Biophys. 2010, 501, 23–30. [Google Scholar] [CrossRef]
- Amezouar, F.; Badri, W.; Hsaine, M.; Bourhim, N.; Fougrach, H. Chemical composition, antioxidant and antibacterial activities of leaves essential oil and ethanolic extract of Moroccan Warionia saharae Benth. & Coss. J. Appl. Pharm. Sci. 2012, 2, 212–217. [Google Scholar] [CrossRef]
- Teke, G.N.; Elisée, K.N.; Roger, K.J. Chemical composition, antimicrobial properties and toxicity evaluation of the essential oil of Cupressus lusitanica Mill. leaves from Cameroon. BMC Complement. Altern. Med. 2013, 13, 130. [Google Scholar] [CrossRef] [Green Version]
- Martins, M.R.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, antimicrobial and toxicological properties of Schinus molle L. essential oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. Int. J. Food Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
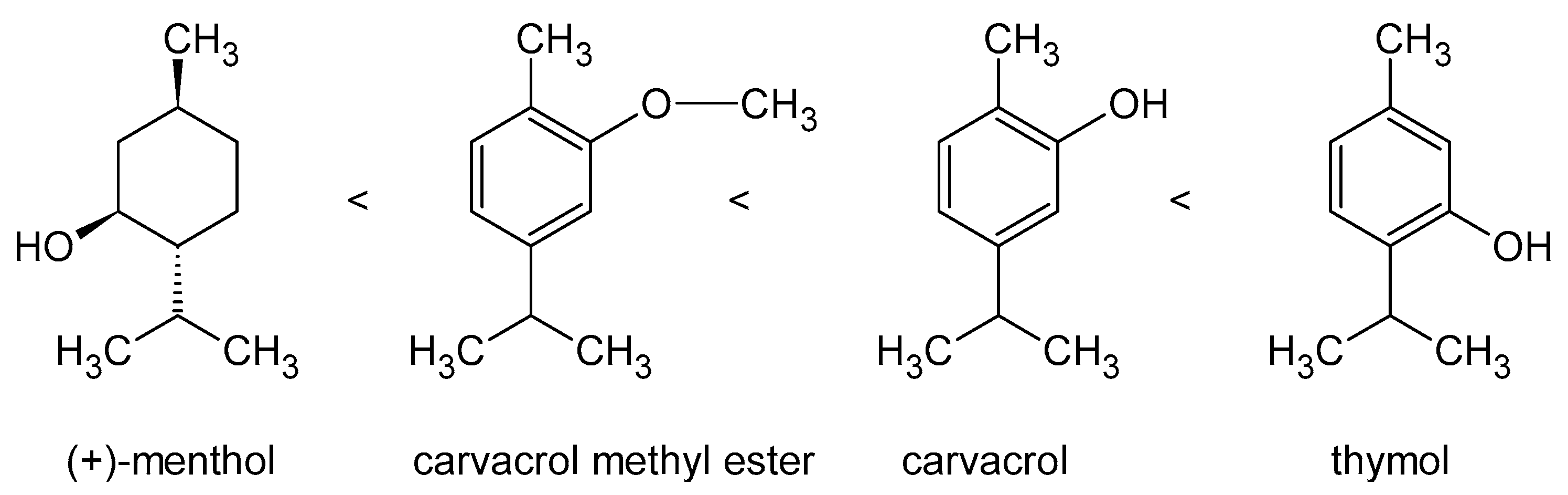
- Ultee, A.; Bennik, M.H.J.; Moezelaar, R. The phenolic hydroxyl group of carvacrol is essential for action against the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 2002, 68, 1561–1568. [Google Scholar] [CrossRef] [Green Version]
- Cermak, P.; Olsovska, J.; Mikyska, A.; Dusek, M.; Kadleckova, Z.; Vanicek, J.; Nyc, O.; Sigler, K.; Bostikova, V.; Bostik, P. Strong antimicrobial activity of xanthohumol and other derivatives from hops (Humulus lupulus L.) on gut anaerobic bacteria. APMIS 2017, 125, 1033–1038. [Google Scholar] [CrossRef]
- Stompor, M.; Żarowska, B. Antimicrobial activity of xanthohumol and its selected structural analogues. Molecules 2016, 21, 608. [Google Scholar] [CrossRef]
- Campos, F.M.; Couto, J.A.; Figueiredo, A.R.; Tóth, I.V.; Rangel, A.O.S.S.; Hogg, T.A. Cell membrane damage induced by phenolic acids on wine lactic acid bacteria. Int. J. Food Microbiol. 2009, 135, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Stojković, D.; Petrović, J.; Soković, M.; Glamočlija, J.; Kukić-Marković, J.; Petrović, S. In situ antioxidant and antimicrobial activities of naturally occurring caffeic acid, p-coumaric acid and rutin, using food systems. J. Sci. Food Agric. 2013, 93, 3205–3208. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. Available online: https://pubmed.ncbi.nlm.nih.gov/23211303 (accessed on 1 December 2022). [PubMed]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A systematic review of plants with antibacterial activities: A taxonomic and phylogenetic perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef]
- Yang, C.; Han, B.; Zheng, Y.; Liu, L.; Li, C.; Sheng, S.; Zhang, J.; Wang, J.; Wu, F. The Quality changes of postharvest mulberry fruit treated by chitosan-g-caffeic acid during cold storage. J. Food Sci. 2016, 81, c881–c888. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yang, H. Postharvest chitosan-g-salicylic acid application alleviates chilling injury and preserves cucumber fruit quality during cold storage. Food Chem. 2015, 174, 558–563. [Google Scholar] [CrossRef]
- Hoa, V.-B.; Song, D.-H.; Seol, K.-H.; Kang, S.-M.; Kim, H.-W.; Kim, J.-H.; Cho, S.-H. Coating with chitosan containing lauric acid (C12:0) significantly extends the shelf-life of aerobically—Packaged beef steaks during refrigerated storage. Meat Sci. 2022, 184, 108696. [Google Scholar] [CrossRef]
- Saucedo-Pompa, S.; Rojas-Molina, R.; Aguilera-Carbó, A.F.; Saenz-Galindo, A.; de La Garza, H.; Jasso-Cantú, D.; Aguilar, C.N. Edible film based on candelilla wax to improve the shelf life and quality of avocado. Food Res. Int. 2009, 42, 511–515. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Liu, Y.; Yong, H.; Zong, S.; Jin, C.; Liu, J. Effect of ferulic acid-grafted-chitosan coating on the quality of pork during refrigerated storage. Foods 2021, 10, 1374. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Zhang, X.; Kan, J.; Jin, C. Effect of gallic acid grafted chitosan film packaging on the postharvest quality of white button mushroom (Agaricus bisporus). Postharvest Biol. Technol. 2019, 147, 39–47. [Google Scholar] [CrossRef]
- Braga, L.R.; Pérezb, L.M.; del, V. Soazo, M.; Machado, F. Evaluation of the antimicrobial, antioxidant and physicochemical properties of Poly(Vinyl chloride) films containing quercetin and silver nanoparticles. LWT 2019, 101, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Malvano, F.; Montone, A.M.I.; Capuano, F.; Coletti, C.; Roveri, N.; Albanese, D.; Capparelli, R. Effects of active alginate edible coating enriched with hydroxyapatite-quercetin complexes during the cold storage of fresh chicken fillets. Food Packag. Shelf Life 2022, 32, 100847. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of bioactive functional poly(lactic acid)/curcumin composite film for food packaging application. Int. J. Biol. Macromol. 2020, 162, 1780–1789. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Curcumin incorporated poly(butyleneadipate-co-terephthalate) film with improved water vapor barrier and antioxidant properties. Materials 2020, 13, 4369. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Carboxymethyl cellulose-based antioxidant and antimicrobial active packaging film incorporated with curcumin and zinc oxide. Int. J. Biol. Macromol. 2020, 148, 666–676. [Google Scholar] [CrossRef]
- Subbuvel, M.; Kavan, P. Preparation and characterization of polylactic acid/fenugreek essential oil/curcumin composite films for food packaging applications. Int. J. Biol. Macromol. 2022, 194, 470–483. [Google Scholar] [CrossRef]
- Muller, J.; Quesada, A.C.; González-Martínez, C.; Chiralt, A. Antimicrobial properties and release of cinnamaldehyde in bilayer films based on polylactic acid (PLA) and starch. Eur. Polym. J. 2017, 73, 316–325. [Google Scholar] [CrossRef]
- Silva, A.; Duarte, A.; Sousa, S.; Ramos, A.; Domingues, F.C. Characterization and antimicrobial activity of cellulose derivatives films incorporated with a resveratrol inclusion complex. LWT 2016, 73, 481–489. [Google Scholar] [CrossRef]
- Aminzare, M.; Moniri, R.; Azar, H.H.; Mehrasbi, M.R. Evaluation of antioxidant and antibacterial interactions between resveratrol and eugenol in carboxymethyl cellulose biodegradable film. Food Sci. Nutr. 2022, 10, 155–168. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, S.J.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Ramos, M.; Fortunati, E.; Beltrán, A.; Peltzer, M.; Cristofaro, F.; Visai, L.; Valente, A.J.M.; Jiménez, A.; Kenny, J.M.; Garrigós, M.C. Controlled release, disintegration, antioxidant, and antimicrobial properties of poly (lactic acid)/thymol/nanoclay composites. Polymers 2020, 12, 1878. [Google Scholar] [CrossRef] [PubMed]
