Elevated Expression of ADAM10 in Skeletal Muscle of Patients with Idiopathic Inflammatory Myopathies Could Be Responsible for FNDC5/Irisin Unbalance
Abstract
:1. Introduction
2. Results
2.1. General Characteristics of Patients
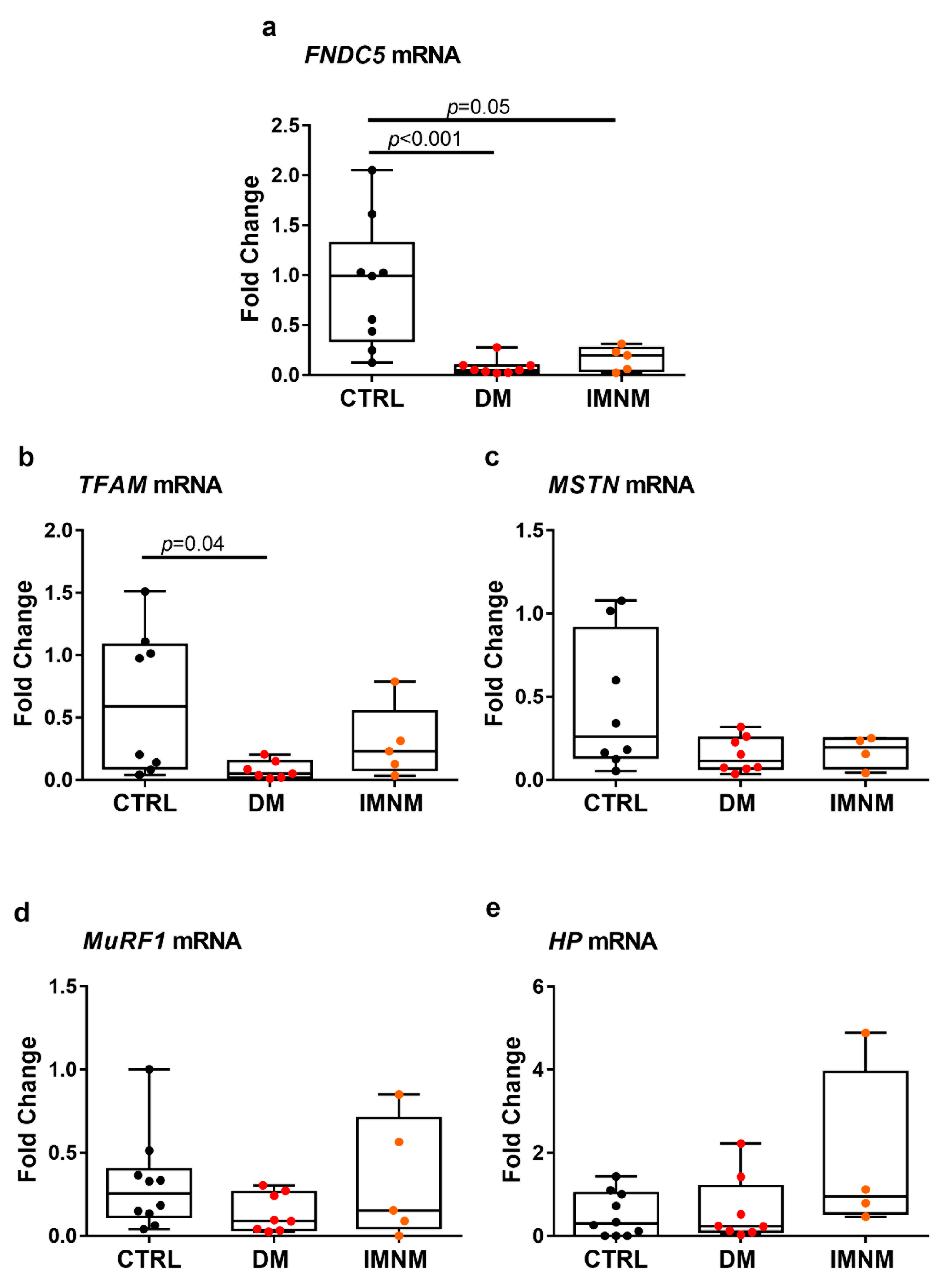
2.2. DM and IMNM Patients Showed Reduced Expression of FNDC5 mRNA in Muscle Biopsies
2.3. Skeletal Muscle Fibers of DM and IMNM Patients Showed Reduced FNDC5 Immunoreactivity
2.4. Irisin Levels in Patients with DM and IMNM Are Higher Than in Control Subjects
2.5. Up-Regulated Levels of ADAM10 mRNA in Skeletal Muscles of DM and IMNM Patients
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Immunohistochemistry
4.3. Real-Time PCR
4.4. Determination of Serum Irisin
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malik, A.; Hayat, G.; Kalia, J.S.; Guzman, M.A. Idiopathic Inflammatory Myopathies: Clinical Approach and Management. Front. Neurol. 2016, 7, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalakas, M.C.; Hohlfeld, R. Polymyositis and dermatomyositis. Lancet 2003, 362, 971–982. [Google Scholar] [CrossRef] [PubMed]
- Bernatsky, S.; Joseph, L.; Pineau, C.A.; Bélisle, P.; Boivin, J.F.; Banerjee, D.; Clarke, A.E. Estimating the prevalence of polymyositis and dermatomyositis from administrative data: Age, sex and regional differences. Ann. Rheum. Dis. 2009, 68, 1192–1196. [Google Scholar] [CrossRef] [PubMed]
- Dobloug, C.; Garen, T.; Bitter, H.; Stjärne, J.; Stenseth, G.; Grøvle, L.; Sem, M.; Gran, J.T.; Molberg, Ø. Prevalence and clinical characteristics of adult polymyositis and dermatomyositis; data from a large and unselected Norwegian cohort. Ann. Rheum. Dis. 2015, 74, 1551–1556. [Google Scholar] [CrossRef] [Green Version]
- Svensson, J.; Arkema, E.V.; Lundberg, I.E.; Holmqvist, M. Incidence and prevalence of idiopathic inflammatory myopathies in Sweden: A nationwide population-based study. Rheumatology 2017, 56, 802–810. [Google Scholar] [CrossRef] [Green Version]
- Wilson, F.C.; Ytterberg, S.R.; St Sauver, J.L.; Reed, A.M. Epidemiology of sporadic inclusion body myositis and polymyositis in Olmsted County, Minnesota. J. Rheumatol. 2008, 35, 445–447. [Google Scholar]
- Hoogendijk, J.E.; Amato, A.A.; Lecky, B.R.; Choy, E.H.; Lundberg, I.E.; Rose, M.R.; Vencovsky, J.; de Visser, M.; Hughes, R.A. 119th ENMC international workshop: Trial design in adult idiopathic inflammatory myopathies, with the exception of inclusion body myositis, 10-12 October 2003, Naarden, The Netherlands. Neuromuscul. Disord. 2004, 14, 337–345. [Google Scholar] [CrossRef]
