Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs
Abstract
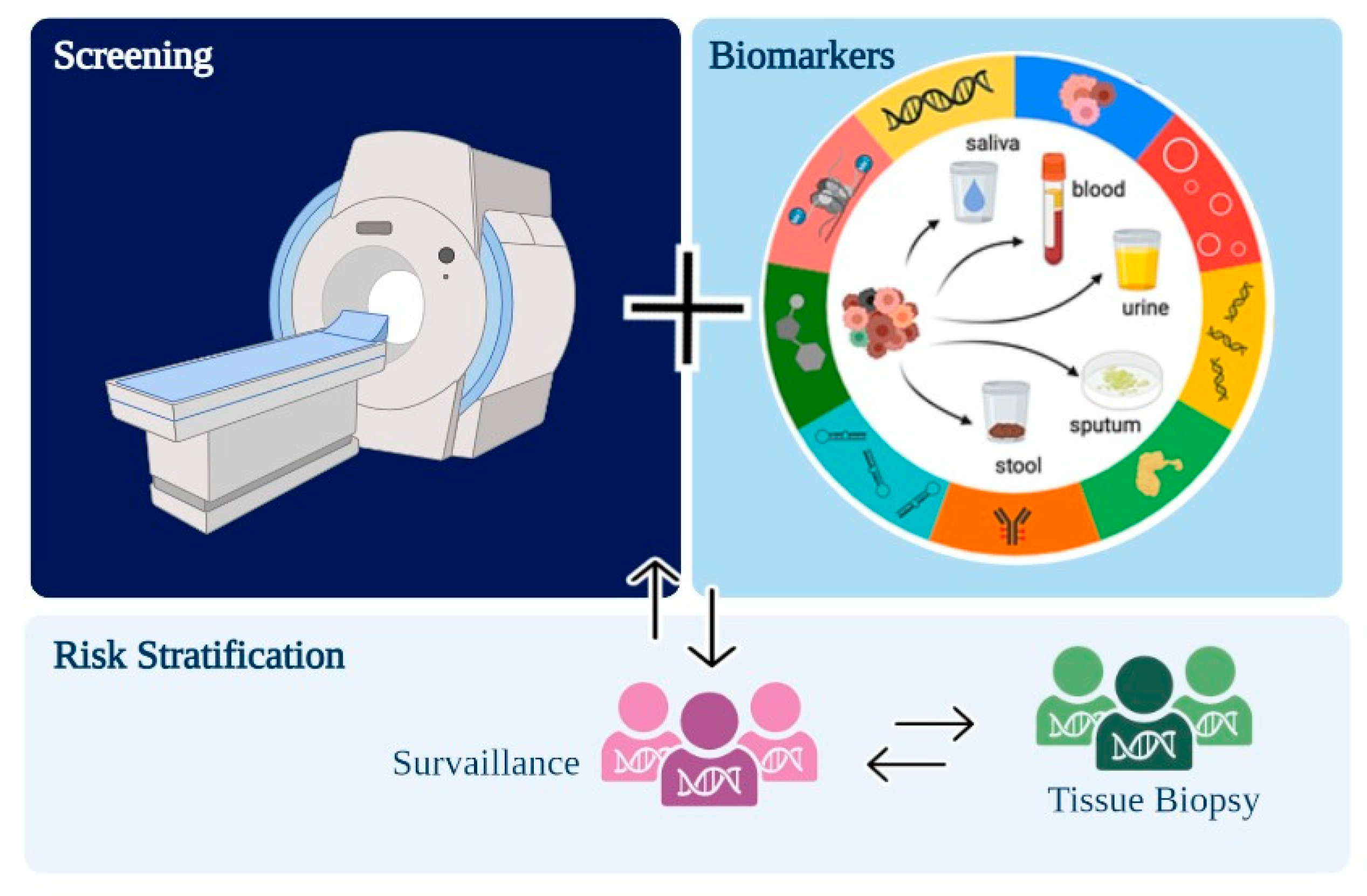
:1. Introduction
2. Sample Types and Analytes for Liquid Biopsy from NSCLC
3. NSCLC Biomarkers for Detection in Liquid Biopsy Samples
3.1. Circulating-Tumor DNA (ctDNA) and Cell-Free DNA (cfDNA)
3.2. cfDNA Methylation Biomarkers
3.3. Circulating Tumor Cells (CTCs)
3.4. Extracellular Vesicles
3.5. MicroRNA/CircRNA
3.6. Metabolites and Proteins
3.7. Autoantibodies against Tumor-Associated Antigens
4. Clinical Application of Liquid Biopsy for NSCLC
4.1. Prognosis
4.2. Precision Medicine and Disease Monitoring
4.3. Early Detection as an Emerging Application of Liquid Biopsy for NSCLC
5. Challenges and Limitations
6. Open Issues e Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Henschke, C.I.; International Early Lung Cancer Action Program Investigators. Survival of patients with clinical stage I lung cancer diagnosed by computed tomography screening for lung cancer. Clin. Cancer Res. AACR 2007, 13, 4949–4950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, G.W.; Ostroff, J.S.; Goffin, J.R. Lung cancer screening, cancer treatment, and addressing the continuum of health risks caused by tobacco. Am. Soc. Clin. Oncol. Educ. Book 2016, 36, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2017; National Cancer Institute: Bethesda, MD, USA, 2020; Volume 4.