- Pleva, P.; Bartošová, L.; Mácalová, D.; Zálešáková, L.; Sedlaríková, J.; Janalíková, M. biofilm formation reduction by eugenol and thymol on biodegradable food packaging material. Foods 2022, 11, 2. [Google Scholar] [CrossRef]
- Del Toro-Sanchez, C.L.; Ayala-Zavala, J.F.; Machi, L.; Santacruz, H.; Villegas-Ochoa, M.A.; Alvarez-Parrilla, E.; Gonzalez-Aguilar, G.A. Controlled release of antifungal volatiles of thyme essential oil from β-cyclodextrin capsules. J. Incl. Phenom. Macrocycl. Chem. 2010, 67, 431–441. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Pérez-Pérez, J.C.; Varillas-Torres, J.M.; Navarro-Cruz, A.R.; Hernández-Carranza, P.; Munguía-Pérez, R.; Cid-Pérez, T.S.; Avila-Sosa, R. Starch edible films/coatings added with carvacrol and thymol: In vitro and in vivo evaluation against Colletotrichum gloeosporioides. Foods 2021, 10, 175. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.S.; Ali, E.F.; Mostafa, N.Y.; Mazrou, R. Shelf-life extension of sweet basil leaves by edible coating with thyme volatile oil encapsulated chitosan nanoparticles. Int. J. Biol. Macromol. 2021, 177, 517–525. [Google Scholar] [CrossRef]
- Ardjoum, N.; Chibani, N.; Shankar, S.; Fadhel, Y.B.; Djidjelli, H.; Lacroix, M. Development of antimicrobial films based on poly(lactic acid) incorporated with Thymus vulgaris essential oil and ethanolic extract of Mediterranean propolis. Int. J. Biol. Macromol. 2021, 185, 535–542. [Google Scholar] [CrossRef]
- Hou, C.-Y.; Hazeena, S.H.; Hsieh, S.-L.; Li, B.-H.; Chen, M.-H.; Wang, P.-Y.; Zheng, B.-Q.; Liang, Y.-S. Effect of D-limonene nanoemulsion edible film on banana (Musa sapientum Linn.) Post-Harvest Preservation. Molecules 2022, 27, 6157. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Ding, D.; Shao, H.; Peng, Q.; Huang, Y. Antibacterial activity and physical properties of fish gelatin-chitosan edible films supplemented with D-limonene. Int. J. Polym. Sci. 2017, 1837171. [Google Scholar] [CrossRef] [Green Version]
- Joshi, P.; Becerra-Mora, N.; Vargas-Lizarazo, A.Y.; Kohlo, P.; Fisher, D.J.; Choudhary, R. Use of edible alginate and limonene-liposome coatings for shelf-life improvement of blackberries. Future Foods 2021, 4, 100091. [Google Scholar] [CrossRef]
- Sedlarikova, J.; Janalikova, M.; Peer, P.; Pavlatkova, L.; Minarik, A.; Pleva, P. Zein-based films containing monolaurin/eugenol or essential oils with potential for bioactive packaging application. Int. J. Mol. Sci. 2022, 23, 384. [Google Scholar] [CrossRef] [PubMed]
- Zinoviadou, K.G.; Koutsoumanis, K.P.; Biliaderis, C.G. Physico-chemical properties of whey protein isolate films containing oregano oil and their antimicrobial action against spoilage flora of fresh beef. Meat Sci. 2009, 82, 338–345. [Google Scholar] [CrossRef]
- Langroodi, M.A.; Nematollahi, A.; Sayadi, M. Chitosan coating incorporated with grape seed extract and Origanum vulgare essential oil: An active packaging for turkey meat preservation. J. Food Meas. Charact. 2021, 15, 2790–2804. [Google Scholar] [CrossRef]
- Sun, X.; Cameron, R.G.; Plotto, A.; Zhong, T.; Ference, C.M.; Bai, J. The Effect of controlled-release carvacrol on safety and quality of blueberries stored in perforated packaging. Foods 2021, 10, 1487. [Google Scholar] [CrossRef]
- Santos, A.R.; da Silva, A.F.; Amaral, V.C.; Ribeiro, A.B.; de Abreu Filho, B.A.; Mikcha, J.M. Application of edible coating with starch and carvacrol in minimally processed pumpkin. J. Food Sci. Technol. 2016, 53, 1975–1983. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Wagle, B.R.; Upadhyay, A.; Arsi, K.; Upadhyaya, I.; Donoghue, D.J.; Donoghue, A.M. Edible Coatings Fortified With Carvacrol Reduce Campylobacter jejuni on Chicken Wingettes and Modulate Expression of Select Virulence Genes. Front. Microbiol. 2019, 10, 583. [Google Scholar] [CrossRef]
- Lu, W.; Cui, R.; Zhu, B.; Qin, Y.; Cheng, G.; Li, L.; Yuan, M. Influence of clove essential oil immobilized in mesoporous silica nanoparticles on the functional properties of poly(lactic acid) biocomposite food packaging film. J. Mater. Res. Technol. 2021, 11, 1152–1161. [Google Scholar] [CrossRef]
- Lieu, M.D.; Ngo, N.N.H.; Lieu, T.L.; Nguyen, K.T.; Dang, T.K.T. The Efficacy of combined application of edible coatings and essential oil in mango preservation. Vietnam J. Sci. Technol. 2018, 56, 458–467. [Google Scholar] [CrossRef] [Green Version]
- Alarcón-Moyano, J.K.; Bustos, R.O.; Herrera, M.L.; Matiacevich, S.B. Alginate edible films containing microencapsulated lemongrass oil or citral: Effect of encapsulating agent and storage time on physical and antimicrobial properties. J. Food Sci. Technol. 2015, 54, 2878–2889. [Google Scholar] [CrossRef]
- Šumiga, B.; Šumiga, B.; Ravnjak, D.; Podgornik, B.B. Antimicrobial paper coatings containing microencapsulated Cymbopogon citrates oil. Coatings 2019, 9, 470. [Google Scholar] [CrossRef] [Green Version]
- Bustos, C.R.O.; Alberti, R.F.V.; Matiacevich, S.B. Edible antimicrobial films based on microencapsulated lemongrass oil. J. Food Sci. Technol. 2015, 53, 832–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motelica, L.; Ficai, D.; Ficai, A.; Truscă, R.-D.; Ilie, C.-I.; Oprea, O.-C.; Andronescu, E. Innovative antimicrobial chitosan/ZnO/AgNPs/Citronella essential oil nanocomposite—Potential coating for grapes. Foods 2020, 9, 1801. [Google Scholar] [CrossRef] [PubMed]
- Jahdkaran, E.; Hosseini, S.E.; Nafchi, A.M.; Nouri, L. The effects of methylcellulose coating containing carvacrol or menthol on the physicochemical, mechanical, and antimicrobial activity of polyethylene films. Food Sci. Nutr. 2021, 9, 2768–2778. [Google Scholar] [CrossRef]
- Utami Kawiji, R.; Khasanah, L.U.; Solikhah, R. The effect of edible coating enriched with kaffir lime leaf essential oil (Citrus hystrixDC)on beef sausage quality during frozen storage(-18°±2°C). IOP Conf. Ser. Mater. Sci. Eng. 2018, 333, 12070. [Google Scholar] [CrossRef]
- Zhao, R.; Guan, W.; Zheng, P.; Tian, F.; Zhang, Z.; Sun, Z.; Cai, L. Development of edible composite film based on chitosan nanoparticles and their application in packaging of fresh red sea bream fillets. Food Control 2022, 132, 108545. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Soto-Valdez, H.; González-León, A.; Álvarez-Parrilla, E.; Martín-Belloso, O.; González-Aguilar, G.A. Microencapsulation of cinnamon leaf (Cinnamomum zeylanicum) and garlic (Allium sativum) oils in β-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2007, 60, 359–368. Available online: https://link.springer.com/article/10.1007/s10847-007-9385-1 (accessed on 28 December 2022). [CrossRef]
- Amankwaah, C.; Li, J.; Lee, J.; Pascall, M.A. Antimicrobial activity of chitosan-based films enriched with green tea extracts on murine norovirus, Escherichia coli, and Listeria innocua. Int. J. Food Sci. 2020, 2020, 3941924. [Google Scholar] [CrossRef]
