SABRE Hyperpolarization with up to 200 bar Parahydrogen in Standard and Quickly Removable Solvents
Abstract
1. Introduction
2. Results
2.1. Dissolution of Hydrogen in Deuterated Methanol
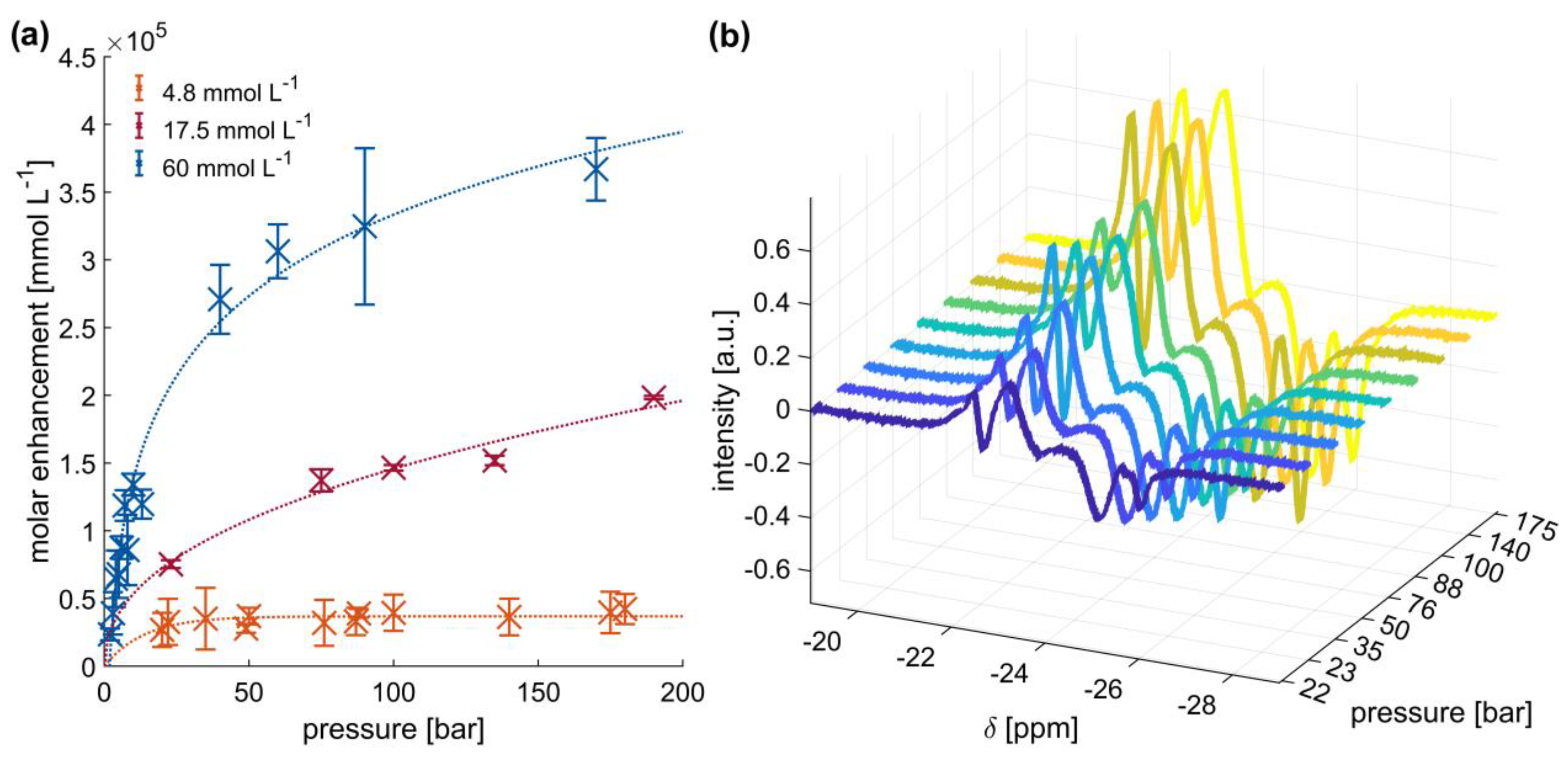
2.2. High-Pressure SABRE Hyperpolarization of Pyrazine in d-Methanol
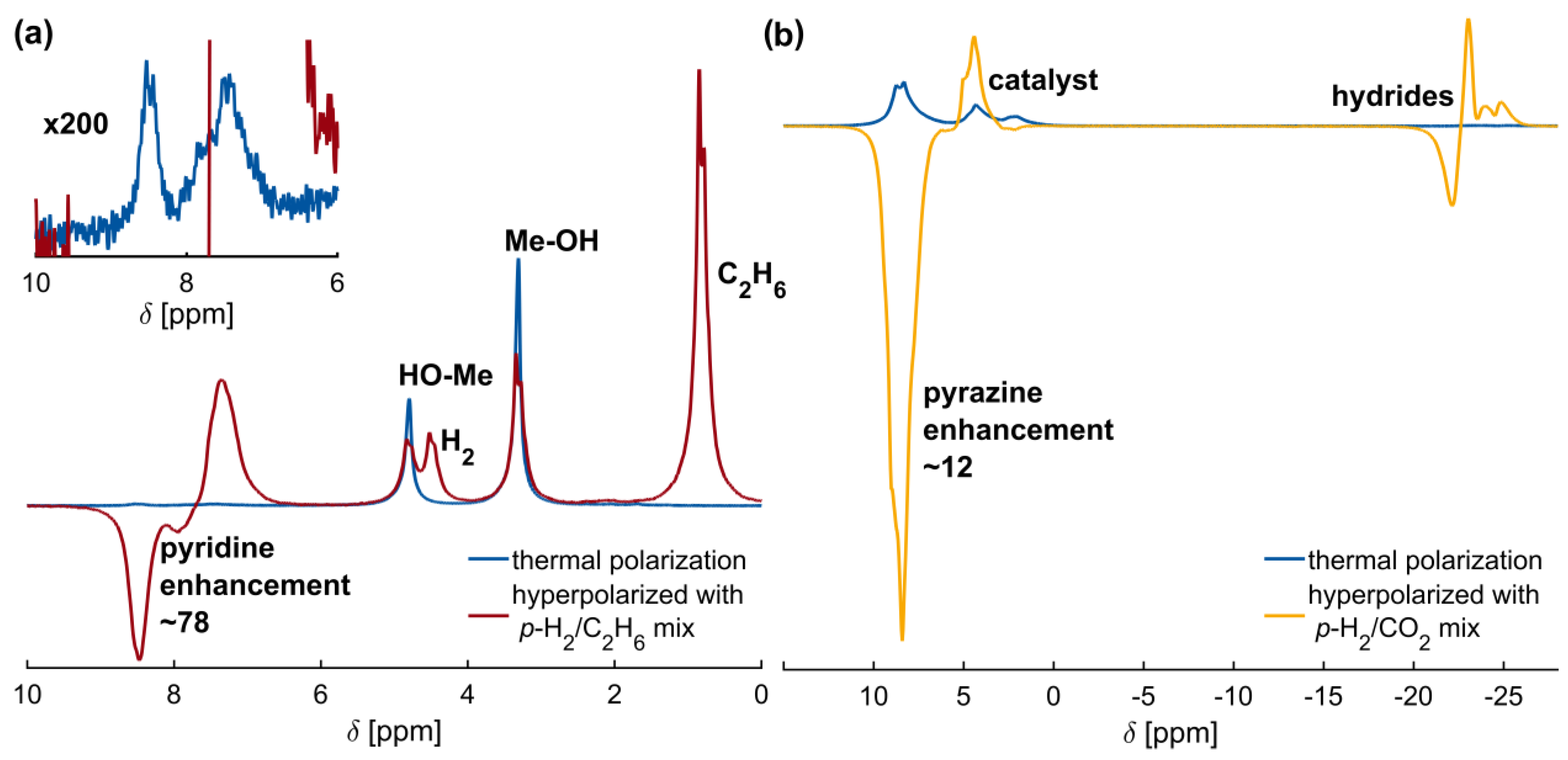
2.3. High-Pressure SABRE Hyperpolarization with Quickly Removable Solvents
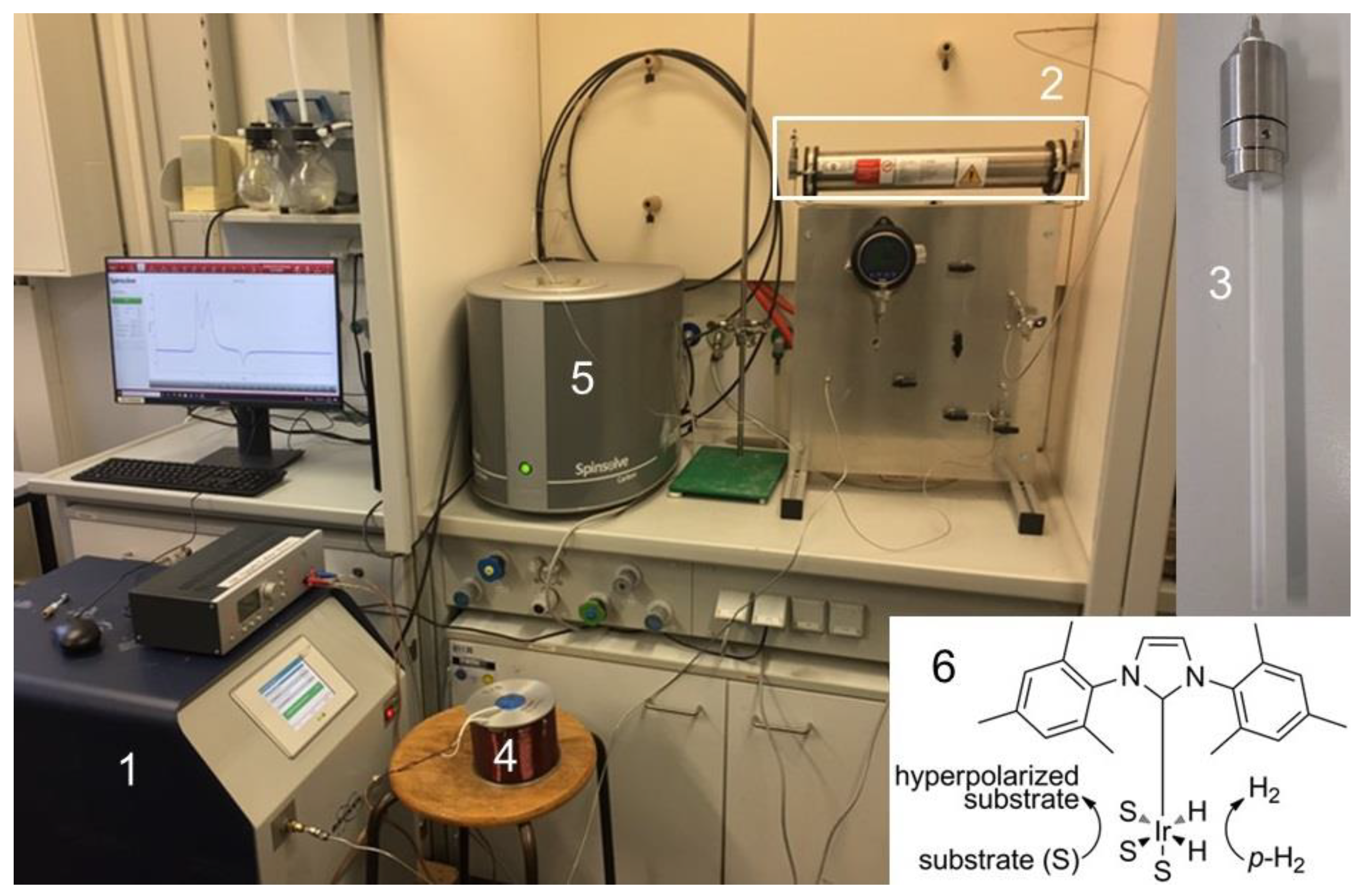
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kemsley, J. Sensitizing NMR. Chem. Eng. News Arch. 2008, 86, 12–15. [Google Scholar] [CrossRef]
- Abraham, M.; McCausland, M.A.H.; Robinson, F.N.H. Dynamic Nuclear Polarization. Phys. Rev. Lett. 1959, 2, 449–451. [Google Scholar] [CrossRef]
- Barskiy, D.A.; Kovtunov, K.V.; Koptyug, I.V.; He, P.; Groome, K.A.; Best, Q.A.; Shi, F.; Goodson, B.M.; Shchepin, R.V.; Truong, M.L.; et al. In situ and ex situ low-field NMR spectroscopy and MRI endowed by SABRE hyperpolarization. ChemPhysChem 2014, 15, 4100–4107. [Google Scholar] [CrossRef]
- Richardson, P.M.; Parrott, A.J.; Semenova, O.; Nordon, A.; Duckett, S.B.; Halse, M.E. SABRE hyperpolarization enables high-sensitivity 1H and 13C benchtop NMR spectroscopy. Analyst 2018, 143, 3442–3450. [Google Scholar] [CrossRef] [PubMed]
- Eshuis, N.; Hermkens, N.; van Weerdenburg, B.J.A.; Feiters, M.C.; Rutjes, F.P.J.T.; Wijmenga, S.S.; Tessari, M. Toward nanomolar detection by NMR through SABRE hyperpolarization. J. Am. Chem. Soc. 2014, 136, 2695–2698. [Google Scholar] [CrossRef]
- Adams, R.W.; Aguilar, J.A.; Atkinson, K.D.; Cowley, M.J.; Elliott, P.I.P.; Duckett, S.B.; Green, G.G.R.; Khazal, I.G.; López-Serrano, J.; Williamson, D.C. Reversible interactions with para-hydrogen enhance NMR sensitivity by polarization transfer. Science 2009, 323, 1708–1711. [Google Scholar] [CrossRef] [PubMed]
