The ANGPTL3-4-8 Axis in Normal Gestation and in Gestational Diabetes, and Its Potential Involvement in Fetal Growth
Abstract
:1. Introduction
2. Results
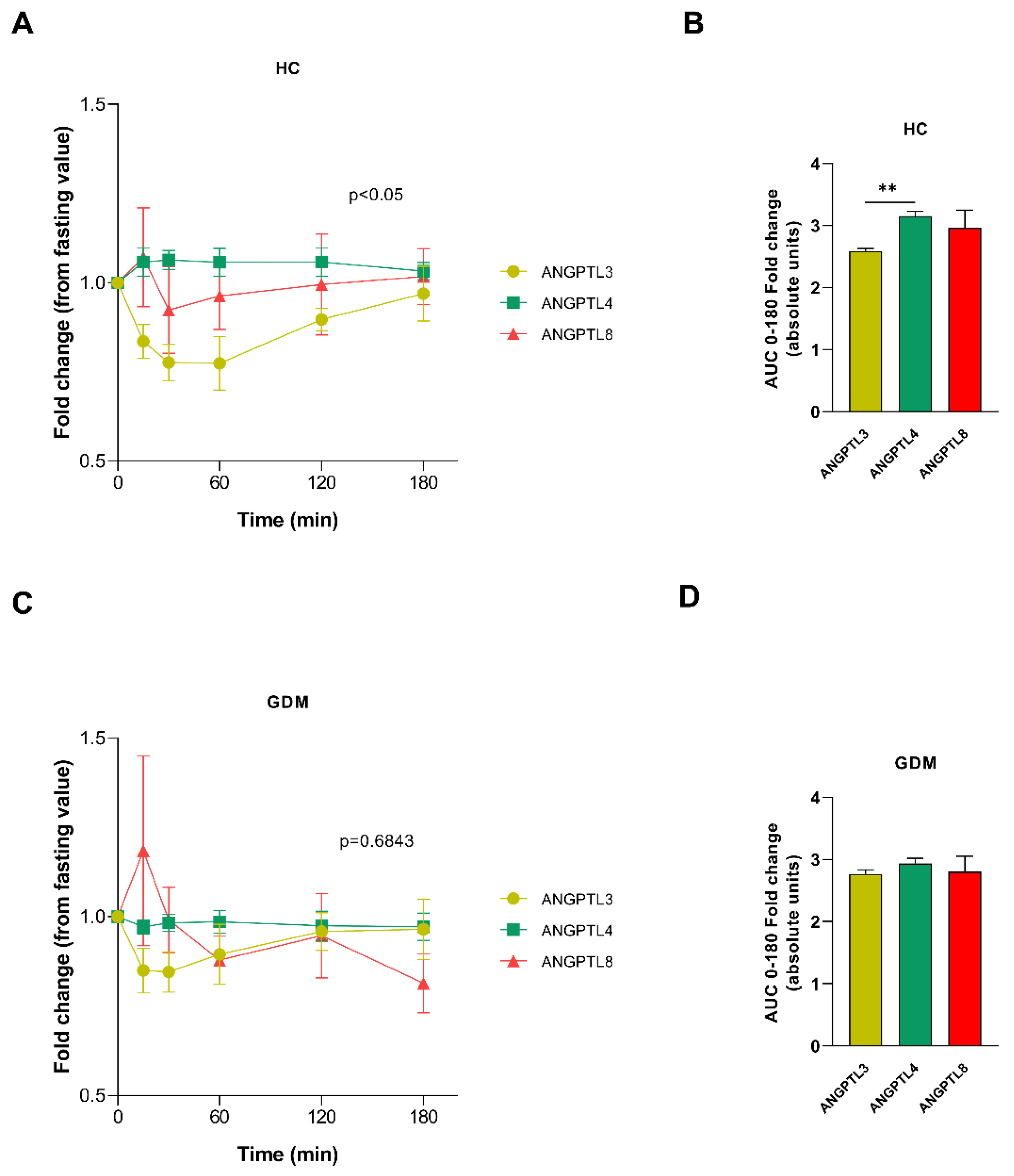
2.1. ANGPTL Secretion Pattern after Meal Test
2.2. Placental Expression and Location-Dependent Changes in Healthy Control Pregnancy and GDM
2.3. Differences in ANGPTL3, 4, and 8, LPL, and Proteins Involved in Lipid Transport on Both Sides of the Placenta According to Glucose Tolerance Status
2.4. ANGPTL3, 4, and 8 and Maternal and Offspring Characteristics
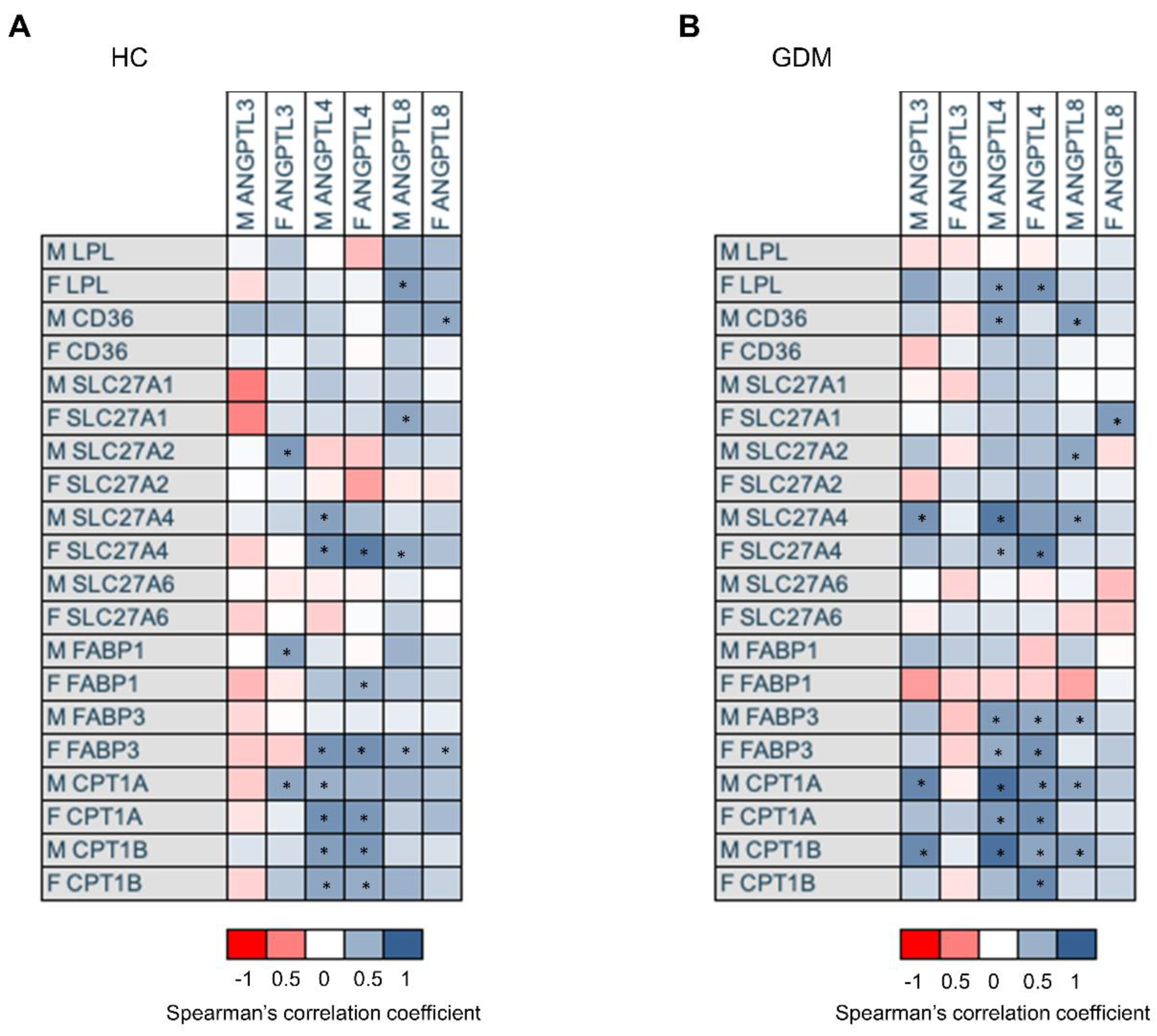
2.5. Association of mRNA Gene Expression of ANGPTL3, 4, and 8, with mRNA Expression of Genes Involved in Placental Lipid Transport According to Glucose Tolerance Status
3. Discussion
4. Methods
4.1. Study Population
4.2. Meal Test
4.3. Cord Blood and Placental Collection and Processing
4.4. Laboratory Measurements
4.5. ANGTPL3, 4 and 8 Determinations
4.6. Data Calculations
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, R. The ANGPTL3-4-8 Model, a Molecular Mechanism for Triglyceride Trafficking. Open Biol. 2015, 6, 150272. [Google Scholar] [CrossRef] [PubMed]
- Kadomatsu, T.; Oike, Y. Roles of Angiopoietin-like Proteins in Regulation of Stem Cell Activity. J. Biochem. 2019, 165, 309–315. [Google Scholar] [CrossRef]
- Hato, T.; Tabata, M.; Oike, Y. The Role of Angiopoietin-like Proteins in Angiogenesis and Metabolism. Trends Cardiovasc. Med. 2008, 18, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, X.; Ji, D. Molecular Mechanism of the Effect of Angiopoietin-like Protein 8 on the Proliferation, Invasion and Migration of Placental Trophoblasts in Preeclampsia. Exp. Ther. Med. 2020, 19, 3461–3468. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Du, Q.; Zhang, L.; Li, L.; Wang, N.; Wu, B.; Li, P. Silencing of ANGPTL8 Alleviates Insulin Resistance in Trophoblast Cells. Front. Endocrinol. 2021, 12, 635321. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.; Yin, W.; Kozlitina, J.; Pennacchio, L.A.; Boerwinkle, E.; Hobbs, H.H.; Cohen, J.C. Rare Loss-of-Function Mutations in ANGPTL Family Members Contribute to Plasma Triglyceride Levels in Humans. J. Clin. Investig. 2009, 119, 70–79. [Google Scholar] [CrossRef]