- Goldfarb, L.G.; Vicart, P.; Goebel, H.H.; Dalakas, M.C. Desmin myopathy. Brain 2004, 127, 723–734. [Google Scholar] [CrossRef]
- McHugh, N.J.; Tansley, S.L. Autoantibodies in myositis. Nat. Rev. Rheumatol. 2018, 14, 290–302. [Google Scholar] [CrossRef]
- Fujimoto, M.; Watanabe, R.; Ishitsuka, Y.; Okiyama, N. Recent advances in dermatomyositis-specific autoantibodies. Curr. Opin. Rheumatol. 2016, 28, 636–644. [Google Scholar] [CrossRef]
- Selva-O’Callaghan, A.; Pinal-Fernandez, I.; Trallero-Araguás, E.; Milisenda, J.C.; Grau-Junyent, J.M.; Mammen, A.L. Classification and management of adult inflammatory myopathies. Lancet Neurol. 2018, 17, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Furst, D.E.; Amato, A.A.; Iorga, Ş.R.; Gajria, K.; Fernandes, A.W. Epidemiology of adult idiopathic inflammatory myopathies in a U.S. managed care plan. Muscle Nerve 2012, 45, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Immune-Mediated Necrotizing Myopathy. Curr. Rheumatol. Rep. 2018, 20, 21. [Google Scholar] [CrossRef] [PubMed]
- Alexanderson, H.; Boström, C. Exercise therapy in patients with idiopathic inflammatory myopathies and systemic lupus erythematosus—A systematic literature review. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101547. [Google Scholar] [CrossRef] [PubMed]
- Munters, L.A.; Loell, I.; Ossipova, E.; Raouf, J.; Dastmalchi, M.; Lindroos, E.; Chen, Y.W.; Esbjörnsson, M.; Korotkova, M.; Alexanderson, H.; et al. Endurance Exercise Improves Molecular Pathways of Aerobic Metabolism in Patients With Myositis. Arthritis Rheumatol. 2016, 68, 1738–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemo Munters, L.; Dastmalchi, M.; Andgren, V.; Emilson, C.; Bergegård, J.; Regardt, M.; Johansson, A.; Orefelt Tholander, I.; Hanna, B.; Lidén, M. Improvement in health and possible reduction in disease activity using endurance exercise in patients with established polymyositis and dermatomyositis: A multicenter randomized controlled trial with a 1-year open extension followup. Arthritis Care Res. 2013, 65, 1959–1968. [Google Scholar] [CrossRef]
- Ma, J.; Chen, K. The role of Irisin in multiorgan protection. Mol. Biol. Rep. 2021, 48, 763–772. [Google Scholar] [CrossRef]
- Colaianni, G.; Storlino, G.; Sanesi, L.; Colucci, S.; Grano, M. Myokines and Osteokines in the Pathogenesis of Muscle and Bone Diseases. Curr. Osteoporos Rep. 2020, 18, 401–407. [Google Scholar] [CrossRef]
- Colaianni, G.; Notarnicola, A.; Sanesi, L.; Brunetti, G.; Lippo, L.; Celi, M.; Moretti, L.; Pesce, V.; Vicenti, G.; Moretti, B.; et al. Irisin levels correlate with bone mineral density in soccer players. J. Biol. Regul. Homeost. Agents 2017, 31, 21–28. [Google Scholar]
