Recent Advances on Surface-Modified GBM Targeted Nanoparticles: Targeting Strategies and Surface Characterization
Abstract
:1. Introduction
2. Targeting the BBB
3. Targeting GBM Cell Receptors
4. Dual Targeting of the BBB and GBM Cells
5. Targeting the Tumor Microenvironment (TME)
6. Surface Characterization
6.1. Electrophoretic Light Scattering
6.2. Fourier-Transform Infrared Spectroscopy
6.3. Raman Spectroscopy
6.4. Nuclear Magnetic Resonance Spectroscopy
6.5. Surface Plasmon Resonance Spectroscopy
6.6. X-ray Photoelectron Spectroscopy
6.7. Thermogravimetric Analysis
6.8. Scanning Electron Microscopy and Transmission Electron Microscopy
6.9. Other Methods
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, K.D.; Ostrom, Q.T.; Kruchko, C.; Patil, N.; Tihan, T.; Cioffi, G.; Fuchs, H.E.; Waite, K.A.; Jemal, A.; Siegel, R.L.; et al. Brain and Other Central Nervous System Tumor Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 381–406. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, A.M.; Taylor, J.W.; Wiencke, J.K.; Wrensch, M.R. Genetic and Molecular Epidemiology of Adult Diffuse Glioma. Nat. Rev. Neurol. 2019, 15, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of Glioblastoma: State of the Art and Future Directions. CA A Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- Delgado-López, P.D.; Corrales-García, E.M. Survival in Glioblastoma: A Review on the Impact of Treatment Modalities. Clin. Transl. Oncol. 2016, 18, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Noch, E.K.; Ramakrishna, R.; Magge, R. Challenges in the Treatment of Glioblastoma: Multisystem Mechanisms of Therapeutic Resistance. World Neurosurg. 2018, 116, 505–517. [Google Scholar] [CrossRef]
- Perrin, S.L.; Samuel, M.S.; Koszyca, B.; Brown, M.P.; Ebert, L.M.; Oksdath, M.; Gomez, G.A. Glioblastoma Heterogeneity and the Tumour Microenvironment: Implications for Preclinical Research and Development of New Treatments. Biochem. Soc. Trans. 2019, 47, 625–638. [Google Scholar] [CrossRef]
- Birolini, G.; Valenza, M.; Ottonelli, I.; Passoni, A.; Favagrossa, M.; Duskey, J.T.; Bombaci, M.; Vandelli, M.A.; Colombo, L.; Bagnati, R.; et al. Insights into Kinetics, Release, and Behavioral Effects of Brain-Targeted Hybrid Nanoparticles for Cholesterol Delivery in Huntington’s Disease. J. Control. Release 2021, 330, 587–598. [Google Scholar] [CrossRef]
- Pederzoli, F.; Ruozi, B.; Duskey, J.; Hagmeyer, S.; Sauer, A.K.; Grabrucker, S.; Coelho, R.; Oddone, N.; Ottonelli, I.; Daini, E.; et al. Nanomedicine Against Aβ Aggregation by β–Sheet Breaker Peptide Delivery: In Vitro Evidence. Pharmaceutics 2019, 11, 572. [Google Scholar] [CrossRef] [Green Version]
- Duskey, J.T.; da Ros, F.; Ottonelli, I.; Zambelli, B.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Enzyme Stability in Nanoparticle Preparations Part 1: Bovine Serum Albumin Improves Enzyme Function. Molecules 2020, 25, 4593. [Google Scholar] [CrossRef]
- Duskey, J.T.; Ottonelli, I.; Rinaldi, A.; Parmeggiani, I.; Zambelli, B.; Wang, L.Z.; Prud’homme, R.K.; Vandelli, M.A.; Tosi, G.; Ruozi, B. Tween® Preserves Enzyme Activity and Stability in PLGA Nanoparticles. Nanomaterials 2021, 11, 2946. [Google Scholar] [CrossRef]
- Mulvihill, J.J.; Cunnane, E.M.; Ross, A.M.; Duskey, J.T.; Tosi, G.; Grabrucker, A.M. Drug Delivery across the Blood–Brain Barrier: Recent Advances in the Use of Nanocarriers. Nanomedicine 2020, 15, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Rigon, L.; Salvalaio, M.; Pederzoli, F.; Legnini, E.; Duskey, J.T.; D’Avanzo, F.; De Filippis, C.; Ruozi, B.; Marin, O.; Vandelli, M.A.; et al. Targeting Brain Disease in MPSII: Preclinical Evaluation of IDS-Loaded PLGA Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2014. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ji, X.; He, D.; Zhang, R.; Liu, Q.; Xin, T. Nanoscale Drug Delivery Systems in Glioblastoma. Nanoscale Res. Lett. 2022, 17, 27. [Google Scholar] [CrossRef]
- Aparicio-Blanco, J.; Sanz-Arriazu, L.; Lorenzoni, R.; Blanco-Prieto, M.J. Glioblastoma Chemotherapeutic Agents Used in the Clinical Setting and in Clinical Trials: Nanomedicine Approaches to Improve Their Efficacy. Int. J. Pharm. 2020, 581, 119283. [Google Scholar] [CrossRef]
- Shabani, L.; Abbasi, M.; Amini, M.; Amani, A.M.; Vaez, A. The Brilliance of Nanoscience over Cancer Therapy: Novel Promising Nanotechnology-Based Methods for Eradicating Glioblastoma. J. Neurol. Sci. 2022, 440, 120316. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, L.D.; Duarte, J.L.; Luiz, M.T.; de Araújo, J.T.C.; Chorilli, M. Drug Delivery Nanosystems in Glioblastoma Multiforme Treatment: Current State of the Art. Curr. Neuropharmacol. 2021, 19, 787–812. [Google Scholar] [CrossRef]
- Amaral, M.; Cruz, N.; Rosa, A.; Nogueira, B.; Costa, D.; Santos, F.; Brazão, M.; Policarpo, P.; Mateus, R.; Kobozev, Y.; et al. An Update of Advanced Nanoplatforms for Glioblastoma Multiforme Management. EXCLI J. 2021, 20, 1544. [Google Scholar] [CrossRef]
- Li, J.; Zhao, J.; Tan, T.; Liu, M.; Zeng, Z.; Zeng, Y.; Zhang, L.; Fu, C.; Chen, D.; Xie, T. Nanoparticle Drug Delivery System for Glioma and Its Efficacy Improvement Strategies: A Comprehensive Review. Int. J. Nanomed. 2020, 15, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the Clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef] [PubMed]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the Blood-Brain Tumor Barrier for Effective Glioblastoma Treatment. Drug Resist. Updat. 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.-M. Transferrin Receptor-Targeted Theranostic Gold Nanoparticles for Photosensitizer Delivery in Brain Tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, K.; Knobloch, T.; Kreuter, J. Targeting the Insulin Receptor: Nanoparticles for Drug Delivery across the Blood–Brain Barrier (BBB). J. Drug Target. 2011, 19, 125–132. [Google Scholar] [CrossRef]