- TomHon, P.; Akeroyd, E.; Lehmkuhl, S.; Chekmenev, E.Y.; Theis, T. Automated pneumatic shuttle for magnetic field cycling and parahydrogen hyperpolarized multidimensional NMR. J. Magn. Reson. 2020, 312, 106700. [Google Scholar] [CrossRef]
- Lehmkuhl, S.; Wiese, M.; Schubert, L.; Held, M.; Küppers, M.; Wessling, M.; Blümich, B. Continuous hyperpolarization with parahydrogen in a membrane reactor. J. Magn. Reson. 2018, 291, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bordonali, L.; Nordin, N.; Fuhrer, E.; MacKinnon, N.; Korvink, J.G. Parahydrogen based NMR hyperpolarisation goes micro: An alveolus for small molecule chemosensing. Lab Chip 2019, 19, 503–512. [Google Scholar] [CrossRef]
- TomHon, P.M.; Han, S.; Lehmkuhl, S.; Appelt, S.; Chekmenev, E.Y.; Abolhasani, M.; Theis, T. A Versatile Compact Parahydrogen Membrane Reactor. Chemphyschem 2021, 22, 2526–2534. [Google Scholar] [CrossRef]
- Truong, M.L.; Theis, T.; Coffey, A.M.; Shchepin, R.V.; Waddell, K.W.; Shi, F.; Goodson, B.M.; Warren, W.S.; Chekmenev, E.Y. 15N Hyperpolarization by Reversible Exchange Using SABRE-SHEATH. J. Phys. Chem. C 2015, 119, 8786–8797. [Google Scholar] [CrossRef] [PubMed]
- Lehmkuhl, S.; Emondts, M.; Schubert, L.; Spannring, P.; Klankermayer, J.; Blümich, B.; Schleker, P.P.M. Hyperpolarizing Water with Parahydrogen. ChemPhysChem 2017, 18, 2426–2429. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Adams, R.W.; Duckett, S.B.; Mewis, R.E.; Williamson, D.C.; Green, G.G.R. The theory and practice of hyperpolarization in magnetic resonance using parahydrogen. Prog. Nucl. Magn. Reson. Spectrosc. 2012, 67, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Xu, J.; Gillen, J.; McMahon, M.T.; Artemov, D.; Tyburn, J.-M.; Lohman, J.A.B.; Mewis, R.E.; Atkinson, K.D.; Green, G.G.R.; et al. Optimization of SABRE for polarization of the tuberculosis drugs pyrazinamide and isoniazid. J. Magn. Reson. 2013, 237, 73–78. [Google Scholar] [CrossRef]