- Yang, H.; He, B.; Yallampalli, C.; Gao, H. Fetal Macrosomia in a Hispanic/Latinx Predominant Cohort and Altered Expressions of Genes Related to Placental Lipid Transport and Metabolism. Int. J. Obes. 2020, 44, 1743–1752. [Google Scholar] [CrossRef]
- Fu, Z.; Abou-Samra, A.B.; Zhang, R. A Lipasin/Angptl8 Monoclonal Antibody Lowers Mouse Serum Triglycerides Involving Increased Postprandial Activity of the Cardiac Lipoprotein Lipase. Sci. Rep. 2015, 5, 18502. [Google Scholar] [CrossRef]
- Chen, Y.Q.; Pottanat, T.G.; Siegel, R.W.; Ehsani, M.; Qian, Y.W.; Zhen, E.Y.; Regmi, A.; Roell, W.C.; Guo, H.; Jane Luo, M.; et al. Angiopoietin-like Protein 8 Differentially Regulates ANGPTL3 and ANGPTL4 during Postprandial Partitioning of Fatty Acids. J. Lipid Res. 2020, 61, 1203–1220. [Google Scholar] [CrossRef]
- Sylvers-Davie, K.L.; Davies, B.S.J. Regulation of Lipoprotein Metabolism by ANGPTL3, ANGPTL4, and ANGPTL8. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E493–E508. [Google Scholar] [CrossRef]
- Herrera, E.; Desoye, G. Maternal and Fetal Lipid Metabolism under Normal and Gestational Diabetic Conditions. Horm. Mol. Biol. Clin. Investig. 2016, 26, 109–127. [Google Scholar] [CrossRef] [PubMed]
- Herrera, E.; Ortega-Senovilla, H. Lipid Metabolism during Pregnancy and Its Implications for Fetal Growth. Curr. Pharm. Biotechnol. 2014, 15, 24–31. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Graf, K.; Kulbacka, I.; Kjos, S.L.; Dudenhausen, J.; Vetter, K.; Herrera, E. Maternal Lipids as Strong Determinants of Fetal Environment and Growth in Pregnancies with Gestational Diabetes Mellitus. Diabetes Care 2008, 31, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Schaefer-Graf, U.M.; Meitzner, K.; Ortega-Senovilla, H.; Graf, K.; Vetter, K.; Abou-Dakn, M.; Herrera, E. Differences in the Implications of Maternal Lipids on Fetal Metabolism and Growth between Gestational Diabetes Mellitus and Control Pregnancies. Diabet. Med. 2011, 28, 1053–1059. [Google Scholar] [CrossRef]