- Colaianni, G.; Faienza, M.F.; Sanesi, L.; Brunetti, G.; Pignataro, P.; Lippo, L.; Bortolotti, S.; Storlino, G.; Piacente, L.; D’Amato, G.; et al. Irisin serum levels are positively correlated with bone mineral status in a population of healthy children. Pediatr. Res. 2019, 85, 484–488. [Google Scholar] [CrossRef]
- Faienza, M.F.; Brunetti, G.; Sanesi, L.; Colaianni, G.; Celi, M.; Piacente, L.; D’Amato, G.; Schipani, E.; Colucci, S.; Grano, M. High irisin levels are associated with better glycemic control and bone health in children with Type 1 diabetes. Diabetes Res. Clin. Pract. 2018, 141, 10–17. [Google Scholar] [CrossRef]
- Palermo, A.; Sanesi, L.; Colaianni, G.; Tabacco, G.; Naciu, A.M.; Cesareo, R.; Pedone, C.; Lelli, D.; Brunetti, G.; Mori, G.; et al. A Novel Interplay Between Irisin and PTH: From Basic Studies to Clinical Evidence in Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2019, 104, 3088–3096. [Google Scholar] [CrossRef]
- Colaianni, G.; Errede, M.; Sanesi, L.; Notarnicola, A.; Celi, M.; Zerlotin, R.; Storlino, G.; Pignataro, P.; Oranger, A.; Pesce, V.; et al. Irisin Correlates Positively With BMD in a Cohort of Older Adult Patients and Downregulates the Senescent Marker p21 in Osteoblasts. J. Bone Min. Res. 2021, 36, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Maak, S.; Norheim, F.; Drevon, C.A.; Erickson, H.P. Progress and Challenges in the Biology of FNDC5 and Irisin. Endocr. Rev. 2021, 42, 436–456. [Google Scholar] [CrossRef]
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.A.; Novick, S.J.; et al. Irisin Mediates Effects on Bone and Fat via αV Integrin Receptors. Cell 2018, 175, 1756–1768.e1717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Q.; Kou, W.; Xu, X.; Zhou, S.; Luan, P.; Xu, X.; Li, H.; Zhuang, J.; Wang, J.; Zhao, Y.; et al. FNDC5/Irisin inhibits pathological cardiac hypertrophy. Clin. Sci. 2019, 133, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Wetzel, S.; Seipold, L.; Saftig, P. The metalloproteinase ADAM10: A useful therapeutic target? Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2071–2081. [Google Scholar] [CrossRef]
- Dehmel, T.; Janke, A.; Hartung, H.P.; Goebel, H.H.; Wiendl, H.; Kieseier, B.C. The cell-specific expression of metalloproteinase-disintegrins (ADAMs) in inflammatory myopathies. Neurobiol. Dis. 2007, 25, 665–674. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Fujimoto, M.; Vencovsky, J.; Aggarwal, R.; Holmqvist, M.; Christopher-Stine, L.; Mammen, A.L.; Miller, F.W. Idiopathic inflammatory myopathies. Nat. Rev. Dis. Prim. 2021, 7, 86. [Google Scholar] [CrossRef]