- Lloyd, L.S.; Asghar, A.; Burns, M.J.; Charlton, A.; Coombes, S.; Cowley, M.J.; Dear, G.J.; Duckett, S.B.; Genov, G.R.; Green, G.G.R.; et al. Hyperpolarisation through reversible interactions with parahydrogen. Catal. Sci. Technol. 2014, 4, 3544–3554. [Google Scholar] [CrossRef]
- Spannring, P.; Reile, I.; Emondts, M.; Schleker, P.P.M.; Hermkens, N.K.J.; van der Zwaluw, N.G.J.; van Weerdenburg, B.J.A.; Tinnemans, P.; Tessari, M.; Blümich, B.; et al. A New Ir-NHC Catalyst for Signal Amplification by Reversible Exchange in D2O. Chem. Eur. J. 2016, 22, 9277–9282. [Google Scholar] [CrossRef]
- Zhivonitko, V.V.; Skovpin, I.V.; Koptyug, I.V. Strong 31P nuclear spin hyperpolarization produced via reversible chemical interaction with parahydrogen. Chem. Commun. 2015, 51, 2506–2509. [Google Scholar] [CrossRef]
- Moreno, K.X.; Nasr, K.; Milne, M.; Sherry, A.D.; Goux, W.J. Nuclear spin hyperpolarization of the solvent using signal amplification by reversible exchange (SABRE). J. Magn. Reson. 2015, 257, 15–23. [Google Scholar] [CrossRef]
- Colell, J.F.P.; Emondts, M.; Logan, A.W.J.; Shen, K.; Bae, J.; Shchepin, R.V.; Ortiz, G.X.; Spannring, P.; Wang, Q.; Malcolmson, S.J.; et al. Direct Hyperpolarization of Nitrogen-15 in Aqueous Media with Parahydrogen in Reversible Exchange. J. Am. Chem. Soc. 2017, 139, 7761–7767. [Google Scholar] [CrossRef]
- Shi, F.; He, P.; Best, Q.A.; Groome, K.; Truong, M.L.; Coffey, A.M.; Zimay, G.; Shchepin, R.V.; Waddell, K.W.; Chekmenev, E.Y.; et al. Aqueous NMR Signal Enhancement by Reversible Exchange in a Single Step Using Water-Soluble Catalysts. J. Phys. Chem. C 2016, 120, 12149–12156. [Google Scholar] [CrossRef]
- Olaru, A.M.; Burns, M.J.; Green, G.G.R.; Duckett, S.B. SABRE hyperpolarisation of vitamin B3 as a function of pH. Chem. Sci. 2017, 8, 2257–2266. [Google Scholar] [CrossRef]