- Ortega-Senovilla, H.; van Poppel, M.N.M.; Desoye, G.; Herrera, E. Angiopoietin-like Protein 4 (ANGPTL4) Is Related to Gestational Weight Gain in Pregnant Women with Obesity. Sci. Rep. 2018, 8, 12428. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Perez, B.; Ejarque, M.; Gutierrez, C.; Nuñez-Roa, C.; Roche, K.; Vila-Bedmar, R.; Ballesteros, M.; Redondo-Angulo, I.; Planavila, A.; Villarroya, F.; et al. Angiopoietin-like Protein 8 (ANGPTL8) in Pregnancy: A Brown Adipose Tissue-Derived Endocrine Factor with a Potential Role in Fetal Growth. Transl. Res. 2016, 178, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garces, M.F.; Rodriguez-Navarro, H.A.; Buell-Acosta, J.D.; Burgos-Cardenas, A.J.; Franco-Vega, R.; Maldonado-Acosta, L.M.; Eslava-Schmalbach, J.; Parada-Baños, A.J.; Castro-Pinzon, A.; Sanchez, E.; et al. Maternal Serum Angiopoietin-Like 3 Levels in Healthy and Mild Preeclamptic Pregnant Women. Front. Endocrinol. 2021, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Shetty, S.K.; Spitler, K.M.; Wattez, J.S.; Davies, B.S.J.; Shao, J. Obesity Reduces Maternal Blood Triglyceride Concentrations by Reducing Angiopoietin-Like Protein 4 Expression in Mice. Diabetes 2020, 69, 1100–1109. [Google Scholar] [CrossRef]
- Ortega-Senovilla, H.; Schaefer-Graf, U.; Meitzner, K.; Abou-Dakn, M.; Herrera, E. Decreased Concentrations of the Lipoprotein Lipase Inhibitor Angiopoietin-like Protein 4 and Increased Serum Triacylglycerol Are Associated with Increased Neonatal Fat Mass in Pregnant Women with Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2013, 98, 3430–3437. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, X.; Chen, X.; Feng, Y.; Guo, H.; Li, S.; Dai, T.; Jiang, R.; Zhang, X.; Fang, C.; et al. Angiopoietin-like Protein 8 in Early Pregnancy Improves the Prediction of Gestational Diabetes. Diabetologia 2018, 61, 574–580. [Google Scholar] [CrossRef]
- Li, M.; Hu, J.; Yao, L.; Gao, M. Decreased ANGPTL4 Impairs Endometrial Angiogenesis during Peri-implantation Period in Patients with Recurrent Implantation Failure. J. Cell. Mol. Med. 2020, 24, 10730–10743. [Google Scholar] [CrossRef] [PubMed]