- Thun, M.J.; Henley, S.J.; Calle, E.E. Tobacco use and cancer: An epidemiologic perspective for geneticists. Oncogene 2002, 21, 7307–7325. [Google Scholar] [CrossRef] [Green Version]
- Ridge, C.A.; McErlean, A.M.; Ginsberg, M.S. (Eds.) Epidemiology of lung cancer. In Seminars in Interventional Radiology; Thieme Medical Publishers: New York, NY, USA, 2013. [Google Scholar]
- Markowitz, S.B.; Levin, S.M.; Miller, A.; Morabia, A. Asbestos, Asbestosis, Smoking, and Lung Cancer. New Findings from the North American Insulator Cohort. Am. J. Respir. Crit. Care Med. 2013, 188, 90–96. [Google Scholar] [CrossRef]
- Shankar, A.; Dubey, A.; Saini, D.; Singh, M.; Prasad, C.P.; Roy, S.; Bharati, S.J.; Rinki, M.; Singh, N.; Seth, T.; et al. Environmental and occupational determinants of lung cancer. Transl. Lung Cancer Res. 2019, 8, S31–S49. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.D.; EPIC Study; Hung, R.J.; Gaborieau, V.; Boffetta, P.; Chabrier, A.; Byrnes, G.; Zaridze, D.; Mukeria, A.; Szeszenia-Dabrowska, N.; et al. Lung cancer susceptibility locus at 5p15.33. Nat. Genet. 2008, 40, 1404–1406. [Google Scholar] [CrossRef] [PubMed]
- McKay, J.D.; SpiroMeta Consortium; Hung, R.J.; Han, Y.; Zong, X.; Carreras-Torres, R.; Christiani, D.C.; Caporaso, N.E.; Johansson, M.; Xiao, X.; et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat. Genet. 2017, 49, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Cruz, C.S.D.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef]
- Howlader, N.N.A.M.; Noone, A.M.; Krapcho, M.E.; Miller, D.; Brest, A.; Yu, M.E.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019; Volume 1.
- Planchard, D.; Smit, E.F.; Groen, H.J.M.; Mazieres, J.; Besse, B.; Helland, Å.; Giannone, V.; D’Amelio, A.M., Jr.; Zhang, P.; Mookerjee, B.; et al. Dabrafenib plus trametinib in patients with previously untreated BRAFV600E-mutant metastatic non-small-cell lung cancer: An open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Santana-Davila, R.; Molina, J.R. (Eds.) Non–Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment; Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Leal, L.F.; de Paula, F.E.; De Marchi, P.; Viana, L.D.S.; Pinto, G.D.J.; Carlos, C.D.; Berardinelli, G.N.; Miziara, J.E.; da Silva, C.M.; Silva, E.C.A.; et al. Mutational profile of Brazilian lung adenocarcinoma unveils association of EGFR mutations with high Asian ancestry and independent prognostic role of KRAS mutations. Sci. Rep. 2019, 9, 3209. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, M.; Ding, X.-J.; Cao, Y. Familial risk for lung cancer. Oncol. Lett. 2017, 13, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Tan, A.C.; Tan, D.S.W. Targeted Therapies for Lung Cancer Patients With Oncogenic Driver Molecular Alterations. J. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef]
- Harrison, S.; Judd, J.; Chin, S.; Ragin, C. Disparities in Lung Cancer Treatment. Curr. Oncol. Rep. 2022, 24, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.; Kim, C.-J.; Sunkara, V.; Kim, M.-H.; Cho, Y.-K. Liquid Biopsy in Lung Cancer: Clinical Applications of Circulating Biomarkers (CTCs and ctDNA). Micromachines 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Han, B. Liquid Biopsy Promotes Non-Small Cell Lung Cancer Precision Therapy. Technol. Cancer Res. Treat. 2018, 17, 1533033818801809. [Google Scholar] [CrossRef] [PubMed]