- Gong, Q.; Gordji-Nejad, A.; Blümich, B.; Appelt, S. Trace analysis by low-field NMR: Breaking the sensitivity limit. Anal. Chem. 2010, 82, 7078–7082. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Logan, A.W.J.; Colell, J.F.P.; Bae, J.; Ortiz, G.X.; Theis, T.; Warren, W.S.; Malcolmson, S.J.; Wang, Q. Diazirines as Potential Molecular Imaging Tags: Probing the Requirements for Efficient and Long-Lived SABRE-Induced Hyperpolarization. Angew. Chem. 2017, 129, 12280–12284. [Google Scholar] [CrossRef]
- Reile, I.; Eshuis, N.; Hermkens, N.K.J.; van Weerdenburg, B.J.A.; Feiters, M.C.; Rutjes, F.P.J.T.; Tessari, M. NMR detection in biofluid extracts at sub-μM concentrations via para-H2 induced hyperpolarization. Analyst 2016, 141, 4001–4005. [Google Scholar] [CrossRef]
- Eshuis, N.; Aspers, R.L.E.G.; van Weerdenburg, B.J.A.; Feiters, M.C.; Rutjes, F.P.J.T.; Wijmenga, S.S.; Tessari, M. 2D NMR Trace Analysis by Continuous Hyperpolarization at High Magnetic Field. Angew. Chem. Int. Ed. 2015, 54, 14527–14530. [Google Scholar] [CrossRef]
- Cowley, M.J.; Adams, R.W.; Atkinson, K.D.; Cockett, M.C.R.; Duckett, S.B.; Green, G.G.R.; Lohman, J.A.B.; Kerssebaum, R.; Kilgour, D.; Mewis, R.E. Iridium N-heterocyclic carbene complexes as efficient catalysts for magnetization transfer from para-hydrogen. J. Am. Chem. Soc. 2011, 133, 6134–6137. [Google Scholar] [CrossRef]
- Colell, J.F.P.; Logan, A.W.J.; Zhou, Z.; Shchepin, R.V.; Barskiy, D.A.; Ortiz, G.X.; Wang, Q.; Malcolmson, S.J.; Chekmenev, E.Y.; Warren, W.S.; et al. Generalizing, Extending, and Maximizing Nitrogen-15 Hyperpolarization Induced by Parahydrogen in Reversible Exchange. J. Phys. Chem. C 2017, 121, 6626–6634. [Google Scholar] [CrossRef]
- Mewis, R.E.; Atkinson, K.D.; Cowley, M.J.; Duckett, S.B.; Green, G.G.R.; Green, R.A.; Highton, L.A.R.; Kilgour, D.; Lloyd, L.S.; Lohman, J.A.B.; et al. Probing signal amplification by reversible exchange using an NMR flow system. Magn. Reson. Chem. 2014, 52, 358–369. [Google Scholar] [CrossRef]