- Alejandro, E.U.; Bernlohr, D.A. Window of Opportunity: Targeting ANGPTL4 Improves Triglyceride Levels in Maternal Obesity During Pregnancy. Diabetes 2020, 69, 1087–1089. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, X.-J.; Zhang, G.-Y.; Su, D.-X.; Lei, L.; Li, R. ANGPTL4 Participates in Gestational Diabetes Mellitus via Regulating Akt Pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 5056–5062. [Google Scholar] [CrossRef] [PubMed]
- Schmid, A.; Belikan, H.; Höpfinger, A.; Schäffler, A.; Karrasch, T. Impact of Oral Lipid and Glucose Tolerance Tests on the Postprandial Concentrations of Angiopoietin-like Proteins (Angptl) 3 and 4. Eur. J. Nutr. 2022, 61, 1919–1929. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, K. An Updated ANGPTL3-4-8 Model as a Mechanism of Triglyceride Partitioning between Fat and Oxidative Tissues. Prog. Lipid Res. 2022, 85, 101140. [Google Scholar] [CrossRef]
- Alvarez, J.J.; Montelongo, A.; Iglesias, A.; Lasunción, M.A.; Herrera, E. Longitudinal Study on Lipoprotein Profile, High Density Lipoprotein Subclass, and Postheparin Lipases during Gestation in Women. J. Lipid Res. 1996, 37, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Sood, R.; Zehnder, J.L.; Druzin, M.L.; Brown, P.O. Gene Expression Patterns in Human Placenta. Proc. Natl. Acad. Sci. USA 2006, 103, 5478–5483. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.; Wang, S.; Deng, Q.; An, Y.; Xing, Y.; Dai, X.; Li, Z.; Ma, Q.; Wang, K.; et al. Single-Cell Transcriptional Profiling Reveals Cellular and Molecular Divergence in Human Maternal–Fetal Interface. Sci. Rep. 2022, 12, 1–15. [Google Scholar] [CrossRef]
- Shimamura, M.; Matsuda, M.; Yasumo, H.; Okazaki, M.; Fujimoto, K.; Kono, K.; Shimizugawa, T.; Ando, Y.; Koishi, R.; Kohama, T.; et al. Angiopoietin-like Protein3 Regulates Plasma HDL Cholesterol through Suppression of Endothelial Lipase. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 366–372. [Google Scholar] [CrossRef]
- Gauster, M.; Hiden, U.; van Poppel, M.; Frank, S.; Wadsack, C.; Hauguel-de Mouzon, S.; Desoye, G. Dysregulation of Placental Endothelial Lipase in Obese Women with Gestational Diabetes Mellitus. Diabetes 2011, 60, 2457–2464. [Google Scholar] [CrossRef]