- He, C.; Li, J.; Cai, P.; Ahmed, T.; Henderson, J.T.; Foltz, W.D.; Bendayan, R.; Rauth, A.M.; Wu, X.Y. Two-Step Targeted Hybrid Nanoconstructs Increase Brain Penetration and Efficacy of the Therapeutic Antibody Trastuzumab against Brain Metastasis of HER2-Positive Breast Cancer. Adv. Funct. Mater. 2018, 28, 1705668. [Google Scholar] [CrossRef]
- Qiao, R.; Jia, Q.; Hüwel, S.; Xia, R.; Liu, T.; Gao, F.; Galla, H.-J.; Gao, M. Receptor-Mediated Delivery of Magnetic Nanoparticles across the Blood–Brain Barrier. ACS Nano 2012, 6, 3304–3310. [Google Scholar] [CrossRef]
- Wei, X.; Zhan, C.; Shen, Q.; Fu, W.; Xie, C.; Gao, J.; Peng, C.; Zheng, P.; Lu, W. A D-Peptide Ligand of Nicotine Acetylcholine Receptors for Brain-Targeted Drug Delivery. Angew. Chem. Int. Ed. 2015, 54, 3023–3027. [Google Scholar] [CrossRef]
- Jiang, X.; Xin, H.; Ren, Q.; Gu, J.; Zhu, L.; Du, F.; Feng, C.; Xie, Y.; Sha, X.; Fang, X. Nanoparticles of 2-Deoxy-d-Glucose Functionalized Poly(Ethylene Glycol)-Co-Poly(Trimethylene Carbonate) for Dual-Targeted Drug Delivery in Glioma Treatment. Biomaterials 2014, 35, 518–529. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Kuang, Y.; An, S.; Ma, H.; Jiang, C. Choline Transporter-Targeting and Co-Delivery System for Glioma Therapy. Biomaterials 2013, 34, 9142–9148. [Google Scholar] [CrossRef]
- Han, L.; Liu, C.; Qi, H.; Zhou, J.; Wen, J.; Wu, D.; Xu, D.; Qin, M.; Ren, J.; Wang, Q.; et al. Systemic Delivery of Monoclonal Antibodies to the Central Nervous System for Brain Tumor Therapy. Adv. Mater. 2019, 31, 1805697. [Google Scholar] [CrossRef] [PubMed]
- Formicola, B.; Dal Magro, R.; Montefusco-Pereira, C.V.; Lehr, C.-M.; Koch, M.; Russo, L.; Grasso, G.; Deriu, M.A.; Danani, A.; Bourdoulous, S.; et al. The Synergistic Effect of Chlorotoxin-MApoE in Boosting Drug-Loaded Liposomes across the BBB. J. Nanobiotechnol. 2019, 17, 115. [Google Scholar] [CrossRef]
- Galstyan, A.; Markman, J.L.; Shatalova, E.S.; Chiechi, A.; Korman, A.J.; Patil, R.; Klymyshyn, D.; Tourtellotte, W.G.; Israel, L.L.; Braubach, O.; et al. Blood–Brain Barrier Permeable Nano Immunoconjugates Induce Local Immune Responses for Glioma Therapy. Nat. Commun. 2019, 10, 3850. [Google Scholar] [CrossRef] [Green Version]
- Jnaidi, R.; Almeida, A.J.; Gonçalves, L.M. Solid Lipid Nanoparticles and Nanostructured Lipid Carriers as Smart Drug Delivery Systems in the Treatment of Glioblastoma Multiforme. Pharmaceutics 2020, 12, 860. [Google Scholar] [CrossRef]
- Wadajkar, A.S.; Dancy, J.G.; Hersh, D.S.; Anastasiadis, P.; Tran, N.L.; Woodworth, G.F.; Winkles, J.A.; Kim, A.J. Tumor-targeted Nanotherapeutics: Overcoming Treatment Barriers for Glioblastoma. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1439. [Google Scholar] [CrossRef]
- Luiz, M.T.; Delello Di Filippo, L.; Tofani, L.B.; de Araújo, J.T.C.; Dutra, J.A.P.; Marchetti, J.M.; Chorilli, M. Highlights in Targeted Nanoparticles as a Delivery Strategy for Glioma Treatment. Int. J. Pharm. 2021, 604, 120758. [Google Scholar] [CrossRef]
- Raucher, D. Tumor Targeting Peptides: Novel Therapeutic Strategies in Glioblastoma. Curr. Opin. Pharmacol. 2019, 47, 14–19. [Google Scholar] [CrossRef]
- Guo, P.; Moses-Gardner, A.; Huang, J.; Smith, E.R.; Moses, M.A. ITGA2 as a Potential Nanotherapeutic Target for Glioblastoma. Sci. Rep. 2019, 9, 6195. [Google Scholar] [CrossRef] [Green Version]
- Duskey, J.T.; Rinaldi, A.; Ottonelli, I.; Caraffi, R.; De Benedictis, C.A.; Sauer, A.K.; Tosi, G.; Vandelli, M.A.; Ruozi, B.; Grabrucker, A.M. Glioblastoma Multiforme Selective Nanomedicines for Improved Anti-Cancer Treatments. Pharmaceutics 2022, 14, 1450. [Google Scholar] [CrossRef]
- Zhao, Y.-L.; Song, J.-N.; Zhang, M. Role of Caveolin-1 in the Biology of the Blood-Brain Barrier. Rev. Neurosci. 2014, 25, 247–254. [Google Scholar] [CrossRef]
- Kang, R.H.; Jang, J.-E.; Huh, E.; Kang, S.J.; Ahn, D.-R.; Kang, J.S.; Sailor, M.J.; Yeo, S.G.; Oh, M.S.; Kim, D.; et al. A Brain Tumor-Homing Tetra-Peptide Delivers a Nano-Therapeutic for More Effective Treatment of a Mouse Model of Glioblastoma. Nanoscale Horiz. 2020, 5, 1213–1225. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.H.; Park, J.; Kim, J.; Chowdhury, T.; Oh, J.H.; Kim, J.; Shin, J.; Kim, M.; Park, C.-K.; Lee, S.; et al. A Deep Dive: SIWV Tetra-Peptide Enhancing the Penetration of Nanotherapeutics into the Glioblastoma. ACS Biomater. Sci. Eng. 2022, 8, 4163–4174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, J.; Li, Z.; Zhang, W.; Wang, F.; Zhang, B. Recent Advances in CXCL12/CXCR4 Antagonists and Nano-Based Drug Delivery Systems for Cancer Therapy. Pharmaceutics 2022, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-T.; Huang, H.-C.; Chung, C.-W.; Chiang, C.-C.; Hsia, T.; Wu, H.-F.; Huang, R.-L.; Chiang, C.-S.; Wang, J.; Lu, T.-T.; et al. CXCR4-Targeted Nitric Oxide Nanoparticles Deliver PD-L1 SiRNA for Immunotherapy against Glioblastoma. J. Control. Release 2022, 352, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Chang, Y.-H.; Rajesh, R. Targeted Delivery of Etoposide, Carmustine and Doxorubicin to Human Glioblastoma Cells Using Methoxy Poly(Ethylene Glycol)-poly(Ε-caprolactone) Nanoparticles Conjugated with Wheat Germ Agglutinin and Folic Acid. Mater. Sci. Eng. C 2019, 96, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Lu, L.; Zhou, J.; Ran, D.; Wang, S.; Xu, Q.; Xu, W.; Wang, J.; Liu, Y.; Xie, C.; et al. All-Stage Targeted Therapy for Glioblastoma Based on Lipid Membrane Coated Cabazitaxel Nanocrystals. J. Control. Release 2022, 345, 685–695. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Y.; Gao, L.; Mosel, S.; Ehlers, M.; Zellermann, E.; Jiang, H.; Knauer, S.K.; Wang, L.; Schmuck, C. From Supramolecular Vesicles to Micelles: Controllable Construction of Tumor-Targeting Nanocarriers Based on Host–Guest Interaction between a Pillar[5]Arene-Based Prodrug and a RGD-Sulfonate Guest. Small 2018, 14, 1803952. [Google Scholar] [CrossRef]