- Montaño-Sánchez, E.; Del Mar Torres-Martínez, B.; Vargas-Sánchez, R.D.; Huerta-Leidenz, N.; Sánchez-Escalante, A.; Beriain, M.J.; Torrescano-Urrutia, G.R. Effects of chitosan coating with green tea aqueous extract on lipid oxidation and microbial growth in pork chops during chilled storage. Foods 2020, 9, 766. [Google Scholar] [CrossRef]
- Mauro, M.; Pinto, P.; Settanni, L.; Puccio, V.; Vazzana, M.; Hornsby, B.L.; Fabbrizio, A.; Di Stefano, V.; Barone, G.; Arizza, V. Chitosan film functionalized with grape seed oil—Preliminary evaluation of antimicrobial activity. Sustainability 2022, 14, 5410. [Google Scholar] [CrossRef]
- Amankwaah, C.; Li, J.; Lee, J.; Pascall, M.A. Development of antiviral and bacteriostatic chitosan-based food packaging material with grape seed extract for murine norovirus, Escherichia coli and Listeria innocua control. Food Sci. Nutr. 2020, 8, 6174–6181. [Google Scholar] [CrossRef]
- Onsare, J.G.; Arora, D.S. Antibiofilm potential of flavonoids extracted from Moringa oleifera seed coat against Staphylococcus Aureus Pseudomonas Aeruginosa Candida Albicans. J. Appl. Microbiol. 2014, 118, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Pagliarulo, C.; Sansone, F.; Moccia, S.; Russo, G.L.; Aquino, R.P.; Salvatore, P.; Di Stasio, M.; Volpe, M.G. Preservation of strawberries with an antifungal edible coating using peony extracts in chitosan. Food Bioproc. Tech. 2016, 9, 1951–1960. [Google Scholar] [CrossRef]
- Ramírez-Guerra, H.E.; Castillo-Yañez, F.J.; Montaño-Cota, E.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Canizales-Rodríguez, F.; Torres-Arreola, W.; Montoya-Camacho, N.; Ocaño-Higuera, V.M. Protective effect of an edible tomato plant extract/chitosan coating on the quality and shelf life of Sierra fish fillets. J. Chem. 2018, 2018, 2436045. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Yue, J.; Gong, X.; Qian, B.; Wang, H.; Deng, Y.; Zhao, Y. Blueberry leaf extracts incorporated chitosan coatings for preserving postharvest quality of fresh blueberries. Postharvest Biol. Technol. 2014, 92, 46–53. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Mohammadi, M.; Lorenzo, J.M. Chitosan nanoparticles as a promising nanomaterial for encapsulation of pomegranate (Punica granatum l.) peel extract as a natural source of antioxidants. Nanomaterials 2021, 11, 1439. [Google Scholar] [CrossRef]
- Khorshidian, N.; Khanniri, E.; Koushki, M.R.; Sohrabvandi, S.; Yousefi, M. An Overview of antimicrobial activity of lysozyme and its functionality in cheese. Front. Nutr. 2022, 9, 833618. [Google Scholar] [CrossRef]
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of lysozyme, an innate immune defense factor, as an alternative antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Silvetti, T.; Brasca, M.; Lodi, R.; Vanoni, L.; Chiolerio, F.; de Groot, M.; Bravi, A. Effects of lysozyme on the microbiological stability and organoleptic properties of unpasteurized beer. J. Inst. Brew. 2010, 116, 33–40. [Google Scholar] [CrossRef]
- Pilevar, Z.; Abhari, K.; Tahmasebi, H.; Beikzadeh, S.; Afshari, R.; Eskandari, S.; Bozorg, M.J.A.; Hosseini, H. Antimicrobial properties of lysozyme in meat and meat products: Possibilities and challenges. Acta Sci. Anim. Sci. 2022, 44, e55262. [Google Scholar] [CrossRef]
- Gill, A.O.; Holley, R.A. Inhibition of bacterial growth on ham and bologna by lysozyme, nisin and EDTA. Food Res. Int. 2000, 33, 83–90. [Google Scholar] [CrossRef]
- Van Landschoot, A. Anti-bacterial activity of lysozyme in pitching yeast and effect of lysozyme on yeast fermentation. In Proceedings of the 32nd International Congress of the European Brewery Convention, Hamburg, Germany, 10–14 May 2009. [Google Scholar]
- Duan, J.; Park, S.-I.; Daeschel, M.A.; Zhao, Y. antimicrobial chitosan-lysozyme (cl) films and coatings for enhancing microbial safety of Mozzarella cheese. J. Food Sci. 2007, 72, M355–M362. [Google Scholar] [CrossRef] [PubMed]
- De S. Medeiros, B.G.; Souza, M.P.; Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Vicente, A.A.; Carneiro-da-Cunha, M.G. Physical characterisation of an alginate/lysozyme nano-laminate coating and its evaluation on ‘Coalho’ Cheese shelf life. Food Bioprocess Technol. 2014, 7, 1088–1098. [Google Scholar] [CrossRef] [Green Version]
- Mehyar, G.F.; Al Nabulsi, A.A.; Saleh, M.; Olaimat, A.N.; Holley, R.A. Effects of chitosan coating containing lysozyme or natamycin on shelf-life, microbial quality, and sensory properties of Halloumi cheese brined in normal and reduced salt solutions. J. Food Process. Preserv. 2017, 42, e13324. [Google Scholar] [CrossRef]
- Haniyah, Y.S.; Purwadi; Radiati, L.E.; Thohari, I.; Setyowati, E.; Manab, A. Gouda cheese microbial controlling during ripening using composite edible film containing lysozyme. Int. J. Curr. Microbiol. App. Sci. 2016, 5, 748–756. [Google Scholar] [CrossRef]
- Li, Q.; Xu, J.; Zhang, D.; Zhong, K.; Sun, T.; Li, X.; Li, J. Preparation of a bilayer edible film incorporated with lysozyme and its effect on fish spoilage bacteria. J. Food Saf. 2020, 40, e12832. [Google Scholar] [CrossRef]
- Rao, M.S.; Chander, R.; Sharma, A. Synergistic effect of chitooligosaccharides and lysozyme for meat preservation. LWT 2008, 41, 1995–2001. [Google Scholar] [CrossRef]
- Park, S.-I.; Daeschel, M.A.; Zhao, Y. Functional properties of antimicrobial lysozyme-chitosan composite films. J. Food Sci. 2004, 69, M215–M221. [Google Scholar] [CrossRef]
- Fagerlund, A.; Langsrud, S.; Heir, E.; Mikkelsen, M.I.; Møretrø, T. Biofilm matrix composition affects the susceptibility of food associated staphylococci to cleaning and disinfection agents. Front. Microbiol. 2016, 7, 856. [Google Scholar] [CrossRef] [Green Version]
- Galié, S.; García-Gutiérrez, C.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Biofilms in the food industry: Health aspects and control methods. Front. Microbiol. 2018, 9, 898. [Google Scholar] [CrossRef]
- Pavlukhina, S.V.; Kaplan, J.B.; Xu, L.; Chang, W.; Yu, X.; Madhyastha, S.; Yakandawala, N.; Mentbayeva, A.; Khan, B.; Sukhishvili, S.A. Non-eluting enzymatic antibiofilm coatings. ACS Appl. Mater. Interfaces 2012, 4, 4708–4716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA required for bacterial biofilm formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Krom, B.P.; van der Mei, H.C.; Busscher, H.J.; Sharma, P.K. DNA-mediated bacterial aggregation is dictated by acid–base interactions. Soft Matter 2011, 7, 2927. [Google Scholar] [CrossRef]
- Swartjes, J.J.T.M.; Das, T.; Sharifi, S.; Subbiahdoss, G.; Sharma, P.K.; Krom, B.P.; Busscher, H.J.; van der Mei, H.C. A Functional DNase I coating to prevent adhesion of bacteria and the formation of biofilm. Adv. Funct. Mater. 2013, 23, 2843–2849. [Google Scholar] [CrossRef]