- Dücker, E.B.; Kuhn, L.T.; Münnemann, K.; Griesinger, C. Similarity of SABRE field dependence in chemically different substrates. J. Magn. Reson. 2012, 214, 159–165. [Google Scholar] [CrossRef]
- Hövener, J.-B.; Schwaderlapp, N.; Lickert, T.; Duckett, S.B.; Mewis, R.E.; Highton, L.A.R.; Kenny, S.M.; Green, G.G.R.; Leibfritz, D.; Korvink, J.G.; et al. A hyperpolarized equilibrium for magnetic resonance. Nat. Commun. 2013, 4, 2946. [Google Scholar] [CrossRef]
- Suefke, M.; Lehmkuhl, S.; Liebisch, A.; Blümich, B.; Appelt, S. Para-hydrogen raser delivers sub-millihertz resolution in nuclear magnetic resonance. Nat. Phys. 2017, 13, 568–572. [Google Scholar] [CrossRef]
- Truong, M.L.; Shi, F.; He, P.; Yuan, B.; Plunkett, K.N.; Coffey, A.M.; Shchepin, R.V.; Barskiy, D.A.; Kovtunov, K.V.; Koptyug, I.V.; et al. Irreversible catalyst activation enables hyperpolarization and water solubility for NMR signal amplification by reversible exchange. J. Phys. Chem. B 2014, 118, 13882–13889. [Google Scholar] [CrossRef] [PubMed]
- Chapman, B.; Joalland, B.; Meersman, C.; Ettedgui, J.; Swenson, R.E.; Krishna, M.C.; Nikolaou, P.; Kovtunov, K.V.; Salnikov, O.G.; Koptyug, I.V.; et al. Low-Cost High-Pressure Clinical-Scale 50% Parahydrogen Generator Using Liquid Nitrogen at 77 K. Anal. Chem. 2021, 93, 8476–8483. [Google Scholar] [CrossRef] [PubMed]
- Birchall, J.R.; Coffey, A.M.; Goodson, B.M.; Chekmenev, E.Y. High-Pressure Clinical-Scale 87% Parahydrogen Generator. Anal. Chem. 2020, 92, 15280–15284. [Google Scholar] [CrossRef] [PubMed]
- Ellermann, F.; Pravdivtsev, A.; Hövener, J.-B. Open-source, partially 3D-printed, high-pressure (50-bar) liquid-nitrogen-cooled parahydrogen generator. Magn. Reson. 2021, 2, 49–62. [Google Scholar] [CrossRef]