- Qi, N.; Zhang, S.; Zhou, X.; Duan, W.; Gao, D.; Feng, J.; Li, A. Combined Integrin Avβ3 and Lactoferrin Receptor Targeted Docetaxel Liposomes Enhance the Brain Targeting Effect and Anti-Glioma Effect. J. Nanobiotechnol. 2021, 19, 446. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Yao, S.; Wang, S.; Wang, R.; Xie, C.; Guo, H.; Lu, W. Stapled RAP12 Peptide Ligand of LRP1 for Micelles-Based Multifunctional Glioma-Targeted Drug Delivery. J. Chem. Eng. 2021, 403, 126296. [Google Scholar] [CrossRef]
- Li, Y.; Pan, Y.; Wang, Y.; Jiang, Z.; Akakuru, O.U.; Li, M.; Zhang, X.; Yuan, B.; Xing, J.; Luo, L.; et al. A D-Peptide Ligand of Neuropeptide Y Receptor Y1 Serves as Nanocarrier Traversing of the Blood Brain Barrier and Targets Glioma. Nano Today 2022, 44, 101465. [Google Scholar] [CrossRef]
- Han, M.; Xing, H.; Chen, L.; Cui, M.; Zhang, Y.; Qi, L.; Jin, M.; Yang, Y.; Gao, C.; Gao, Z.; et al. Efficient Antiglioblastoma Therapy in Mice through Doxorubicin-Loaded Nanomicelles Modified Using a Novel Brain-Targeted RVG-15 Peptide. J. Drug Target 2021, 29, 1016–1028. [Google Scholar] [CrossRef]
- Xin, X.; Liu, W.; Zhang, Z.-A.; Han, Y.; Qi, L.-L.; Zhang, Y.-Y.; Zhang, X.-T.; Duan, H.-X.; Chen, L.-Q.; Jin, M.-J.; et al. Efficient Anti-Glioma Therapy Through the Brain-Targeted RVG15-Modified Liposomes Loading Paclitaxel-Cholesterol Complex. Int. J. Nanomed. 2021, 16, 5755–5776. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chai, Z.; Lu, J.; Xie, C.; Ran, D.; Wang, S.; Zhou, J.; Lu, W. αvβ3-Targeted Liposomal Drug Delivery System with Attenuated Immunogenicity Enabled by Linear Pentapeptide for Glioma Therapy. J. Control. Release 2020, 322, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Huo, T.; Yang, Y.; Qian, M.; Jiang, H.; Du, Y.; Zhang, X.; Xie, Y.; Huang, R. Versatile Hollow COF Nanospheres via Manipulating Transferrin Corona for Precise Glioma-Targeted Drug Delivery. Biomaterials 2020, 260, 120305. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.K.; Behera, C.; Chakraborty, S.; Sandha, K.K.; Goswami, A.; Gupta, P.N. Tumor Micro-Environment Targeted Collagenase-Modified Albumin Nanoparticles for Improved Drug Delivery. J. Drug Deliv. Sci. Technol. 2022, 71, 103366. [Google Scholar] [CrossRef]
- Skóra, B.; Piechowiak, T.; Szychowski, K.A. Epidermal Growth Factor-Labeled Liposomes as a Way to Target the Toxicity of Silver Nanoparticles into EGFR-Overexpressing Cancer Cells in Vitro. Toxicol. Appl. Pharmacol. 2022, 443, 116009. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xiang, L.; Fang, C.; Tan, Y.; Li, Y.; Gong, T.; Wu, Q.; Gong, T.; Zhang, Z. Dual-Targeting of Tumor Cells and Tumor-Associated Macrophages by Palmitic Acid Modified Albumin Nanoparticles for Antitumor and Antimetastasis Therapy. ACS Appl. Mater. Interfaces 2022, 14, 14887–14902. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, H.; Qiu, W.; Han, Y.; Liu, H.; Li, Z. Biomimetic Nanoparticles Directly Remodel Immunosuppressive Microenvironment for Boosting Glioblastoma Immunotherapy. Bioact. Mater. 2022, 16, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Fan, Y.; Lv, Y.; Liu, J.; Yu, N.; Kong, D.; Sun, H.; Li, J. A Prodrug Hydrogel with Tumor Microenvironment and Near-Infrared Light Dual-Responsive Action for Synergistic Cancer Immunotherapy. Acta Biomater. 2022, 149, 334–346. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Zhao, X.; Yang, C.; Shi, M.; Zhang, B.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. Binary Regulation of the Tumor Microenvironment by a PH-Responsive Reversible Shielding Nanoplatform for Improved Tumor Chemo-Immunotherapy. Acta Biomater. 2022, 138, 505–517. [Google Scholar] [CrossRef]
- Izraely, S.; Witz, I.P. Site-Specific Metastasis: A Cooperation between Cancer Cells and the Metastatic Microenvironment. Int. J. Cancer 2021, 148, 1308–1322. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Kim, J.; Kim, H.; Choi, N.; Chung, S. Microfluidic Reconstitution of Tumor Microenvironment for Nanomedical Applications. Adv. Healthc. Mater. 2021, 10, 2002122. [Google Scholar] [CrossRef]
- Wang, W.; Jin, Y.; Liu, X.; Chen, F.; Zheng, X.; Liu, T.; Yang, Y.; Yu, H. Endogenous Stimuli-Activatable Nanomedicine for Immune Theranostics for Cancer. Adv. Funct. Mater. 2021, 31, 2100386. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR Effect and beyond: Strategies to Improve Tumor Targeting and Cancer Nanomedicine Treatment Efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Leroux, J.-C. Editorial: Drug Delivery: Too Much Complexity, Not Enough Reproducibility. Angew. Chem. Int. Ed. 2017, 56, 15170–15171. [Google Scholar] [CrossRef] [Green Version]
- Danhier, F. To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Nakamura, Y.; Mochida, A.; Choyke, P.L.; Kobayashi, H. Nanodrug Delivery: Is the Enhanced Permeability and Retention Effect Sufficient for Curing Cancer? Bioconjug. Chem. 2016, 27, 2225–2238. [Google Scholar] [CrossRef] [PubMed]
- de Lázaro, I.; Mooney, D.J. A Nanoparticle’s Pathway into Tumours. Nat. Mater. 2020, 19, 486–487. [Google Scholar] [CrossRef]
- Golombek, S.K.; May, J.-N.; Theek, B.; Appold, L.; Drude, N.; Kiessling, F.; Lammers, T. Tumor Targeting via EPR: Strategies to Enhance Patient Responses. Adv. Drug Deliv. Rev. 2018, 130, 17–38. [Google Scholar] [CrossRef]
- Park, J.; Choi, Y.; Chang, H.; Um, W.; Ryu, J.H.; Kwon, I.C. Alliance with EPR Effect: Combined Strategies to Improve the EPR Effect in the Tumor Microenvironment. Theranostics 2019, 9, 8073–8090. [Google Scholar] [CrossRef]