- Kim, S.-H.; Park, C.; Lee, E.-J.; Bang, W.-S.; Kim, Y.-J.; Kim, J.-S. Biofilm formation of Campylobacter strains isolated from raw chickens and its reduction with DNase I treatment. Food Control 2017, 71, 94–100. [Google Scholar] [CrossRef]
- Hu, W.S.; Nam, D.M.; Kim, J.-S.; Koo, O.K. Synergistic anti-biofilm effects of Brassicaceae plant extracts in combination with proteinase K against Escherichia coli O157:H7. Nat. Res. 2020, 10, 21090. [Google Scholar] [CrossRef]
- Kim, N.J.; Kim, J.S. Enhanced inactivation of Salmonella enterica Enteritidis biofilms on the stainless steel surface by proteinase K in the combination with chlorine. Food Control 2022, 132, 108519. [Google Scholar] [CrossRef]
- Li, Y.; Dong, R.; Ma, L.; Qian, Y.; Liu, Z. Combined anti-biofilm enzymes strengthen the eradicate effect of Vibrio parahaemolyticus biofilm: Mechanism on cpsA-J expression and application on different carriers. Food 2022, 11, 1305. [Google Scholar] [CrossRef]
- Cavadini, C.; Hertel, C.; Hammes, W.P. Application of lysostaphin–producing lactobacilli to control staphylococcal food poisoning in meat products. J. Food Prot. 1998, 61, 419–424. [Google Scholar] [CrossRef]
- Van Hekken, D.L.; Wall, R.J.; Somkuti, G.A.; Powell, M.A.; Tunick, M.J.; Tomasula, P.M. Fate of lysostaphin in milk from individual cows through pasteurization and cheesemaking. J. Dairy Sci. 2009, 92, 444–457. [Google Scholar] [CrossRef] [Green Version]
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A natural antimicrobial protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef] [Green Version]
- Jenssen, H.; Hancock, R. Antimicrobial properties of lactoferrin. Biochimie 2009, 91, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.G.R.; Alencar, W.M.P.; Iacuzio, R.; Silva, N.C.C.; Picone, C.S.F. Synthesis, characterization and application of antibacterial lactoferrin nanoparticles. Curr. Res. Nutr. Food Sci. 2022, 5, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Rollini, M.; Nielsen, T.; Musatti, A.; Limbo, S.; Piergiovanni, L.; Munoz, P.H.; Gavara, R. Antimicrobial performance of two different packaging materials on the microbiological quality of fresh salmon. Coatings 2016, 6, 6. [Google Scholar] [CrossRef]
- Brown, C.A.; Wang, B.; Oh, J.-H. Antimicrobial activity of lactoferrin against foodborne pathogenic bacteria incorporated into edible chitosan film. J. Food Prot. 2008, 71, 319–324. [Google Scholar] [CrossRef]
- Padrão, J.; Gonçalves, S.; Silva, J.P.; Sencadas, V.; Lanceros-Méndez, S.; Pinheiro, A.C.; Vicente, A.A.; Rodrigues, L.R.; Fernando Dourado, F. Bacterial cellulose-lactoferrin as an antimicrobial edible packaging. Food Hydrocoll. 2016, 58, 126e140. [Google Scholar] [CrossRef] [Green Version]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial actions and applications of chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Goy, R.C.; Morais, S.T.B.; Assis, O.B.G. Evaluation of the antimicrobial activity of chitosan and its quaternized derivative on E. coli and S. aureus growth. Rev. Bras. Farmacogn. 2016, 26, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Yan, D.; Li, Y.; Liu, Y.; Li, N.; Zhang, X.; Yan, C. Antimicrobial properties of chitosan and chitosan derivatives in the treatment of enteric infections. Molecules 2021, 26, 7136. [Google Scholar] [CrossRef]
- Atay, H.Y. Antibacterial activity of chitosan-based systems. In Functional Chitosan; Jana, S., Ed.; Springer: Singapore, Germany, 2020; Volume 6, pp. 457–489. [Google Scholar] [CrossRef]
- Goy, R.C.; de Britto, D.; Assis, O.B.G. A Review of the antimicrobial activity of chitosan. Polímeros Ciência Tecnol. 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Kim, K.D.; Chun, S.C. Antibacterial activity of chitosan nanoparticles: A review. Process 2020, 8, 1173. [Google Scholar] [CrossRef]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and antimicrobial activity of chitosan and its derivatives: A concise review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Garba, B.; Ren, Y.; Yao, M.; Xia, X.; Li, M.; Wang, Y. Antifungal activity of chitosan against Aspergillus ochraceus and its possible mechanisms of action. Int. J. Biol. Macromol. 2020, 158, 1063–1070. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L.V. Molecular mechanisms of chitosan interactions with fungi and plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poverenov, E.; Arnon-Rips, H.; Zaitsev, Y.; Bar, V.; Danay, O.; Horev, B.; Bilbao-Sainz, C.; McHugh, T.; Rodov, V. Potential of chitosan from mushroom waste to enhance quality and storability of fresh-cut melons. Food Chem. 2018, 268, 233–241. [Google Scholar] [CrossRef]
- Alqahtani, F.; Aleanizy, F.; El Tahir, E.; Alhabib, H.; Alsaif, R.; Shazly, G.; AlQahtani, H.; Alsarra, I.; Mahdavi, J. Antibacterial activity of chitosan nanoparticles against pathogenic N. gonorrhea. Int. J. Nanomed. 2020, 15, 7877–7887. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M.M. Insights into chitosan antibiofilm activity against methicillin-resistant Staphylococcus aureus. J. Appl. Microbiol. 2017, 122, 1547–1557. [Google Scholar] [CrossRef]
- Guanzon, T.B.; Denney, B.M.; Aurestila, B.J.D.; Manliguez, A.M.E.; Codera, K.F.; Cabrera, J.P.; Gayam, G.G.P.; Cabriles, J.C.; Lozada, M.M. Inhibition of methicillin resistant Staphylococcus aureus (MRSA) biofilm proliferation by chitosan isolated from Portunus pelagicus (Linnaeus 1758). Arch. Microbiol. Immunol. 2022, 6, 101–114. [Google Scholar] [CrossRef]
- Economou, V.; Tsitsos, A.; Theodoridis, A.; Ambrosiadis, I.; Arsenos, G. Effects of chitosan coatings on controlling Listeria monocytogenes and methicillin-resistant Staphylococcus aureus in beef and mutton Cuts. Appl. Sci. 2022, 12, 11345. [Google Scholar] [CrossRef]
- Best, E.; James-Meyer, L.; Cogan, T. Chitosan-coated carboxylic acids show antimicrobial activity against antibiotic-resistant Gram-negative and positive pathogens. bioRxiv 2022. [Google Scholar] [CrossRef]
- Essa, E.E.; Hamza, D.; Khalil, M.M.H.; Zaher, H.; Salah, D.; Alnemari, A.M.; Rady, M.H.; Mo‘men, S.A.A. The antibacterial activity of Egyptian wasp chitosan-based nanoparticles against important antibiotic-resistant pathogens. Molecules 2022, 27, 7189. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Pepping, L.; Dekker, M.; Fogliano, V.; Heising, J. Using particle size and fat content to control the release of Allyl isothiocyanate from ground mustard seeds for its application in antimicrobial packaging. Food Chem. 2020, 308, 125573. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.-S.; Bang, J.; Kim, H.; Beuchat, L.R.; Cho, S.Y.; Ryu, J.-H. Development of an antimicrobial sachet containing encapsulated allyl isothiocyanate to inactivate Escherichia coli O157:H7 on spinach leaves. Int. J. Food Microbiol. 2012, 159, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.F.; Meca, G.; Bocate, K.C.P.; Nazareth, T.M.; Bordin, K.; Luciano, F.B. Development of food packaging system containing allyl isothiocyanate against Penicillium nordicum in chilled pizza: Preliminary study. J. Food Process. Preserv. 2017, 42, e13436. [Google Scholar] [CrossRef]