- Pisapia, P.; Malapelle, U.; Troncone, G. Liquid Biopsy and Lung Cancer. Acta Cytol. 2019, 63, 489–496. [Google Scholar] [CrossRef]
- Hoseok, I.; Cho, J.-Y. Lung cancer biomarkers. Adv. Clin. Chem. 2015, 72, 107–170. [Google Scholar]
- Buder, A.; Tomuta, C.; Filipits, M. The potential of liquid biopsies. Curr. Opin. Oncol. 2016, 28, 130–134. [Google Scholar] [CrossRef]
- Castro-Giner, F.; Gkountela, S.; Donato, C.; Alborelli, I.; Quagliata, L.; Ng, C.K.Y.; Piscuoglio, S.; Aceto, N. Cancer Diagnosis Using a Liquid Biopsy: Challenges and Expectations. Diagnostics 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.C.M.; Massie, C.; Garcia-Corbacho, J.; Mouliere, F.; Brenton, J.D.; Caldas, C.; Pacey, S.; Baird, R.; Rosenfeld, N. Liquid biopsies come of age: Towards implementation of circulating tumour DNA. Nat. Rev. Cancer 2017, 17, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Bracht, J.W.P.; Mayo-De-Las-Casas, C.; Berenguer, J.; Karachaliou, N.; Rosell, R. The Present and Future of Liquid Biopsies in Non-Small Cell Lung Cancer: Combining Four Biosources for Diagnosis, Prognosis, Prediction, and Disease Monitoring. Curr. Oncol. Rep. 2018, 20, 70. [Google Scholar] [CrossRef] [PubMed]
- Trombetta, D.; Sparaneo, A.; Fabrizio, F.P.; Muscarella, L.A. Liquid biopsy and NSCLC. Lung Cancer Manag. 2016, 5, 91–104. [Google Scholar] [CrossRef]
- Chan, M.H.M.; Chow, K.M.; Chan, A.T.C.; Leung, C.B.; Chan, L.Y.S.; Chow, K.C.K.; Lam, C.W.; Lo, Y.M.D. Quantitative Analysis of Pleural Fluid Cell-free DNA as a Tool for the Classification of Pleural Effusions. Clin. Chem. 2003, 49, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Pu, D.; Liang, H.; Wei, F.; Akin, D.; Feng, Z.; Yan, Q.; Li, Y.; Zhen, Y.; Xu, L.; Dong, G.; et al. Evaluation of a novel saliva-based epidermal growth factor receptor mutation detection for lung cancer: A pilot study. Thorac. Cancer 2016, 7, 428–436. [Google Scholar] [CrossRef]
- Wang, Y.; Springer, S.; Zhang, M.; McMahon, K.W.; Kinde, I.; Dobbyn, L.; Ptak, J.; Brem, H.; Chaichana, K.; Gallia, G.L.; et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc. Natl. Acad. Sci. USA 2015, 112, 9704–9709. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Barzi, A.; Sartore-Bianchi, A.; Cassingena, A.; Siravegna, G.; Karp, D.D.; Piha-Paul, S.A.; Subbiah, V.; Tsimberidou, A.M.; Huang, H.J.; et al. Mutation-Enrichment Next-Generation Sequencing for Quantitative Detection of KRAS Mutations in Urine Cell-Free DNA from Patients with Advanced CancersKRAS Mutations in Urine cfDNA. Clin. Cancer Res. 2017, 23, 3657–3666. [Google Scholar] [CrossRef] [Green Version]
- Benlloch, S.; Martí-Ciriquián, J.L.; Galbis-Caravajal, J.M.; Martín, C.; Sánchez-Payá, J.; Rodríguez-Paniagua, J.M.; Romero, S.; Massutí, B. Cell-Free DNA Concentration in Pleural Fluid and Serum: Quantitative Approach and Potential Prognostic Factor in Patients with Cancer and Pleural Effusions. Clin. Lung Cancer 2006, 8, 140–145. [Google Scholar] [CrossRef]
- Abbosh, C.; Birkbak, N.J.; Wilson, G.A.; Jamal-Hanjani, M.; Constantin, T.; Salari, R.; Le Quesne, J.; Moore, D.A.; Veeriah, S.; Rosenthal, R.; et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature 2017, 545, 446–451. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, H.; Watanabe, Y.; Itoh, F. Cell-Free DNA. In Biomarkers in Cancer Therapy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 11–24. [Google Scholar]