- Kim, J.; Hong, J.; Lee, J.; Fakhraei Lahiji, S.; Kim, Y.-H. Recent Advances in Tumor Microenvironment-Targeted Nanomedicine Delivery Approaches to Overcome Limitations of Immune Checkpoint Blockade-Based Immunotherapy. J. Control. Release 2021, 332, 109–126. [Google Scholar] [CrossRef]
- Li, J.; Burgess, D.J. Nanomedicine-Based Drug Delivery towards Tumor Biological and Immunological Microenvironment. Acta Pharm. Sin. B 2020, 10, 2110–2124. [Google Scholar] [CrossRef]
- Fu, S.; Xu, X.; Ma, Y.; Zhang, S.; Zhang, S. RGD Peptide-Based Non-Viral Gene Delivery Vectors Targeting Integrin Avβ3 for Cancer Therapy. J. Drug Target 2019, 27, 1–11. [Google Scholar] [CrossRef]
- Lv, Y.; Xu, C.; Zhao, X.; Lin, C.; Yang, X.; Xin, X.; Zhang, L.; Qin, C.; Han, X.; Yang, L.; et al. Nanoplatform Assembled from a CD44-Targeted Prodrug and Smart Liposomes for Dual Targeting of Tumor Microenvironment and Cancer Cells. ACS Nano 2018, 12, 1519–1536. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Shin, M.; Kim, S.A.; Lee, S.; Kim, H.; Koo, H.; Kim, B.-S.; Song, H.K.; Kim, S.H.; Choi, K.; et al. In Vivo Fluorescence Imaging for Cancer Diagnosis Using Receptor-Targeted Epidermal Growth Factor-Based Nanoprobe. Biomaterials 2013, 34, 9149–9159. [Google Scholar] [CrossRef] [PubMed]
- Zalba, S.; Contreras, A.M.; Merino, M.; Navarro, I.; de Ilarduya, C.T.; Trocóniz, I.F.; Koning, G.; Garrido, M.J. EGF-Liposomes Promote Efficient EGFR Targeting in Xenograft Colocarcinoma Model. Nanomedicine 2016, 11, 465–477. [Google Scholar] [CrossRef]
- Zhang, Y.; Elechalawar, C.K.; Yang, W.; Frickenstein, A.N.; Asfa, S.; Fung, K.-M.; Murphy, B.N.; Dwivedi, S.K.; Rao, G.; Dey, A.; et al. Disabling Partners in Crime: Gold Nanoparticles Disrupt Multicellular Communications within the Tumor Microenvironment to Inhibit Ovarian Tumor Aggressiveness. Mater. Today 2022, 56, 79–95. [Google Scholar] [CrossRef]
- Ambasta, R.K.; Sharma, A.; Kumar, P. Nanoparticle Mediated Targeting of VEGFR and Cancer Stem Cells for Cancer Therapy. Vasc. Cell 2011, 3, 26. [Google Scholar] [CrossRef] [Green Version]
- Gysler, S.M.; Drapkin, R. Tumor Innervation: Peripheral Nerves Take Control of the Tumor Microenvironment. J. Clin. Investig. 2021, 131, e147276. [Google Scholar] [CrossRef]
- Hernandez, S.; Serrano, A.G.; Solis Soto, L.M. The Role of Nerve Fibers in the Tumor Immune Microenvironment of Solid Tumors. Adv. Biol. 2022, 6, 2200046. [Google Scholar] [CrossRef] [PubMed]
- Zahalka, A.H.; Arnal-Estapé, A.; Maryanovich, M.; Nakahara, F.; Cruz, C.D.; Finley, L.W.S.; Frenette, P.S. Adrenergic Nerves Activate an Angio-Metabolic Switch in Prostate Cancer. Science 2017, 358, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, J.; Sun, C.; Wang, Z.; Chu, Z.; Liu, C.; Xu, X.; Xia, M.; Zhao, M.; Wang, C. Sequential Receptor–Mediated Mixed-Charge Nanomedicine to Target Pancreatic Cancer, Inducing Immunogenic Cell Death and Reshaping the Tumor Microenvironment. Int. J. Pharm. 2021, 601, 120553. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Liu, P.; Pan, W.; Singh, S.R.; Wei, Y. Hypoxia and Hypoxia Inducible Factors in Tumor Metabolism. Cancer Lett. 2015, 356, 263–267. [Google Scholar] [CrossRef]
- Rinaldi, A.; Caraffi, R.; Grazioli, M.V.; Oddone, N.; Giardino, L.; Tosi, G.; Vandelli, M.A.; Calzà, L.; Ruozi, B.; Duskey, J.T. Applications of the ROS-Responsive Thioketal Linker for the Production of Smart Nanomedicines. Polymers 2022, 14, 687. [Google Scholar] [CrossRef]
- Oddone, N.; Pederzoli, F.; Duskey, J.T.; De Benedictis, C.A.; Grabrucker, A.M.; Forni, F.; Angela Vandelli, M.; Ruozi, B.; Tosi, G. ROS-Responsive “Smart” Polymeric Conjugate: Synthesis, Characterization and Proof-of-Concept Study. Int. J. Pharm. 2019, 570, 118655. [Google Scholar] [CrossRef]
- Oddone, N.; Boury, F.; Garcion, E.; Grabrucker, A.M.; Martinez, M.C.; Da Ros, F.; Janaszewska, A.; Forni, F.; Vandelli, M.A.; Tosi, G.; et al. Synthesis, Characterization, and In Vitro Studies of an Reactive Oxygen Species (ROS)-Responsive Methoxy Polyethylene Glycol-Thioketal-Melphalan Prodrug for Glioblastoma Treatment. Front. Pharmacol. 2020, 11, 574. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, H.; Huang, G.; Liu, J.; He, W.; Huang, Q. ES-MION-Based Dual-Modality PET/MRI Probes for Acidic Tumor Microenvironment Imaging. ACS Omega 2022, 7, 3442–3451. [Google Scholar] [CrossRef]
- Koo, S.; Park, O.K.; Kim, J.; Han, S.I.; Yoo, T.Y.; Lee, N.; Kim, Y.G.; Kim, H.; Lim, C.; Bae, J.-S.; et al. Enhanced Chemodynamic Therapy by Cu–Fe Peroxide Nanoparticles: Tumor Microenvironment-Mediated Synergistic Fenton Reaction. ACS Nano 2022, 16, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, N.; Lee, J.-S.; Liman, R.A.D.; Ruallo, J.M.S.; Villaflores, O.B.; Ger, T.-R.; Hsiao, C.-D. Potential Toxicity of Iron Oxide Magnetic Nanoparticles: A Review. Molecules 2020, 25, 3159. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Liu, Y.; Huang, J.; Chen, K.; Huang, J.; Xiao, K. Uptake, Distribution, Clearance, and Toxicity of Iron Oxide Nanoparticles with Different Sizes and Coatings. Sci. Rep. 2018, 8, 2082. [Google Scholar] [CrossRef] [Green Version]
- Kalbasi, A.; Ribas, A. Tumour-Intrinsic Resistance to Immune Checkpoint Blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, X.; Xu, J.; Qiu, N.; Zhai, G. Nanotechnology for Boosting Cancer Immunotherapy and Remodeling Tumor Microenvironment: The Horizons in Cancer Treatment. ACS Nano 2021, 15, 12567–12603. [Google Scholar] [CrossRef] [PubMed]