- Fornaro, M.; Girolamo, F.; Cavagna, L.; Franceschini, F.; Giannini, M.; Amati, A.; Lia, A.; Tampoia, M.; D’Abbicco, D.; Maggi, L.; et al. Severe muscle damage with myofiber necrosis and macrophage infiltrates characterize anti-Mi2 positive dermatomyositis. Rheumatology 2021, 60, 2916–2926. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oranger, A.; Storlino, G.; Dicarlo, M.; Zerlotin, R.; Pignataro, P.; Sanesi, L.; Narici, M.; Pišot, R.; Simunič, B.; Colaianni, G.; et al. Impact of 10-day bed rest on serum levels of irisin and markers of musculoskeletal metabolism. Faseb J. 2023, 37, e22668. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J. Targeting the MSTN signaling pathway to treat muscle loss and metabolic dysfunction. J. Clin. Investig. 2021, 131, e148372. [Google Scholar] [CrossRef] [PubMed]
- Theilen, N.T.; Kunkel, G.H.; Tyagi, S.C. The Role of Exercise and TFAM in Preventing Skeletal Muscle Atrophy. J. Cell Physiol. 2017, 232, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Mongelli, T.; Cuscito, C.; Pignataro, P.; Lippo, L.; Spiro, G.; Notarnicola, A.; Severi, I.; Passeri, G.; Mori, G.; et al. Irisin prevents and restores bone loss and muscle atrophy in hind-limb suspended mice. Sci. Rep. 2017, 7, 2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.S.; Kim, H.C.; Zhang, D.; Yeom, H.; Lim, S.K. The novel myokine irisin: Clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine 2019, 64, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Colaianni, G.; Oranger, A.; Dicarlo, M.; Lovero, R.; Storlino, G.; Pignataro, P.; Fontana, A.; Di Serio, F.; Ingravallo, A.; Caputo, G.; et al. Irisin Serum Levels and Skeletal Muscle Assessment in a Cohort of Charcot-Marie-Tooth Patients. Front. Endocrinol. 2022, 13, 886243. [Google Scholar] [CrossRef]
- Lundberg, I.E.; Tjärnlund, A.; Bottai, M.; Werth, V.P.; Pilkington, C.; Visser, M.; Alfredsson, L.; Amato, A.A.; Barohn, R.J.; Liang, M.H.; et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Ann. Rheum. Dis. 2017, 76, 1955–1964. [Google Scholar] [CrossRef]



| CTRL (N = 40) | DM (N = 32) | IMNM (N = 9) | |
|---|---|---|---|
| Age (years) | 58.03 ± 17.98 | 57.09 ± 19.25 | 57.78 ± 12.98 |
| Sex (Number) | 8 M; 33 F | 6 M; 26 F | 2 M; 7 F |
| Irisin (ng/mL) | 8.99 ± 1.51 | 10.25 (7.52; 13.37) | 12.66 ± 4.73 |
| MMT8 Unilaterally (potential score 0–80) | n/a | 73 (66; 79) | 65.11 ± 9.47 |