- Cescon, D.W.; Bratman, S.V.; Chan, S.M.; Siu, L.L. Circulating tumor DNA and liquid biopsy in oncology. Nat. Cancer 2020, 1, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, A.; Wagner, J.; Gorges, T.M.; Taenzer, A.; Uzunoglu, F.G.; Driemel, C.; Stoecklein, N.H.; Knoefel, W.T.; Angenendt, S.; Hauch, S.; et al. Characterization of different CTC subpopulations in non-small cell lung cancer. Sci. Rep. 2016, 6, 28010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaffer, C. Circulating Tumor Cells and the Liquid Biopsy: Processed appropriately, liquid biopsy samples and circulating biomarkers may aid cancer diagnosis and predict treatment outcomes. Genet. Eng. Biotechnol. News 2019, 39, 42–44. [Google Scholar] [CrossRef]
- Jia, S.; Zhang, R.; Li, Z.; Li, J. Clinical and biological significance of circulating tumor cells, circulating tumor DNA, and exosomes as biomarkers in colorectal cancer. Oncotarget 2017, 8, 55632. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.A.; Arora, S.; Prakasam, G.; Calin, G.A.; Syed, M.A. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol. Asp. Med. 2019, 70, 3–20. [Google Scholar] [CrossRef]
- Li, L.; Fu, K.; Zhou, W.; Snyder, M. Applying circulating tumor DNA methylation in the diagnosis of lung cancer. Precis. Clin. Med. 2019, 2, 45–56. [Google Scholar] [CrossRef]
- Moss, J.; Magenheim, J.; Neiman, D.; Zemmour, H.; Loyfer, N.; Korach, A.; Samet, Y.; Maoz, M.; Druid, H.; Arner, P.; et al. Comprehensive human cell-type methylation atlas reveals origins of circulating cell-free DNA in health and disease. Nat. Commun. 2018, 9, 5068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Q.; Zhao, W.; Wang, L.; Guo, F.; Song, D.; Zhang, Q.; Zhang, D.; Fan, Y.; Wang, J. Integration of metabolomic and transcriptomic profiles to identify biomarkers in serum of lung cancer. J. Cell. Biochem. 2019, 120, 11981–11989. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Li, Z.; Lazar, L.; Fang, Z.; Tang, C.; Zhao, J. Metabolomics workflow for lung cancer: Discovery of biomarkers. Clin. Chim. Acta 2019, 495, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Seow, W.J.; Shu, X.-O.; Nicholson, J.; Holmes, E.; Walker, D.I.; Hu, W.; Cai, Q.; Gao, Y.-T.; Xiang, Y.-B.; Moore, S.C.; et al. Association of Untargeted Urinary Metabolomics and Lung Cancer Risk Among Never-Smoking Women in China. JAMA Netw. Open 2019, 2, e1911970. [Google Scholar] [CrossRef] [Green Version]
- Yokota, H.; Guo, J.; Matoba, M.; Higashi, K.; Tonami, H.; Nagao, Y. Lactate, choline, and creatine levels measured by vitro 1H-MRS as prognostic parameters in patients with non-small-cell lung cancer. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2007, 25, 992–999. [Google Scholar]
- Chapman, C.; Murray, A.; Chakrabarti, J.; Thorpe, A.; Woolston, C.; Sahin, U.; Barnes, A.; Robertson, J. Autoantibodies in breast cancer: Their use as an aid to early diagnosis. Ann. Oncol. 2007, 18, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Yu, R.; Wang, H.; Yan, N.; Yuan, Q.; Ma, Y.; Slamon, D.; Hou, D.; Wang, H.; Wang, Q. Significance of tumor-associated autoantibodies in the early diagnosis of lung cancer. Clin. Respir. J. 2018, 12, 2020–2028. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Rao, M.; Qu, J.; Luo, D. Applications of liquid biopsy in lung cancer-diagnosis, prognosis prediction, and disease monitoring. Am. J. Transl. Res. 2018, 10, 3911. [Google Scholar] [PubMed]
- Miyanaga, A.; Masuda, M.; Yamada, T. Biomarkers of Lung Cancer: Liquid Biopsy Comes of Age. In Biomarkers in Cancer Therapy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 105–113. [Google Scholar]