- Hövener, J.-B.; Bär, S.; Leupold, J.; Jenne, K.; Leibfritz, D.; Hennig, J.; Duckett, S.B.; von Elverfeldt, D. A continuous-flow, high-throughput, high-pressure parahydrogen converter for hyperpolarization in a clinical setting. NMR Biomed. 2013, 26, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Coffey, A.M.; Colon, R.D.; Chekmenev, E.Y.; Waddell, K.W. A pulsed injection parahydrogen generator and techniques for quantifying enrichment. J. Magn. Reson. 2012, 214, 258–262. [Google Scholar] [CrossRef]
- Duchowny, A.; Ortiz Restrepo, S.A.; Adams, M.; Thelen, R.; Adams, A. Refined high-pressure tube design for improved resolution in high-pressure NMR spectroscopy. Analyst 2022, 147, 3827–3832. [Google Scholar] [CrossRef]
- Duchowny, A.; Mohnke, O.; Thern, H.; Dupuy, P.M.; Widerøe, H.C.; Faanes, A.; Paulsen, A.; Küppers, M.; Blümich, B.; Adams, A. Composition analysis of natural gas by combined benchtop NMR spectroscopy and mechanistical multivariate regression. Energy Rep. 2022, 8, 3661–3670. [Google Scholar] [CrossRef]
- Duchowny, A.; Dupuy, P.M.; Widerøe, H.C.; Berg, O.J.; Faanes, A.; Paulsen, A.; Thern, H.; Mohnke, O.; Küppers, M.; Blümich, B.; et al. Versatile high-pressure gas apparatus for benchtop NMR: Design and selected applications. J. Magn. Reson. 2021, 329, 107025. [Google Scholar] [CrossRef]
- Burueva, D.B.; Romanov, A.S.; Salnikov, O.G.; Zhivonitko, V.V.; Chen, Y.-W.; Barskiy, D.A.; Chekmenev, E.Y.; Hwang, D.W.; Kovtunov, K.V.; Koptyug, I.V. Extending the Lifetime of Hyperpolarized Propane Gas through Reversible Dissolution. J. Phys. Chem. C 2017, 121, 4481–4487. [Google Scholar] [CrossRef]
- Alderson, T.R.; Charlier, C.; Torchia, D.A.; Anfinrud, P.; Bax, A. Monitoring Hydrogen Exchange During Protein Folding by Fast Pressure Jump NMR Spectroscopy. J. Am. Chem. Soc. 2017, 139, 11036–11039. [Google Scholar] [CrossRef]