- Lindegaard, M.L.S.; Damm, P.; Mathiesen, E.R.; Nielsen, L.B. Placental Triglyceride Accumulation in Maternal Type 1 Diabetes Is Associated with Increased Lipase Gene Expression. J. Lipid Res. 2006, 47, 2581–2588. [Google Scholar] [CrossRef]
- Gauster, M.; Hiden, U.; Blaschitz, A.; Frank, S.; Lang, U.; Alvino, G.; Cetin, I.; Desoye, G.; Wadsack, C. Dysregulation of Placental Endothelial Lipase and Lipoprotein Lipase in Intrauterine Growth-Restricted Pregnancies. J. Clin. Endocrinol. Metab. 2007, 92, 2256–2263. [Google Scholar] [CrossRef]
- Belcastro, L.; Ferreira, C.S.; Saraiva, M.A.; Mucci, D.B.; Murgia, A.; Lai, C.; Vigor, C.; Oger, C.; Galano, J.M.; Pinto, G.D.A.; et al. Decreased Fatty Acid Transporter FABP1 and Increased Isoprostanes and Neuroprostanes in the Human Term Placenta: Implications for Inflammation and Birth Weight in Maternal Pre-Gestational Obesity. Nutrients 2021, 13, 2768. [Google Scholar] [CrossRef]
- Dubé, E.; Gravel, A.; Martin, C.; Desparois, G.; Moussa, I.; Ethier-Chiasson, M.; Forest, J.C.; Giguére, Y.; Masse, A.; Lafond, J. Modulation of Fatty Acid Transport and Metabolism by Maternal Obesity in the Human Full-Term Placenta. Biol. Reprod. 2012, 87, 14. [Google Scholar] [CrossRef] [PubMed]
- Georgiadi, A.; Lichtenstein, L.; Degenhardt, T.; Boekschoten, M.V.; van Bilsen, M.; Desvergne, B.; Müller, M.; Kersten, S. Induction of Cardiac Angptl4 by Dietary Fatty Acids Is Mediated by Peroxisome Proliferator-Activated Receptor β/δ and Protects against Fatty Acid-Induced Oxidative Stress. Circ. Res. 2010, 106, 1712–1721. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, L.; Mattijssen, F.; de Wit, N.J.; Georgiadi, A.; Hooiveld, G.J.; van der Meer, R.; He, Y.; Qi, L.; Köster, A.; Tamsma, J.T.; et al. Angptl4 Protects against Severe Proinflammatory Effects of Saturated Fat by Inhibiting Fatty Acid Uptake into Mesenteric Lymph Node Macrophages. Cell Metab. 