- Ngamcherdtrakul, W.; Bejan, D.S.; Cruz-Muñoz, W.; Reda, M.; Zaidan, H.Y.; Siriwon, N.; Marshall, S.; Wang, R.; Nelson, M.A.; Rehwaldt, J.P.C.; et al. Targeted Nanoparticle for Co-Delivery of HER2 SiRNA and a Taxane to Mirror the Standard Treatment of HER2+ Breast Cancer: Efficacy in Breast Tumor and Brain Metastasis. Small 2022, 18, 2107550. [Google Scholar] [CrossRef]
- Walens, A.; DiMarco, A.V.; Lupo, R.; Kroger, B.R.; Damrauer, J.S.; Alvarez, J.V. CCL5 Promotes Breast Cancer Recurrence through Macrophage Recruitment in Residual Tumors. eLife 2019, 8, e43653. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, X.; Huang, L. Macrophage-Mediated Tumor Cell Phagocytosis: Opportunity for Nanomedicine Intervention. Adv. Funct. Mater. 2021, 31, 2006220. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of Response, Resistance, and Toxicity to Immune Checkpoint Blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- Kubli, S.P.; Berger, T.; Araujo, D.V.; Siu, L.L.; Mak, T.W. Beyond Immune Checkpoint Blockade: Emerging Immunological Strategies. Nat. Rev. Drug Discov. 2021, 20, 899–919. [Google Scholar] [CrossRef] [PubMed]
- Alghamri, M.S.; Banerjee, K.; Mujeeb, A.A.; Mauser, A.; Taher, A.; Thalla, R.; McClellan, B.L.; Varela, M.L.; Stamatovic, S.M.; Martinez-Revollar, G.; et al. Systemic Delivery of an Adjuvant CXCR4–CXCL12 Signaling Inhibitor Encapsulated in Synthetic Protein Nanoparticles for Glioma Immunotherapy. ACS Nano 2022, 16, 8729–8750. [Google Scholar] [CrossRef] [PubMed]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B Cell Regulation in Cancer and Anti-Tumor Immunity. Cell Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef]
- Shen, L.; Li, J.; Liu, Q.; Das, M.; Song, W.; Zhang, X.; Tiruthani, K.; Dorosheva, O.; Hu, H.; Lai, S.K.; et al. Nano-Trapping CXCL13 Reduces Regulatory B Cells in Tumor Microenvironment and Inhibits Tumor Growth. J. Control. Release 2022, 343, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T.K. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaliva, M.; Vamvakaki, M. Nanomaterials Characterization. In Polymer Science and Nanotechnology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 401–433. ISBN 978-0-12-816806-6. [Google Scholar]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef] [PubMed]
- Baer, D.R.; Engelhard, M.H.; Johnson, G.E.; Laskin, J.; Lai, J.; Mueller, K.; Munusamy, P.; Thevuthasan, S.; Wang, H.; Washton, N.; et al. Surface Characterization of Nanomaterials and Nanoparticles: Important Needs and Challenging Opportunities. J. Vac. Sci. Technol. 2013, 31, 050820. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Stellacci, F. Effect of Surface Properties on Nanoparticle–Cell Interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef]
- Smith, A.M.; Johnston, K.A.; Crawford, S.E.; Marbella, L.E.; Millstone, J.E. Ligand Density Quantification on Colloidal Inorganic Nanoparticles. Analyst 2017, 142, 11–29. [Google Scholar] [CrossRef]
- Jayawardena, H.S.N.; Liyanage, S.H.; Rathnayake, K.; Patel, U.; Yan, M. Analytical Methods for Characterization of Nanomaterial Surfaces. Anal. Chem. 2021, 93, 1889–1911. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Elsevier: Amsterdam, The Netherlands, 2017; pp. 43–58. ISBN 978-0-08-100557-6. [Google Scholar]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Silva, A.M.; Martins-Gomes, C.; Coutinho, T.E.; Fangueiro, J.F.; Sanchez-Lopez, E.; Pashirova, T.N.; Andreani, T.; Souto, E.B. Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef] [Green Version]
- Ottonelli, I.; Bighinati, A.; Adani, E.; Loll, F.; Caraffi, R.; Vandelli, M.A.; Boury, F.; Tosi, G.; Duskey, J.T.; Marigo, V.; et al. Optimization of an Injectable Hydrogel Depot System for the Controlled Release of Retinal-Targeted Hybrid Nanoparticles. Pharmaceutics 2023, 15, 25. [Google Scholar] [CrossRef]
- Martens, T.F.; Remaut, K.; Deschout, H.; Engbersen, J.F.J.; Hennink, W.E.; van Steenbergen, M.J.; Demeester, J.; De Smedt, S.C.; Braeckmans, K. Coating Nanocarriers with Hyaluronic Acid Facilitates Intravitreal Drug Delivery for Retinal Gene Therapy. J. Control. Release 2015, 202, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Mester, L.; Govyadinov, A.A.; Chen, S.; Goikoetxea, M.; Hillenbrand, R. Subsurface Chemical Nanoidentification by Nano-FTIR Spectroscopy. Nat. Commun. 2020, 11, 3359. [Google Scholar] [CrossRef]
- Ţucureanu, V.; Matei, A.; Avram, A.M. FTIR Spectroscopy for Carbon Family Study. Crit. Rev. Anal. Chem. 2016, 46, 502–520. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR Analysis of Natural and Synthetic Collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Guerrero-Pérez, M.O.; Patience, G.S. Experimental Methods in Chemical Engineering: Fourier Transform Infrared Spectroscopy—FTIR. Can. J. Chem. Eng. 2020, 98, 25–33. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Li, J.; Tang, J.; Li, B.; Zhang, Y.; Gu, N.; Yang, F. Sphingosine 1-Phosphate Liposomes for Targeted Nitric Oxide Delivery to Mediate Anticancer Effects against Brain Glioma Tumors. Adv. Mater. 2021, 33, 2101701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; van Os, W.L.; Tian, X.; Zu, G.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Development of Curcumin-Loaded Zein Nanoparticles for Transport across the Blood–Brain Barrier and Inhibition of Glioblastoma Cell Growth. Biomater. Sci. 2021, 9, 7092–7103. [Google Scholar] [CrossRef] [PubMed]
- Luque-Michel, E.; Sebastian, V.; Larrea, A.; Marquina, C.; Blanco-Prieto, M.J. Co-Encapsulation of Superparamagnetic Nanoparticles and Doxorubicin in PLGA Nanocarriers: Development, Characterization and in Vitro Antitumor Efficacy in Glioma Cells. Eur. J. Pharm. Biopharm. 2019, 145, 65–75. [Google Scholar] [CrossRef]