- Winther, M.; Nielsen, V. Active packaging of cheese with allyl isothiocyanate, an alternative to modified atmosphere packaging. J. Food Prot. 2006, 69, 2430–2435. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Jin, T.Z. Antimicrobial activity of allyl isothiocyanate used to coat biodegradable composite films as affected by storage and handling conditions. J. Food Prot. 2012, 75, 2234–2237. [Google Scholar] [CrossRef] [PubMed]
- Asare, P.T.; Greppi, A.; Stettler, M.; Schwab, C.; Stevens, M.J.A.; Lacroix, C. Decontamination of minimally-processed fresh lettuce using reuterin produced by Lactobacillus reuteri. Front. Microbiol. 2018, 9, 1421. [Google Scholar] [CrossRef]
- Sun, M.-C.; Hu, Z.-Y.; Li, D.-D.; Chen, Y.-X.; Xi, J.-H.; Zhao, C.-H. Application of the reuterin system as food preservative or health-promoting agent: A critical review. Foods 2022, 11, 4000. [Google Scholar] [CrossRef]
- Schaefer, A.; Auchtung, T.A.; Hermans, K.E.; Whitehead, D.; Borhan, B.; Britton, R.A. The antimicrobial compound reuterin (3- hydroxypropionaldehyde) induces oxidative stress via interaction with thiol groups. Microbiology 2010, 156, 1589–1599. [Google Scholar] [CrossRef] [Green Version]
- Jers, C.; Kalantari, A.; Garg, A.; Mijakovic, I. Production of 3-hydroxypropanoic acid from glycerol by metabolically engineered bacteria. Front. Bioeng. Biotechnol. 2019, 7, 124. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Rivera, Y.; Sánchez-Vega, R.; Gutiérrez-Méndez, N.; León-Félix, J.; Acosta-Muñiz, C.; Sepulveda, D.R. Production of reuterin in a fermented milk product by Lactobacillus reuteri: Inhibition of pathogens, spoilage microorganisms, and lactic acid bacteria. J. Dairy Sci. 2017, 100, 4258–4268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langa, S.; Martín-Cabrejas, I.; Montiel, R.; Landete, J.M.; Medina, M.; Arqués, J.L. Combined antimicrobial activity of reuterin and diacetyl against foodborne pathogens. J. Dairy Sci. 2014, 97, 6116–6121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langa, S.; Martín-Cabrejas, I.; Montiel, R.; Peirotén, Á.; Arqués, J.L.; Medina, M. Protective effect of reuterin-producing Lactobacillus reuteri against Listeria monocytogenes and Escherichia coli O157:H7 in semi-hard cheese. Food Control 2018, 84, 284–289. [Google Scholar] [CrossRef]
- Hernandez-Carrillo, J.G.; Orta-Zavalza, E.; Gonzalez-Rodríguez, J.G.; Montoya-Torres, C.; Sepúlveda-Ahumada, D.R.; Ortiz-Riviera, Y. Evaluation of the effectivity of reuterin in pectin edible coatings to extend the shelf-life of strawberries during cold storage. Food Packag. Shelf Life 2021, 30, 100760. [Google Scholar] [CrossRef]
- Angiolillo, L.; Conte, A.; Del Nobile, M.A. A new method to bio-preserve sea bass fillets. Int. J. Food Microbiol. 2018, 271, 60–66. [Google Scholar] [CrossRef]
- Zhang, L.; Ben Said, L.; Diarra, M.S.; Fliss, I. Inhibitory activity of natural synergetic antimicrobial consortia against Salmonella enterica on broiler chicken carcasses. Front. Microbiol. 2021, 12, 656956. [Google Scholar] [CrossRef]
- Naimi, S.; Zirah, S.; Greppi, A.; Lacroix, C.; Rebuffat, S.; Fliss, I. Impact of microcin J25 on the porcine microbiome in a continuous culture model. Front. Microbiol. 2022, 13, 930392. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Biron, E.; Said, L.B.; Subirade, M.; Fliss, I. Bacteriocin-based synergetic consortia: A promising strategy to enhance antimicrobial activity and broaden the spectrum of inhibition. Microbiol. Spectr. 2022, 10, e00406–e00421. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Waheed, S.M.; Pradhan, D. Efficacy of reuterin and bacteriocins nisin and pediocin in the preservation of raw milk from dairy farms. Food Technol. Biotechnol. 2020, 58, 359–369. [Google Scholar] [CrossRef]
- Arqués, J.L.; Rodríguez, E.; Nuñez, M.; Medina, M. Inactivation of Gram-negative pathogens in refrigerated milk by reuterin in combination with nisin or the lactoperoxidase system. Eur. Food Res. Technol. 2007, 227, 77–82. [Google Scholar] [CrossRef]
- Połaska, M.; Sokołowska, B. Bacteriophages—A new hope or a huge problem in the food industry. AIMS Microbiol. 2019, 5, 324–346. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouvea, D.M.; Mendonça, R.C.S.; Soto, M.L.; Cruz, R.S. Acetate cellulose film with bacteriophages for potential antimicrobial use in food packaging. LWT 2015, 63, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Chibeu, A.; Agius, L.; Gao, A.; Sabour, P.M.; Kropinski, A.M.; Balamurugan, S. Efficacy of bacteriophage LISTEX™P100 combined with chemical antimicrobials in reducing Listeria monocytogenes in cooked turkey and roast beef. Int. J. Food Microbiol. 2013, 167, 208–214. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Ryu, S. Bacteriophage and endolysin engineering for biocontrol of food pathogens/pathogens in the food: Recent advances and future trends. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Saeghi, S.S.; Hossain, M.A.; Al-Touby, S.S.J. Characterization of antioxidant and antibacterial compounds from aerial parts of Haplophyllum tuberculatum. J. Bioresour. Bioprod. 2022, 7, 52–62. [Google Scholar] [CrossRef]
- Nascimento, G.G.F.; Locatelli, J.; Freitas, P.S.; Silva, G.L. Antibacterial activity of plant extracts and phytochemicals on antibiotic-resistant bacteria. Braz. J. Microbiol. 2000, 31, 247–256. [Google Scholar] [CrossRef]
- Tura, G.T.; Eshete, W.B.; Tucho, G.T. Antibacterial efficacy of local plants and their contribution to public health in rural Ethiopia. Antimicrob. Resist. Infect. Control 2017, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Ahn, J.-W.; Walter, E.S.; White, A.E.; McQueen, R.B.; Hoffmann, S. Identifying sepsis from foodborne hospitalization: Incidence and hospitalization cost by pathogen. Clin. Infect. Dis. 2022, 75, 857–866. [Google Scholar] [CrossRef] [PubMed]

| Metal Nanoparticles | Polymer/Matrix | Characteristics | References |
|---|---|---|---|
| Ag NPs | LDPE | antimicrobial activity against mold and total bacteria in barberries packaged in Ag-LDPE film with 1 wt% AgNP | [37] |
| agar hydrogel | the Ag-based packaging system was effective in inhibiting the growth of Pseudomonas spp. in Fior di Latte cheese | [38] | |