| Cpk (60–190 U/L) | n/a | 395 (76; 1559) | 3327 (1155; 4068) |
| PCR (>5–10 mg/L) | n/a | 6.22 ± 4.76 | 7.94 ± 4.25 |
| Auto-antibodies | n/a | Mi-2 (40%DM) MDA-5 (19%DM) TIF1-γ (15.6%DM) NXP-2 (9.4%DM) SAE-1/2 (9.4% DM) SSA/Ro52 (3.1%DM) | HMGCR (80%IMNM)SRP (11%IMNM) |
| Gene Name and ID | Forward (5′–3′) | Reverse (5′–3′) | Product Length |
|---|---|---|---|
| GAPDH NM_001256799.3 | aatgggcagccgttaggaaa | gcccaatacgaccaaatcagag | 166 |
| B2M NM_004048.4 | agatgagtatgcctgccgtg | ttcaaacctccatgatgctgc | 97 |
| FNDC5 NM_001171940.2 | tcatcgtcgtggtcctgttc | tcaatgatgtcatactggcggc | 70 |
| MSTN NM_005259.3 | caggcactggtatttggcag | aacggattcagcccatcttctc | 163 |
| HP NM_001126102.3 | cgccacagaaggagatggag | ttgggcttcccacatactgc | 108 |
| MuRF1 NM_032588.4 | gagccaccttcctcttgactg | ctcagggcgtctgctatgtg | 145 |
| ADAM10 NM_001110.4 | tcatggtgaaacgcataagaatca | ccagaccaagtacgccatca | 183 |
| TFAM NM_001270782.2 | cgggttccagttgtgattgc | acacaaaactgaagggggagc | 196 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zerlotin, R.; Fornaro, M.; Errede, M.; Pignataro, P.; Suriano, C.; Ruggieri, M.; Colucci, S.; Iannone, F.; Grano, M.; Colaianni, G. Elevated Expression of ADAM10 in Skeletal Muscle of Patients with Idiopathic Inflammatory Myopathies Could Be Responsible for FNDC5/Irisin Unbalance. Int. J. Mol. Sci. 2023, 24, 2469. https://doi.org/10.3390/ijms24032469
Zerlotin R, Fornaro M, Errede M, Pignataro P, Suriano C, Ruggieri M, Colucci S, Iannone F, Grano M, Colaianni G. Elevated Expression of ADAM10 in Skeletal Muscle of Patients with Idiopathic Inflammatory Myopathies Could Be Responsible for FNDC5/Irisin Unbalance. International Journal of Molecular Sciences. 2023; 24(3):2469. https://doi.org/10.3390/ijms24032469
Chicago/Turabian StyleZerlotin, Roberta, Marco Fornaro, Mariella Errede, Patrizia Pignataro, Clelia Suriano, Maddalena Ruggieri, Silvia Colucci, Florenzo Iannone, Maria Grano, and Graziana Colaianni. 2023. "Elevated Expression of ADAM10 in Skeletal Muscle of Patients with Idiopathic Inflammatory Myopathies Could Be Responsible for FNDC5/Irisin Unbalance" International Journal of Molecular Sciences 24, no. 3: 2469. https://doi.org/10.3390/ijms24032469
APA StyleZerlotin, R., Fornaro, M., Errede, M., Pignataro, P., Suriano, C., Ruggieri, M., Colucci, S., Iannone, F., Grano, M., & Colaianni, G. (2023). Elevated Expression of ADAM10 in Skeletal Muscle of Patients with Idiopathic Inflammatory Myopathies Could Be Responsible for FNDC5/Irisin Unbalance. International Journal of Molecular Sciences, 24(3), 2469. https://doi.org/10.3390/ijms24032469