- Budama-Kilinc, Y.; Kecel-Gunduz, S.; Cakir-Koc, R.; Aslan, B.; Bicak, B.; Kokcu, Y.; Ozel, A.E.; Akyuz, S. Structural Characterization and Drug Delivery System of Natural Growth-Modulating Peptide Against Glioblastoma Cancer. Int. J. Pept. Res. Ther. 2021, 27, 2015–2028. [Google Scholar] [CrossRef]
- Mazarei, M.; Mohammadi Arvejeh, P.; Mozafari, M.R.; Khosravian, P.; Ghasemi, S. Anticancer Potential of Temozolomide-Loaded Eudragit-Chitosan Coated Selenium Nanoparticles: In Vitro Evaluation of Cytotoxicity, Apoptosis and Gene Regulation. Nanomaterials 2021, 11, 1704. [Google Scholar] [CrossRef]
- Ortiz-Islas, E.; Sosa-Arróniz, A.; Manríquez-Ramírez, M.E.; Rodríguez-Pérez, C.E.; Tzompantzi, F.; Padilla, J.M. Mesoporous Silica Nanoparticles Functionalized with Folic Acid for Targeted Release Cis-Pt to Glioblastoma Cells. Rev. Adv. Mater. Sci. 2021, 60, 25–37. [Google Scholar] [CrossRef]
- Carissimi, G.; Montalbán, M.G.; Víllora, G.; Barth, A. Direct Quantification of Drug Loading Content in Polymeric Nanoparticles by Infrared Spectroscopy. Pharmaceutics 2020, 12, 912. [Google Scholar] [CrossRef] [PubMed]
- Paudel, A.; Raijada, D.; Rantanen, J. Raman Spectroscopy in Pharmaceutical Product Design. Adv. Drug Deliv. Rev. 2015, 89, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gualerzi, A.; Picciolini, S.; Carlomagno, C.; Terenzi, F.; Ramat, S.; Sorbi, S.; Bedoni, M. Raman Profiling of Circulating Extracellular Vesicles for the Stratification of Parkinson’s Patients. Nanomedicine 2019, 22, 102097. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.S.; Chung, A.J.; Erickson, D. Surface Enhanced Raman Spectroscopy and Its Application to Molecular and Cellular Analysis. Microfluid Nanofluid 2009, 6, 285–297. [Google Scholar] [CrossRef]
- Sahli, F.; Courcelle, M.; Palama, T.; Djaker, N.; Savarin, P.; Spadavecchia, J. Temozolomide, Gemcitabine, and Decitabine Hybrid Nanoconjugates: From Design to Proof-of-Concept (PoC) of Synergies toward the Understanding of Drug Impact on Human Glioblastoma Cells. J. Med. Chem. 2020, 63, 7410–7421. [Google Scholar] [CrossRef]
- Depciuch, J.; Miszczyk, J.; Maximenko, A.; Zielinski, P.M.; Rawojć, K.; Panek, A.; Olko, P.; Parlinska-Wojtan, M. Gold Nanopeanuts as Prospective Support for Cisplatin in Glioblastoma Nano-Chemo-Radiotherapy. Int. J. Mol. Sci. 2020, 21, 9082. [Google Scholar] [CrossRef]
- Marbella, L.E.; Millstone, J.E. NMR Techniques for Noble Metal Nanoparticles. Chem. Mater. 2015, 27, 2721–2739. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Fuentes, M.; Torres, D.; Martín-Pastor, M.; Alonso, M.J. Application of NMR Spectroscopy to the Characterization of PEG-Stabilized Lipid Nanoparticles. Langmuir 2004, 20, 8839–8845. [Google Scholar] [CrossRef]
- Marchetti, A.; Chen, J.; Pang, Z.; Li, S.; Ling, D.; Deng, F.; Kong, X. Understanding Surface and Interfacial Chemistry in Functional Nanomaterials via Solid-State NMR. Adv. Mater. 2017, 29, 1605895. [Google Scholar] [CrossRef]
- Pucci, C.; De Pasquale, D.; Marino, A.; Martinelli, C.; Lauciello, S.; Ciofani, G. Hybrid Magnetic Nanovectors Promote Selective Glioblastoma Cell Death through a Combined Effect of Lysosomal Membrane Permeabilization and Chemotherapy. ACS Appl. Mater. Interfaces 2020, 12, 29037–29055. [Google Scholar] [CrossRef] [PubMed]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G.; et al. PLGA-PEG-ANG-2 Nanoparticles for Blood-Brain Barrier Crossing: Proof-of-Concept Study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emamgholizadeh Minaei, S.; Khoei, S.; Khoee, S.; Karimi, M.R. Tri-Block Copolymer Nanoparticles Modified with Folic Acid for Temozolomide Delivery in Glioblastoma. Int. J. Biochem. Cell Biol. 2019, 108, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Vilella, A.; Tosi, G.; Grabrucker, A.M.; Ruozi, B.; Belletti, D.; Vandelli, M.A.; Boeckers, T.M.; Forni, F.; Zoli, M. Insight on the Fate of CNS-Targeted Nanoparticles. Part I: Rab5-Dependent Cell-Specific Uptake and Distribution. J. Control. Release 2014, 174, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Picciolini, S.; Gualerzi, A.; Carlomagno, C.; Cabinio, M.; Sorrentino, S.; Baglio, F.; Bedoni, M. An SPRi-Based Biosensor Pilot Study: Analysis of Multiple Circulating Extracellular Vesicles and Hippocampal Volume in Alzheimer’s Disease. J. Pharm. Biomed. Anal. 2021, 192, 113649. [Google Scholar] [CrossRef]
- Crielaard, B.J.; Yousefi, A.; Schillemans, J.P.; Vermehren, C.; Buyens, K.; Braeckmans, K.; Lammers, T.; Storm, G. An in Vitro Assay Based on Surface Plasmon Resonance to Predict the in Vivo Circulation Kinetics of Liposomes. J. Control. Release 2011, 156, 307–314. [Google Scholar] [CrossRef]
- Gregori, M.; Taylor, M.; Salvati, E.; Re, F.; Mancini, S.; Balducci, C.; Forloni, G.; Zambelli, V.; Sesana, S.; Michael, M.; et al. Retro-Inverso Peptide Inhibitor Nanoparticles as Potent Inhibitors of Aggregation of the Alzheimer’s Aβ Peptide. Nanomedicine 2017, 13, 723–732. [Google Scholar] [CrossRef] [Green Version]
- Gries, M.; Thomas, N.; Daouk, J.; Rocchi, P.; Choulier, L.; Jubréaux, J.; Pierson, J.; Reinhard, A.; Jouan-Hureaux, V.; Chateau, A.; et al. Multiscale Selectivity and in Vivo Biodistribution of NRP-1-Targeted Theranostic AGuIX Nanoparticles for PDT of Glioblastoma. Int. J. Nanomed. 2020, 15, 8739–8758. [Google Scholar] [CrossRef]
- Gabold, B.; Adams, F.; Brameyer, S.; Jung, K.; Ried, C.L.; Merdan, T.; Merkel, O.M. Transferrin-Modified Chitosan Nanoparticles for Targeted Nose-to-Brain Delivery of Proteins. Drug Deliv. Transl. Res. 2022. [Google Scholar] [CrossRef]
- Krishna, D.N.G.; Philip, J. Review on Surface-Characterization Applications of X-Ray Photoelectron Spectroscopy (XPS): Recent Developments and Challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Pulvirenti, L.; Monforte, F.; Lo Presti, F.; Li Volti, G.; Carota, G.; Sinatra, F.; Bongiorno, C.; Mannino, G.; Cambria, M.T.; Condorelli, G.G. Synthesis of MIL-Modified Fe3O4 Magnetic Nanoparticles for Enhancing Uptake and Efficiency of Temozolomide in Glioblastoma Treatment. Int. J. Mol. Sci. 2022, 23, 2874. [Google Scholar] [CrossRef]