| bio-based coating with MAP | the elongation of the shelf life of Fior di Latte cheese | [39] | |
| polystyrene (PS) matrix | antimicrobial effect against Gram-positive and Gram-negative bacteria and yeast | [40] | |
| carboxymethyl CS | prepared modified antibacterial membranes could kill almost 100% of bacteria under certain conditions and inhibition zone still existed after more than 7 cycles of tests | [41] | |
| CS/montmorillonite nanocomposite films | CS with different molar masses and deacetylation degrees, and their modifications were used; all nanocomposite-AgNPs films inhibited the growth of E. coli and Bacillus subtilis | [42] | |
| Ag NPs and Au NPs | CS film | good antibacterial activity against bacteria (S. aureus, P. aeruginosa), fungi (Aspergillus niger) and yeast (Candida albicans) | [43] |
| Au NPs | PVA crosslink composite films (as a crosslinking agent in film production glyoxal and/or glutaraldehyde (GA) were employed) | the improvement of banana shelf life with PVA-glyoxal-AuNPs composite film | [44] |
| Se NPs | multilayer laminates made of PET and LDPE | active packaging based on selenium nanoparticles prevented the oxidation of tested real food products and extended their shelf life | [45] |
| Se NPs | potato starch film | inhibited growth of Salmonella Typhimurium and E. coli, slightly inhibited B. cereus, did no inhibit Listeria innocua | [46] |
| Metal Oxide Nanoparticles | Polymer/Matrix | Characteristics | References |
|---|---|---|---|
| TiO2 NPs | CS-based coatings | in vitro inhibitory effect on the growth of E. coli and S. aureus | [53] |
| LDPE | reduction in the E. coli colony on fresh lettuce packed with TiO2 nanoparticle-coated films | [54] | |
| various organic and inorganic binders (e.g., PVA, polyethylene glycol, polyurethane) | bactericidal property of TiO2 coatings against E. coli O157:H7 | [55] | |
| TiO2 NPs and Cymbopogon citratus essential oil (CCEO) | CS film | treatments with CCEO and TiO2 extended the shelf life of minced meat as total bacterial count was in acceptable range after 10 days of storage | [56] |
| ZnO NPs and Ag NPs | LDPE films | the microbial load of fresh orange juice kept in packages with Ag and ZnO was below the limit of microbial shelf life (6 log CFU/mL) up to 28 days | [57] |
| ZnO NPs and Ag NPs and essential oils | pullulan films | ZnO NPs were active against S. aureus, L. monocytogenes, E. coli O157:H7, and S. Typhimurium, while Ag NPs were more active against S. aureus than L. monocytogenes; pullulan films containing the compounds effectively inhibited the pathogens associated with vacuum-packaged meat and poultry products stored at 4 °C for up to 3 weeks | [58] |
| ZnO-Ag nanocomposite | PHBV-CS | nanocomposites show great antimicrobial activity in the food packaging of poultry items | [59] |
| Ag-ZnO NPs | PHBV | the bionanocomposite PHBV/Ag–ZnO (10%) was the most potent against S. aureus and E. coli when compared with bionanocomposites Ag-ZnO 5%, 3% and 1% | [60] |
| ZnO NPs | PLA | reduction in E. coli growth by 3.14 log for 0.5% ZnO loading in the PLA coating layer | [61] |
| ZnO-SiO2 | CS, PVA | antibacterial activity against S. aureus S33R and E. coli IRAQ 3; increased the shelf life of bread | [62] |
| SiO2 | PHBV | the maximum antibacterial activity (about 94.7% growth inhibition for E. coli and 92% for S. aureus) was obtained for PHBV/SiO2 (2.0%) | [63] |
| MgO NPs | carboxymethyl-CS | CM-CS/MgO composites exhibited antimicrobial activity against L. monocytogenes and Shewanella baltica | [64] |
| PLA | biofilms with 2% MgO NPs caused progressive damage and death of nearly 46% of E. coli bacterial culture after 12 h treatment | [65] | |
| Aluminum-doped zinc oxide (AZO) | PLA | strong antibacterial activity against E. coli | [66] |
| Al2O3–Ag and TiO2–Ag composite NPs | epoxy polymer | disc diffusion assays proved antimicrobial potential against E. coli DH5α and S. epidermidis NCIMB 12,721 | [67] |
| Peptide | Polymer/Packaging Material | Characteristics | References |
|---|---|---|---|
| Nisin | PLA | significantly inhibited growth of L. monocytogenes and Salmonella Enteritidis in liquid egg white and of E. coli O157:H7 in orange juice | [97] |
| PLA | completely inactivation of L. monocytogenes growth in skim milk and liquid egg white | [98] | |
| PLA | reduction of E. coli O157:H7 count in strawberry puree at 22 °C | [99] | |
| Starch–halloysite nanocomposites | films with 6 g/100 g nisin completely inhibited L. monocytogenes on Minas frescal cheese surface | [100] | |
| cellulose | inhibited growth of L. monocytogenes and S. aureus in minimally processed mangoes | [101] | |
| cellulose | antimicrobial effect in vitro against S. aureus and L. monocytogenes | [102] | |
| plastic film | reduction of L. monocytogenes level in cold-smoked salmon samples | [103] | |
| edible films from whey protein isolates, soy protein isolates, egg albumen and wheat gluten | inhibitory activity against L. monocytogenes strain V7 | [104] | |
| Nisin and ε-polylysine | chitosan coating | decreased growth of microorganism (yeast and mold, total viable counts, total coliforms counts, S. aureus and Pseudomonas spp.) in fresh-cut carrots | [105] |
| Nisin with calcium propionate | edible zein film coatings | strong inhibitory impact on the growth of L. monocytogenes in film-coated ready-to-eat chicken | [106] |
| Nisin and bacteriocin-like substance (BLS) P34 | liposome-encapsulated | reduced population of L. monocytogenes during 21 days of storage of Minas frescal cheese at 7 °C | [107] |
| Nisin combined with Grape Seed Extract (GSE) or Green Tea Extract (GTE) | soy protein film | the greatest inhibitory effect against L. monocytogenes was observed in turkey frankfurters coated with film with 1% GSE and 10,000 IU/mL nisin | [108] |
| Bacteriocin KU24 | in vitro inhibitory effect against methicillin-resistant S. aureus | [109] | |
| Enterocin 416K | LDPE film | significant decrease in L. monocytogenes viable counts in frankfurters | [110] |
| Enterocins A and B | alginate films | in combination with high-pressure processing (HPP) reduction of L. monocytogenes level in sliced cooked ham | [111] |
| Lactocin 705 and AL705 | LDPE | in vitro antimicrobial activity against Lactobacillus plantarum CRL691 and Listeria innocua 7 | [112] |
| Lacticin 3147 and nisin | cellulose-based bioactive inserts and anti-microbial polyethylene/polyamide pouches | reduced the population of lactic acid bacteria in sliced cheese and ham stored in modified atmosphere packaging (MAP) at refrigeration temperatures; nisin-adsorbed bioactive inserts reduced levels of L. innocua and S. aureus | [113] |
| Pediocin | PLA/sawdust particle (SP) biocomposite film | potential inhibition ofL. monocytogenes (99% of total listerial population) on raw sliced pork during the chilled storage | [114] |