- Kuwata, K.; Li, H.; Yamada, H.; Batt, C.A.; Goto, Y.; Akasaka, K. High pressure NMR reveals a variety of fluctuating conformers in beta-lactoglobulin. J. Mol. Biol. 2001, 305, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Royer, C.A.; Roumestand, C. Monitoring protein folding through high pressure NMR spectroscopy. Prog. Nucl. Magn. Reson. Spectrosc. 2017, 102–103, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Brunner, E.; Hültenschmidt, W.; Schlichthärle, G. Fluid mixtures at high pressures IV. Isothermal phase equilibria in binary mixtures consisting of (methanol + hydrogen or nitrogen or methane or carbon monoxide or carbon dioxide). J. Chem. Thermodyn. 1987, 19, 273–291. [Google Scholar] [CrossRef]
- Descamps, C.; Coquelet, C.; Bouallou, C.; Richon, D. Solubility of hydrogen in methanol at temperatures from 248.41 to 308.20 K. Thermochim. Acta 2005, 430, 1–7. [Google Scholar] [CrossRef]
- d’Angelo, J.V.H.; Francesconi, A.Z. Gas−Liquid Solubility of Hydrogen in n -Alcohols (1 ≤ n ≤ 4) at Pressures from 3.6 MPa to 10 MPa and Temperatures from 298.15 K to 525.15 K. J. Chem. Eng. Data 2001, 46, 671–674. [Google Scholar] [CrossRef]
- Bezanehtak, K.; Combes, G.B.; Dehghani, F.; Foster, N.R.; Tomasko, D.L. Vapor−Liquid Equilibrium for Binary Systems of Carbon Dioxide + Methanol, Hydrogen + Methanol, and Hydrogen + Carbon Dioxide at High Pressures. J. Chem. Eng. Data 2002, 47, 161–168. [Google Scholar] [CrossRef]
- Barskiy, D.A.; Pravdivtsev, A.N.; Ivanov, K.L.; Kovtunov, K.V.; Koptyug, I.V. A simple analytical model for signal amplification by reversible exchange (SABRE) process. Phys. Chem. Chem. Phys. 2016, 18, 89–93. [Google Scholar] [CrossRef]
- Campalani, C.; Amadio, E.; Zanini, S.; Dall’Acqua, S.; Panozzo, M.; Ferrari, S.; de Nadai, G.; Francescato, S.; Selva, M.; Perosa, A. Supercritical CO2 as a green solvent for the circular economy: Extraction of fatty acids from fruit pomace. J. CO2 Util. 2020, 41, 101259. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef]