- Venditti, I.; Cartoni, A.; Cerra, S.; Fioravanti, R.; Salamone, T.A.; Sciubba, F.; Tabocchini, M.A.; Dini, V.; Battocchio, C.; Iucci, G.; et al. Hydrophilic Gold Nanoparticles as Anti-PD-L1 Antibody Carriers: Synthesis and Interface Properties. Part. Part. Syst. Charact. 2022, 39, 2100282. [Google Scholar] [CrossRef]
- Saadatkhah, N.; Carillo Garcia, A.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental Methods in Chemical Engineering: Thermogravimetric Analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Mansfield, E.; Tyner, K.M.; Poling, C.M.; Blacklock, J.L. Determination of Nanoparticle Surface Coatings and Nanoparticle Purity Using Microscale Thermogravimetric Analysis. Anal. Chem. 2014, 86, 1478–1484. [Google Scholar] [CrossRef]
- Bannov, A.G.; Popov, M.V.; Kurmashov, P.B. Thermal Analysis of Carbon Nanomaterials: Advantages and Problems of Interpretation. J. Therm. Anal. Calorim. 2020, 142, 349–370. [Google Scholar] [CrossRef]
- Świętek, M.; Ma, Y.-H.; Wu, N.-P.; Paruzel, A.; Tokarz, W.; Horák, D. Tannic Acid Coating Augments Glioblastoma Cellular Uptake of Magnetic Nanoparticles with Antioxidant Effects. Nanomaterials 2022, 12, 1310. [Google Scholar] [CrossRef] [PubMed]
- Shahein, S.A.; Aboul-Enein, A.M.; Higazy, I.M.; Abou-Elella, F.; Lojkowski, W.; Ahmed, E.R.; Mousa, S.A.; AbouAitah, K. Targeted Anticancer Potential against Glioma Cells of Thymoquinone Delivered by Mesoporous Silica Core-Shell Nanoformulations with PH-Dependent Release. Int. J. Nanomed. 2019, 14, 5503–5526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.J. Chapter 1. Characterization of Nanomaterials Using Transmission Electron Microscopy. In Nanoscience & Nanotechnology Series; Kirkland, A.I., Haigh, S.J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 1–29. ISBN 978-1-84973-805-7. [Google Scholar]
- Michen, B.; Geers, C.; Vanhecke, D.; Endes, C.; Rothen-Rutishauser, B.; Balog, S.; Petri-Fink, A. Avoiding Drying-Artifacts in Transmission Electron Microscopy: Characterizing the Size and Colloidal State of Nanoparticles. Sci. Rep. 2015, 5, 9793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eaton, P.; Quaresma, P.; Soares, C.; Neves, C.; de Almeida, M.P.; Pereira, E.; West, P. A Direct Comparison of Experimental Methods to Measure Dimensions of Synthetic Nanoparticles. Ultramicroscopy 2017, 182, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Ghaferi, M.; Raza, A.; Koohi, M.; Zahra, W.; Akbarzadeh, A.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Impact of PEGylated Liposomal Doxorubicin and Carboplatin Combination on Glioblastoma. Pharmaceutics 2022, 14, 2183. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, Y.; Wang, S.; Zou, Y.; Zheng, M.; Shi, B. Brain-Targeting Metastatic Tumor Cell Membrane Cloaked Biomimetic Nanomedicines Mediate Potent Chemodynamic and RNAi Combinational Therapy of Glioblastoma. Adv. Funct. Mater. 2022, 32, 2209239. [Google Scholar] [CrossRef]
- Costagliola di Polidoro, A.; Zambito, G.; Haeck, J.; Mezzanotte, L.; Lamfers, M.; Netti, P.A.; Torino, E. Theranostic Design of Angiopep-2 Conjugated Hyaluronic Acid Nanoparticles (Thera-ANG-CHANPs) for Dual Targeting and Boosted Imaging of Glioma Cells. Cancers 2021, 13, 503. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; He, Z.; Cai, P.; Zhao, Y.; Gao, L.; Yang, W.; Zhao, Y.; Gao, X.; Gao, F. Surface-Functionalized Modified Copper Sulfide Nanoparticles Enhance Checkpoint Blockade Tumor Immunotherapy by Photothermal Therapy and Antigen Capturing. ACS Appl. Mater. Interfaces 2019, 11, 13964–13972. [Google Scholar] [CrossRef] [PubMed]
- Lesiak, B.; Rangam, N.; Jiricek, P.; Gordeev, I.; Tóth, J.; Kövér, L.; Mohai, M.; Borowicz, P. Surface Study of Fe3O4 Nanoparticles Functionalized With Biocompatible Adsorbed Molecules. Front. Chem. 2019, 7, 642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borowik, A.; Butowska, K.; Konkel, K.; Banasiuk, R.; Derewonko, N.; Wyrzykowski, D.; Davydenko, M.; Cherepanov, V.; Styopkin, V.; Prylutskyy, Y.; et al. The Impact of Surface Functionalization on the Biophysical Properties of Silver Nanoparticles. Nanomaterials 2019, 9, 973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- León Félix, L.; Sanz, B.; Sebastián, V.; Torres, T.E.; Sousa, M.H.; Coaquira, J.a.H.; Ibarra, M.R.; Goya, G.F. Gold-Decorated Magnetic Nanoparticles Design for Hyperthermia Applications and as a Potential Platform for Their Surface-Functionalization. Sci. Rep. 2019, 9, 4185. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yan, C.; Gao, S.; Liu, Y. Surface Chemistry of Gold Nanoparticles Determines Interactions with Bovine Serum Albumin. Mater. Sci. Eng. C 2019, 103, 109856. [Google Scholar] [CrossRef]
- Ramalho, M.J.; Bravo, M.; Loureiro, J.A.; Lima, J.; Pereira, M.C. Transferrin-Modified Nanoparticles for Targeted Delivery of Asiatic Acid to Glioblastoma Cells. Life Sci. 2022, 296, 120435. [Google Scholar] [CrossRef]
- Su, X.; Zhang, D.; Zhang, H.; Zhao, K.; Hou, W. Preparation and Characterization of Angiopep-2 Functionalized Ginsenoside-Rg3 Loaded Nanoparticles and the Effect on C6 Glioma Cells. Pharm. Dev. Technol. 2020, 25, 385–395. [Google Scholar] [CrossRef]
- He, W.; Li, X.; Morsch, M.; Ismail, M.; Liu, Y.; Rehman, F.U.; Zhang, D.; Wang, Y.; Zheng, M.; Chung, R.; et al. Brain-Targeted Codelivery of Bcl-2/Bcl-Xl and Mcl-1 Inhibitors by Biomimetic Nanoparticles for Orthotopic Glioblastoma Therapy. ACS Nano 2022, 16, 6293–6308. [Google Scholar] [CrossRef]