| Pediocins | cellulose acetate | inhibiting growth of L. innocua and Salmonella sp. on slices of ham | [115] |
| Plantaricin BM-1 | PET/polyvinylidene chloride/retort casting polypropylene (PPR) plastic multilayer film | decreased the viable counts of L. monocytogenes in meat at 4 °C | [116] |
| Gramicidin A | multilayer biofunctionalized thin films cationic poly(L-lysine) | inhibitory activity against Enterococcus faecalis | [117] |
| Antimicrobial peptide mitochondrial-targeted peptide 1 (MTP1) | PET | reduction in total aerobic mesophilic bacteria (APC) and yeasts on ricotta cheese and meat samples | [118] |
| Antimicrobial Agent | Polymer/Matrix | Characteristics | References |
|---|---|---|---|
| Polyphenols | |||
| Caffeic acid (CA) | CS | CS-CA postharvest treatment of mulberry fruit improved the quality and extend the shelf life during cold storage | [164] |
| Salicylic acid (SA) | CS | CS-SA coating inhibited chilling injury better than SA or CS alone and increased the antioxidant enzyme activities | [165] |
| Lauric acid (LA) | CS edible coating | incorporation LA increased the inhibitory effects against the spoilage bacteria growth, and LA almost completely protected the fresh beef samples against the discoloration after 21 days of storage | [166] |
| Ellagic acid | candelilla wax | significant reduction of the damage caused by Colletotrichum gloesporioides and extended the shelf life of avocado | [167] |
| Ferulic acid (FA) | CS films | FA-CS coating effectively extended the shelf life of refrigerated pork to 7 days; FA-CS had higher antibacterial activity than CS coatings (reduction of total viable counts by 1–2 log) | [168] |
| Gallic acid (GA) | CS and PE films | coating with CS-GA film improved quality of white button mushroom (Agaricus bisporus) in comparison to CS and PE films | [169] |
| Quercetin and Ag NPs | PVC-based film | PVC-based films with quercetin and AgNPs proved to be highly effective in inhibiting bacterial growth of food pathogens (E. coli, S. Typhimurium and L. monocytogenes) | [170] |
| Hydroxyapatite-Quercetin (H-Q) complexes | ALG edible coatings | H-Q alginate coatings inhibited the spoilage bacteria (Pseudomonas spp. and Enterobacteriaceae) growth and preserved the quality of chicken fillets for 11 days at 6 °C | [171] |
| Curcumin | PLA-based composite films | number of bacteria decreased by 1 to 2 logarithmic cycles | [172] |
| Curcumin | PBAT film | only curcumin-PBAT film showed a slight antibacterial activity against E. coli and L. monocytogenes (reduction of 1–2 log CFU) | [173] |
| Curcumin + ZnO | CMC film | only ZnO and ZnO/curcumin films had antibacterial properties | [174] |
| Fenugreek essential oil (FEO) + Curcumin | PLA composite film | good antibacterial and antioxidant properties of PLA-FEO-curcumin composite film | [175] |
| Cinnamaldehyde (CIN) | PLA and starch mono- and bilayer films | monolayer and bilayer films with CIN had bacteriostatic effects to E. coli and L. inocua | [176] |
| Resveratrol (RS) and its inclusion complex (IC) with hydroxypropyl-γ-cyclodextrin | film based on cellulose derivatives (hydroxyethylcellulose and cellulose acetate) | antimicrobial activity of films against Campylobacter jejuni and Campylobacter coli | [177] |
| Resveratrol (RS) and eugenol (EUG) | CMC films | increasing the concentration of RS or EUG in CMC films caused an increase in antimicrobial effects against L. monocytogenes, S. aureus, Salmonella Enteritidis, and E. coli—the antagonistic effects of the combined use of RES and EUG compared with their use alone | [178] |
| Lignin nanoparticles | PVA, CS | the inhibition of the bacterial growth of Erwinia carotovora subsp. carotovora and Xanthomonas arboricola pv. pruni | [179] |
| Essential oils and their ingredients | |||
| Thymol and montmorillonite D43B | PLA | addition of 8 wt.% thymol and 2.5 wt.% D43B significantly increased the antibacterial activity against E. coli and S. aureus 8325-4 at all tested temperatures | [180] |
| Thymol (T) and eugenol (E) | PLA, poly (adipic acid succinate), and poly (succinic acid succinate) (PBS). | antibacterial activity, determined using disk diffusion method, proved that PBS/E was the most efficient against E. coli, S. aureus, Bacillus tequilensis, B, subtilis and B. pumilis, while against Stenotrophomonas maltophilia PLA/E was better | [181] |
| Thyme essential oils (TO) | β-Cyclodextrin capsule | the growth of Alternaria alternata was inhibited significantly by the addition and exposure to TO:β-CD as measured by both the agar dilution and the headspace method | [182] |
| Thymol and carvacrol | edible starch films | carvacrol and thymol presented a fungistatic effect on C. gloeosporioides growth on coated mango and papaya | [183] |
| Thyme oil (TEO) and clove oil (CEO) | PLA-PBAT film | complete killing of S. aureus, that is a reduction from 6.5 log CFU/mL to 0 log CFU/mL, was observed on the 10 wt% CEO incorporated composite film; CEO and TEO composite films inhibited E. coli biofilm by 93.43% and 82.30%, respectively | [184] |
| Thyme volatile oil (TVO), chitosan (and chitosan nanoparticles (CS_NP) | edible coating of CS, CS_NP and thyme volatile oil encapsulated CS_NP (TVO-CS_NP) | edible CS coating considerably extended the shelf life of basil leaves, especially TVO-CS_NPs coating (2.4-fold higher shelf life than the control) | [185] |
| Mediterranean propolis (EEP) and Thymus vulgaris essential oil (TV-EOs) | PLA film | EEP showed the best inhibitory effect on S. aureus and Penicillium sp. (the diameters of the inhibition zones were 12.1 mm and 11.58 mm, respectively); antimicrobial activity of the films showed that films containing 10 wt% EEP inhibited the growth of Candida albicans and the combination of EEP and TV-EOs in the PLA matrix showed a synergistic effect against E. coli | [186] |
| D-limonene nanoemulsion (DLN) and D-limonene essential oil (DLEO) | - | antibacterial activity was proved against S. enterica, E. coli, S. aureus and L. monocytogenes, MBC value of DLN was lower than MBC of DLEO | [187] |
| D-limonene | edible fish gelatin- CS film | strong antibacterial activity against E. coli | [188] |
| Limonene–liposome | ALG coating | the limonene–liposome-treated blackberries had significantly lower visible mold incidence compared to non-coated berries on days 16 and 20 days of storage, whereas the ALG-coated berries had significantly lower visible mold incidence compared to the control on the 16th day only | [189] |