2010, 12, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A.D.; Das, M.K.; Sarkar, A.; Vilasagaram, S.; Basak, S.; Duttaroy, A.K. Tube Formation in the First Trimester Placental Trophoblast Cells: Differential Effects of Angiogenic Growth Factors and Fatty Acids. Cell Biol. Int. 2016, 40, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Song, Q.Y.; Niu, S.X.; Chen, H.J.; Petersen, R.B.; Zhang, Y.; Huang, K. Emerging Roles of Angiopoietin-like Proteins in Inflammation: Mechanisms and Potential as Pharmacological Targets. J. Cell Physiol. 2022, 237, 98–117. [Google Scholar] [CrossRef] [PubMed]
- Pantham, P.; Aye, I.L.M.H.; Powell, T.L. Inflammation in Maternal Obesity and Gestational Diabetes Mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef]
- Grupo Español de Diabetes y Embarazo (GEDE); Grupo Español de Diabetes y Embarazo Asistencia a la gestante con diabetes. Guía de práctica clínica actualizada en 2014|Avances en Diabetología. Av. Diabetol. 2015, 31, 45–59. [Google Scholar] [CrossRef]
- National Diabetes Data Group. Classification and Diagnosis of Diabetes Mellitus and Other Categories of Glucose Intolerance. Diabetes 1979, 28, 1039–1057. [Google Scholar] [CrossRef] [PubMed]
- Mari, A.; Pacini, G.; Murphy, E.; Ludvik, B.; Nolan, J.J. A Model-Based Method for Assessing Insulin Sensitivity from the Oral Glucose Tolerance Test. Diabetes Care 2001, 24, 539–548. [Google Scholar] [CrossRef] [PubMed]


| Healthy Control n = 8 | GDM n = 10 | p-Value | |
|---|---|---|---|
| Age (years) | 32.0 (31.3–35.3) | 34.0 (26.0–36.0) | 0.968 |
| BMI (kg/m2) | 21.9 (18.4–29.8) | 28.0 (26.3–36.3) | 0.041 |
| GWG (kg) | 11.3 (9.4–13.6) | 6.7 (0.5–9.6) | 0.062 |
| Gestational Week at testing | 32.0 (32.3–33.0) | 32.0 (30–34.0) | 0.968 |
| Glucose (mmol/L) | 3.8 (3.7–4.4) | 4.2 (4.1–4.5) | 0.091 |
| Triglycerides (mmol/L) | 1.9 (1.5–2.7) | 2.4 (2.1–3.1) | 0.177 |
| FFAs (nmol/mL) | 77.4 (53.0–98.8) | 76.7 (59.5–1050) | 1 |
| Insulin (pmol/L) | 50.9 (45.2–91.1) | 122.0 (68.2–177.9) | 0.015 |
| ANGPTL3 (ng/mL) | 202 (129–224) | 240 (164–340) | 0.167 |
| ANGPTL4 (ng/mL) | 6.9 (5.9–7.9) | 7.6 (6.2–8.2) | 0.091 |
| ANGPTL8 (pg/mL) | 535 (417–1059) | 531 (437–735) | 0.875 |
| Insulinogenic index | 0.6709 (0.2953–1.739) | 0.8150 (0.6143–1.523) | 0.6058 |
| OGIS Index (mL/min/m2) | 550.5 (484.3–622.8) | 482.0 (446–520.5) | 0.0745 |
| Healthy Control n = 19 | GDM n = 20 | p-Value | |