- Gazaille, C.; Sicot, M.; Akiki, M.; Lautram, N.; Dupont, A.; Saulnier, P.; Eyer, J.; Bastiat, G. Characterization of Biological Material Adsorption to the Surface of Nanoparticles without a Prior Separation Step: A Case Study of Glioblastoma-Targeting Peptide and Lipid Nanocapsules. Pharm. Res. 2021, 38, 681–691. [Google Scholar] [CrossRef] [PubMed]
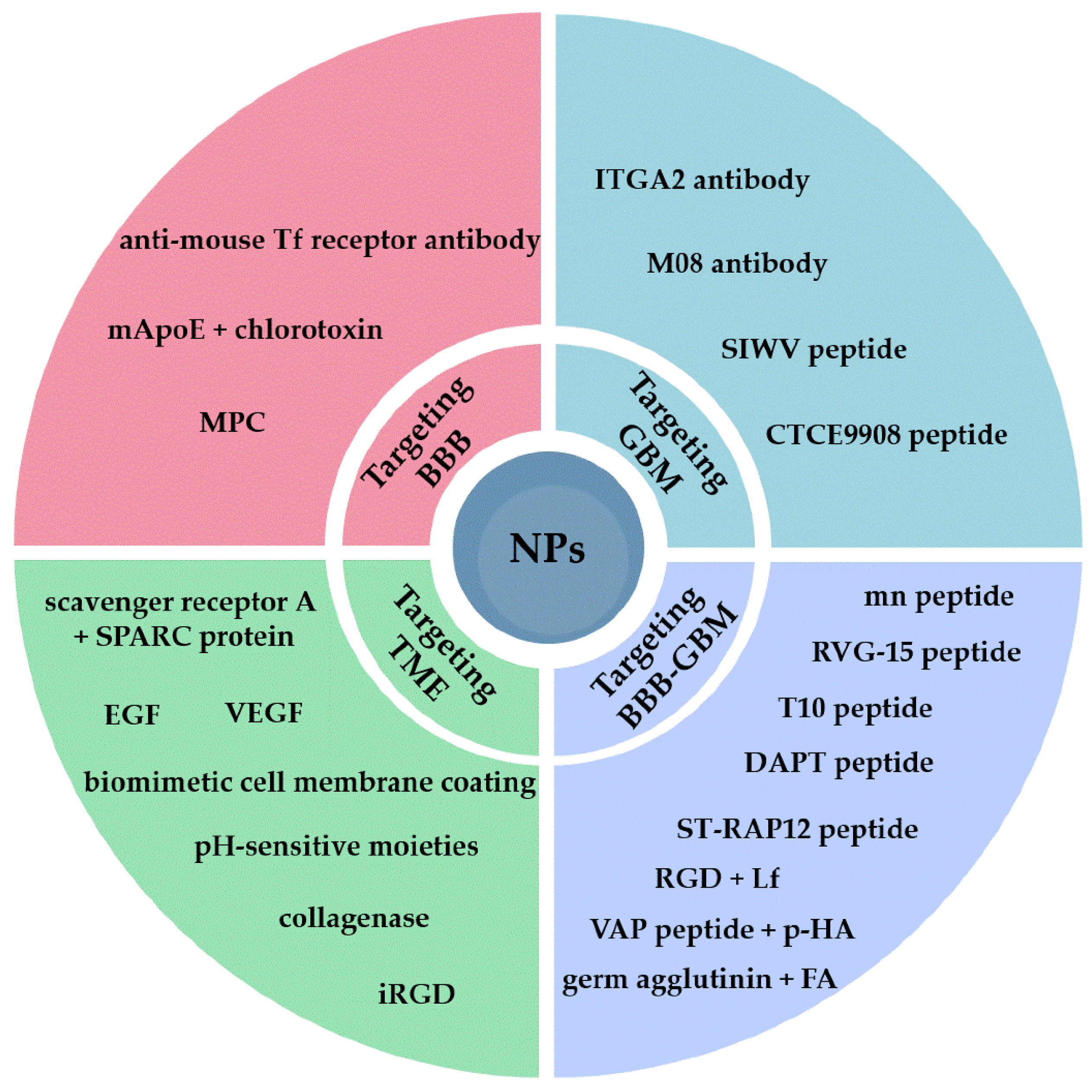


| NP Type | Targeting Moiety | Target | Key Results | Ref. |
|---|---|---|---|---|
| Crosslinked peptide | MPC | Acetylcholine transporter | Successful delivery of nimotuzumab in orthotopic glioma xenograft mice | [32] |
| Liposomes | ApoE derived peptide + chlorotoxin | Lipid transport | Doxorubicin loaded into the liposomes produced reduced viability of GBM U87 cells and did not affect endothelial cells in vitro | [33] |
| Nano-immune conjugate | Tf Receptor antibody | Tf receptor | PMLA backbone conjugated to Tf Receptor antibody for targeting and CTLA-4 and PD-1 antibodies. The system was able to induce antitumor immune response in GBM mice | [34] |
| PEGylated liposomes | ITGA2 antibody | ITGA2 | Doxorubicin loaded liposomes were able to cross the BBTB but not the healthy BBB. ITGA2 blocked GBM cell migration | [39] |
| PLGA NPs | M08 antibody | Cell surface vimentin | NPs loaded with paclitaxel showed increased apoptosis in GBM cells compared to healthy astrocytes | [40] |
| Porous silica NPs | SIWV peptide | Caveolin-mediated transport | Accumulation of NPs in the brain of mice with GBM xenografts, resulting in prolonged survival, with higher GBM selectivity in vitro than in vivo | [42,43] |
| Lipid-CaP NPs | CTCE9908 peptide | CXCR4 | Efficient delivery of siRNA in GBM cultures and GBM mice, resulting in silencing of the PD-1 gene ligand | [45] |
| PEG–PCL NPs | WGA + FA | Sialic acid + FA receptor | NPs were loaded with different anticancer drugs, and the double-ligand strategy showed improved targeting efficacy compared to the single moieties in vitro | [46] |
| PEGylated liposomes | VAP + p-HA | GRP78 protein + dopamine receptors | Enhanced BBB crossing and GBM accumulation in spheroids Apoptotic and antiangiogenic effect in orthotopic GBM mice | [47] |
| PEGylated liposomes | RGD + Lf | Integrin αvβ3 + Lf receptor | Improved BBB crossing and GBM accumulation in spheroids Improved efficacy of docetaxel in vivo compared to nontargeted liposomes | [49] |
| Polymeric micelles | ST-RAP12 peptide | LRP1 receptor | The peptide improved GBM specificity of paclitaxel-loaded micelles, with increased survival rate and inhibited angiogenesis in vivo | [50] |
| DSPE–PEG micelles | DATP | Neuropeptide Y receptor Y1 | Increased BBB crossing in vitro compared to other known ligands The ATP peptide improved photothermal therapy in vivo | [51] |
| PEGylated liposomes | RVG15 peptide | Nicotinic acetylcholine receptor | Improved delivery of paclitaxel across the BBB and accumulation in GBM cells in vivo Inhibition of tumor growth and metastases formation | [53] |
| Liposomes | mnRwr peptide | Integrin αvβ3 | Increased penetration in tumor spheroids compared to RGD peptide, and increased accumulation in GBM mice | [54] |
| PEI-coated silica NPs | T10 peptide | Tf receptor | Induced formation of a Tf corona on the surface of NPs to target the Tf Receptor Efficient BBB crossing and GBM targeting in vivo with prolonged release of doxorubicin | [55] |
| Albumin NPs | Collagenase | Extracellular matrix | Efficient delivery of gemcitabine in tumor spheroids | [56] |
| Liposomes | EGF | EGF receptor | Increased delivery of silver NPs loaded into liposomes, specifically to GBM cells in vitro | [57] |
| Albumin NPs | Scavenger receptor A + SPARC protein | TAMs in TME | Improved ICB therapy with elimination of TAMs from the TME | [58] |
| Copper–selenium NPs | Biomimetic cell membrane | TME | Shift of TAMs to an M1 phenotype, decreased expression of the PD-1 ligand, and increase in memory T cells | [59] |
| Albumin NPs | ROS-sensitive linker + PD-1 ligand antibody | ROS in TME | System enclosed in a hydrogel together with iron oxide NPs for combined photodynamic therapy and immunomodulation | [60] |
| Platinum NPs + dextran NPs | Linkage via pH -sensitive borate ester | Acidic pH in TME | Disassembly of the two NPs improved penetration into GBM and release of loaded sotuletinib to eliminate TAMs | [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodà, F.; Caraffi, R.; Picciolini, S.; Tosi, G.; Vandelli, M.A.; Ruozi, B.; Bedoni, M.; Ottonelli, I.; Duskey, J.T. Recent Advances on Surface-Modified GBM Targeted Nanoparticles: Targeting Strategies and Surface Characterization. Int. J. Mol. Sci. 2023, 24, 2496. https://doi.org/10.3390/ijms24032496
Rodà F, Caraffi R, Picciolini S, Tosi G, Vandelli MA, Ruozi B, Bedoni M, Ottonelli I, Duskey JT. Recent Advances on Surface-Modified GBM Targeted Nanoparticles: Targeting Strategies and Surface Characterization. International Journal of Molecular Sciences. 2023; 24(3):2496. https://doi.org/10.3390/ijms24032496
Chicago/Turabian StyleRodà, Francesca, Riccardo Caraffi, Silvia Picciolini, Giovanni Tosi, Maria Angela Vandelli, Barbara Ruozi, Marzia Bedoni, Ilaria Ottonelli, and Jason Thomas Duskey. 2023. "Recent Advances on Surface-Modified GBM Targeted Nanoparticles: Targeting Strategies and Surface Characterization" International Journal of Molecular Sciences 24, no. 3: 2496. https://doi.org/10.3390/ijms24032496
APA StyleRodà, F., Caraffi, R., Picciolini, S., Tosi, G., Vandelli, M. A., Ruozi, B., Bedoni, M., Ottonelli, I., & Duskey, J. T. (2023). Recent Advances on Surface-Modified GBM Targeted Nanoparticles: Targeting Strategies and Surface Characterization. International Journal of Molecular Sciences, 24(3), 2496. https://doi.org/10.3390/ijms24032496