| Monolaurin, eugenol, oregano, and thyme essential oil | zein-based films | zein films with thyme were not active against the tested microorganisms; significant inhibitory effect was observed against S. aureus, when oregano, monolaurin, or eugenol were added; films with eugenol were active against E. coli, Aspergillus niger and Candida albicans | [190] |
| Oregano oil (OO) | sorbitol-plasticized whey protein isolate (WPI) films | growth rate of total flora (total viable count) and pseudomonads were significantly reduced by a factor of two with the use of OO-films, while the growth of lactic acid bacteria was completely inhibited | [191] |
| Origanum vulgare essential oils (OO) and 1 or 2% of grape seed extract (GSE) | CS coatings | turkey breast meat coated with CS-based coating with GSE and OO showed reduced count of total viable count, Enterobacteriaceae, Pseudomonas spp., lactic acid bacteria (and yeast-mold on day 20 of cold storage | [192] |
| Carvacrol (CRV) | pectin/sodium alginate matrix | microencapsulated CRV and the non-encapsulated CRV treatments significantly reduced the populations of yeast, mold, E. coli and mesophilic aerobic bacteria | [193] |
| edible coating of cassava starch | coatings with CRV reduced the counts of E. coli and Salmonella Typhimurium by ~ 5 log CFU/g; Aeromonas hydrophila was reduced by ~ 8 log CFU/g, and S. aureus was reduced by ~2 log CFU/g on the 7 day of storage of minimally processed pumpkin | [194] | |
| gum arabic (GA) and cCS | CRV, as well as CRV-CS or CRV-GA coatings on poultry reduced C. jejuni from day 0 through 7 by up to 3.0 log10 CFU/sample; CRV-CS coatings reduced total aerobic counts when compared with non-coated samples for a majority of the storage times | [195] | |
| Clove essential oil (CEO)—encapsulated in mesoporous silica nanoparticles (MSN) | PLA and polycaprolactone composite films | the higher the content of MSN/CEO/PLA, the better the antibacterial effect against E. coli and S. aureus | [196] |
| Lemongrass oil, citronella oil and cajeput oil | edible CS or ALG film | lemongrass oil was the most effective inhibitor of A. niger (MIC of 10 μL/mL), citronella oil and cajeput oil MIC were of 20 μL/mL, with elongation of shelf life of mango coated by ALG film with lemon grass oil from 10 to 14 days | [197] |
| Lemongrass oil | ALG edible films | antimicrobial activity of ALG-based films against E. coli ATCC 25922 was not dependent on the type of encapsulating agent; lemongrass oil-ALG film has MIC 0.5%, while ALG film 0.6% | [198] |
| Encapsulated citronella oil (complex coacervation of gelatine with carboxymethylcellulose or with gum Arabic) | paper coatings | the antimicrobial activity of released citronella vapors on E. coli and S. cerevisiae (for paper coating 30 g/m2) | [199] |
| Lemongrass oil (LMO) microcapsules | edible coating based on sodium caseinate as wall material | films containing LMO at concentrations of 1250, 2500 and 5000 ppm were able to inhibit growth of E. coli ATCC 25922 and L. monocytogenes ISP 65–08 in liquid cultures | [200] |
| Citronella essential oil (CEO) | CS film with ZnO and Ag NPs | the strongest antimicrobial activity was exhibited by the CS-ZnO-AgNPs membrane, with the inhibition diameter being ~30 mm for S. aureus and E. coli and over 20 mm for C. albicans | [201] |
| Peppermint (menthol) and Origanum vulgare (carvacrol) essential oil | PE-coated films | increment of menthol and carvacrol concentration in PE film coating leads to an increase in the antimicrobial activity of films against E. coli, S. aureus, L. innocua, and S. cerevisiae | [202] |
| Kaffir Lime Leaf Essential Oil (Citrus hystrix DC) | edible coating with tapioca flour | edible coating, with the addition of kaffir lime leaves essential oils, decreased the microbial growth (total plate count) of beef sausage | [203] |
| Cinnamon-perilla essential oil (C-PEO) | edible composite films based on CS_NP | the shelf life of fresh red sea bream fillets wrapped in tested composite film was extended to 6–8 days | [204] |
| Cinnamon leaf (CLO) and garlic oils (GO) | β-cyclodextrin (β-CD) microcapsules | good antifungal activity of CLO:β-CD and GO:β-CD microcapsules against Alternaria alternata | [205] |
| Plant extracts | |||
| Green tea extract (GTE) | CS film | GTE-CS film inhibited growth of L. inocua and E. coli K12 to undetectable levels in tryptic soy broth after 24 h exposure | [206] |
| Green tea water extracts (GTWE) | CS coating | GTWE inclusion in pork samples reduced growth of mesophilic and psychrotrophic bacteria | [207] |
| Extract from red grape seeds (Vitis vinifera) (GSE) | CS film | GSE-CS film caused strong inhibition of S. aureus, E. coli, L. monocytogenes, Brochothrix thermosphacta, Acinetobacter guillouiae, Enterobacter amnigenus, and P. aeruginosa. | [208] |
| Grape seed extract (GSE) | edible coatings and films based on CS | GSE-chitosan film inhibited growth of L. inocua and E. coli K12 | [209] |
| Flavonoids extracted from Moringa oleifera seed coat | - | exhibited antibiofilm potential against S. aureus, P. aeruginosa and Candida albicans. | [210] |
| Paeonia rockii extracts (PPR) dispersed in chitosan (CS) | polysaccharide gels | coatings has antifungal activity and was able to prolong the shelf life of strawberries to about 16 days | [211] |
| Tomato Plant Extract (TPE) | edible CS coating | both CS and TPE alone reduced the mesophyll count of sierra fillets stored on ice during 15 days compared to control fillets, but a synergistic activity TPE-CS was the best antimicrobial treatment | [212] |
| Blueberry fruit and leaf extracts (BLE) | CS coatings | BLE showed good antimicrobial activity against S. aureus, L. monocytogenes, Salmonella Typhimurium and E. coli, with a minimum inhibition concentration from 25 to 50 g L−1. | [213] |
| Pomegranate Peel Extract (PPE) | CS_NPs | pure PPE and PPE-loaded CS_NPs effectively retarded the growth of S. aureus with MIC of 0.27 and 1.1 mg/mL, respectively; E. coli was not sensitive | [214] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duda-Chodak, A.; Tarko, T.; Petka-Poniatowska, K. Antimicrobial Compounds in Food Packaging. Int. J. Mol. Sci. 2023, 24, 2457. https://doi.org/10.3390/ijms24032457
Duda-Chodak A, Tarko T, Petka-Poniatowska K. Antimicrobial Compounds in Food Packaging. International Journal of Molecular Sciences. 2023; 24(3):2457. https://doi.org/10.3390/ijms24032457
Chicago/Turabian StyleDuda-Chodak, Aleksandra, Tomasz Tarko, and Katarzyna Petka-Poniatowska. 2023. "Antimicrobial Compounds in Food Packaging" International Journal of Molecular Sciences 24, no. 3: 2457. https://doi.org/10.3390/ijms24032457
APA StyleDuda-Chodak, A., Tarko, T., & Petka-Poniatowska, K. (2023). Antimicrobial Compounds in Food Packaging. International Journal of Molecular Sciences, 24(3), 2457. https://doi.org/10.3390/ijms24032457