|---|---|---|---|
| Age (years) | 35.0 (31.0–40.0) | 35.0 (32.5–38.0) | 0.968 |
| BMI (kg/m2) | 22.7 (21.03–31.1) | 27.6 (22.6–29.4) | 0.041 |
| GWG (kg) | 10.0 (5.5–14.0) | 8.1 (5.5–10.7) | 0.062 |
| Gestational Week at delivery | 39.0 (38.0–40.0) | 39.0 (38.0–40.0) | 0.968 |
| Birth weight (g) | 3388 (3080–3590) | 3330 (3125–3537) | 0.091 |
| Birth length (cm) | 49.0 (48.0–50.0) | 49.5 (48.1–51.0) | 0.177 |
| Placental weight (g) | 600 (540–660) | 615 (525–700) | 1 |
| Cord blood glucose (mmol/L) | 4.3 (3.6–4.9) | 4.2 (4.0–5.1) | 0.723 |
| Cord blood Cholesterol (mmol/L) | 1.6 (1.5–2.1) | 1.9 (1.6–2.0) | 0.415 |
| Cord blood Cholesterol HDL (mmol/L) | 0.70 (0.65–0.75) | 0.73 (0.62–0.83) | 0.884 |
| Cord blood Cholesterol LDL (mmol/L) | 0.98 (0.65–0.96) | 0.91 (0.67–0.96) | 0.415 |
| Cord blood Triglycerides (mmol/L) | 0.40 (0.28–0.58) | 0.61 (0.36–0.69) | 0.072 |
| Cord blood Insulin (pmol/L) | 20.8 (10.8–48.1) | 26.6 (7.9–62.4) | 0.947 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klid, S.; Maymó-Masip, E.; Algaba-Chueca, F.; Ballesteros, M.; Inglès-Puig, M.; Guarque, A.; Madeira, A.; Jareño, C.; Vendrell, J.; Fernández-Veledo, S.; et al. The ANGPTL3-4-8 Axis in Normal Gestation and in Gestational Diabetes, and Its Potential Involvement in Fetal Growth. Int. J. Mol. Sci. 2023, 24, 2486. https://doi.org/10.3390/ijms24032486
Klid S, Maymó-Masip E, Algaba-Chueca F, Ballesteros M, Inglès-Puig M, Guarque A, Madeira A, Jareño C, Vendrell J, Fernández-Veledo S, et al. The ANGPTL3-4-8 Axis in Normal Gestation and in Gestational Diabetes, and Its Potential Involvement in Fetal Growth. International Journal of Molecular Sciences. 2023; 24(3):2486. https://doi.org/10.3390/ijms24032486
Chicago/Turabian StyleKlid, Sergiy, Elsa Maymó-Masip, Francisco Algaba-Chueca, Mónica Ballesteros, Montserrat Inglès-Puig, Albert Guarque, Ana Madeira, Carlos Jareño, Joan Vendrell, Sonia Fernández-Veledo, and et al. 2023. "The ANGPTL3-4-8 Axis in Normal Gestation and in Gestational Diabetes, and Its Potential Involvement in Fetal Growth" International Journal of Molecular Sciences 24, no. 3: 2486. https://doi.org/10.3390/ijms24032486