- Shen, Z.; Li, D.; McHugh, M.A. Solubility of Pyrazine and Its Derivatives in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2006, 51, 2056–2064. [Google Scholar] [CrossRef]
- Knecht, S.; Hadjiali, S.; Barskiy, D.A.; Pines, A.; Sauer, G.; Kiryutin, S.A.; Ivanov, K.L.; Yurkovskaya, A.V.; Buntkowsky, G. Indirect Detection of Short-Lived Hydride Intermediates of Iridium N-Heterocyclic Carbene Complexes via Chemical Exchange Saturation Transfer Spectroscopy. J. Phys. Chem. C 2019, 123, 16288–16293. [Google Scholar] [CrossRef]
- Lin, K.; Patrick TomHon, P.; Lehmkuhl, S.; Laasner, R.; Theis, T.; Blum, V. Density Functional Theory Study of Reaction Equilibria in Signal Amplification by Reversible Exchange. ChemPhysChem 2021, 22, 1947–1957. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duchowny, A.; Denninger, J.; Lohmann, L.; Theis, T.; Lehmkuhl, S.; Adams, A. SABRE Hyperpolarization with up to 200 bar Parahydrogen in Standard and Quickly Removable Solvents. Int. J. Mol. Sci. 2023, 24, 2465. https://doi.org/10.3390/ijms24032465
Duchowny A, Denninger J, Lohmann L, Theis T, Lehmkuhl S, Adams A. SABRE Hyperpolarization with up to 200 bar Parahydrogen in Standard and Quickly Removable Solvents. International Journal of Molecular Sciences. 2023; 24(3):2465. https://doi.org/10.3390/ijms24032465
Chicago/Turabian StyleDuchowny, Anton, Johannes Denninger, Lars Lohmann, Thomas Theis, Sören Lehmkuhl, and Alina Adams. 2023. "SABRE Hyperpolarization with up to 200 bar Parahydrogen in Standard and Quickly Removable Solvents" International Journal of Molecular Sciences 24, no. 3: 2465. https://doi.org/10.3390/ijms24032465
APA StyleDuchowny, A., Denninger, J., Lohmann, L., Theis, T., Lehmkuhl, S., & Adams, A. (2023). SABRE Hyperpolarization with up to 200 bar Parahydrogen in Standard and Quickly Removable Solvents. International Journal of Molecular Sciences, 24(3), 2465. https://doi.org/10.3390/ijms24032465