- Diaz, L.A., Jr.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014, 32, 579. [Google Scholar] [CrossRef]
- Davis, A.A.; Cristofanilli, M. Detection of Predictive Biomarkers Using Liquid Biopsies. In Predictive Biomarkers in Oncology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 107–117. [Google Scholar]
- Gale, D.; Lawson, A.R.; Howarth, K.; Madi, M.; Durham, B.; Smalley, S.; Calaway, J.; Blais, S.; Jones, G.; Clark, J.; et al. Development of a highly sensitive liquid biopsy platform to detect clinically-relevant cancer mutations at low allele fractions in cell-free DNA. PLoS ONE 2018, 13, e0194630. [Google Scholar] [CrossRef]
- Revelo, A.E.; Martin, A.; Velasquez, R.; Kulandaisamy, P.C.; Bustamante, J.; Keshishyan, S.; Otterson, G. Liquid biopsy for lung cancers: An update on recent developments. Ann. Transl. Med. 2019, 7, 349. [Google Scholar] [CrossRef]
- Jee, J.; Lebow, E.S.; Murciano-Goroff, Y.R.; Jayakumaran, G.; Shen, R.; Brannon, A.R.; Benayed, R.; Namakydoust, A.; Offin, M.; Paik, P.K.; et al. Overall Survival with Circulating Tumor DNA-Guided Therapy in Advanced Non-Small Cell Lung Cancer; Wolters Kluwer Health: Philadelphia, PA, USA, 2021. [Google Scholar]
- Shendure, J.; Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 2008, 26, 1135–1145. [Google Scholar] [CrossRef]
- Wong, W.H.; Tong, R.S.; Young, A.L.; Druley, T.E. Rare Event Detection Using Error-corrected DNA and RNA Sequencing. J. Vis. Exp. 2018, e57509. [Google Scholar] [CrossRef] [Green Version]
- Teder, H.; Koel, M.; Paluoja, P.; Jatsenko, T.; Rekker, K.; Laisk-Podar, T.; Kukuškina, V.; Velthut-Meikas, A.; Fjodorova, O.; Peters, M.; et al. TAC-seq: Targeted DNA and RNA sequencing for precise biomarker molecule counting. NPJ Genom. Med. 2018, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Villaflor, V.; Won, B.; Nagy, R.; Banks, K.; Lanman, R.B.; Talasaz, A.; Salgia, R. Biopsy-free circulating tumor DNA assay identifies actionable mutations in lung cancer. Oncotarget 2016, 7, 66880. [Google Scholar] [PubMed] [Green Version]
- Sabari, J.K.; Offin, M.; Stephens, D.; Ni, A.; Lee, A.; Pavlakis, N.; Clarke, S.; Diakos, C.I.; Datta, S.; Tandon, N.; et al. A Prospective Study of Circulating Tumor DNA to Guide Matched Targeted Therapy in Lung Cancers. Gynecol. Oncol. 2019, 111, 575–583. [Google Scholar] [CrossRef] [PubMed]
- FuFaD, A. Aprovação Pré-Comercialização P150044, Teste de Mutação Cobas EGFR V2. 2017 FD. 2017. Available online: https://www.accessdata.fda.gov/cdrh_docs/pdf12/p120019s007c.pdf (accessed on 1 September 2022).
- Torres, S.; González, Á.; Tomás, A.J.C.; Fariñas, S.C.; Ferrero, M.; Mirda, D.; Sirera, R.; Jantus-Lewintre, E.; Camps, C. A profile on cobas® EGFR Mutation Test v2 as companion diagnostic for first-line treatment of patients with non-small cell lung cancer. Expert Rev. Mol. Diagn. 2020, 20, 575–582. [Google Scholar]
- Vrba, L.; Oshiro, M.M.; Kim, S.S.; Garland, L.L.; Placencia, C.; Mahadevan, D.; Nelson, M.A.; Futscher, B.W. DNA methylation biomarkers discovered in silico detect cancer in liquid biopsies from non-small cell lung cancer patients. Epigenetics 2020, 15, 419–430. [Google Scholar] [PubMed] [Green Version]
- Duruisseaux, M.; Esteller, M. (Eds.) Lung cancer epigenetics: From knowledge to applications. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Bjaanaes, M.M.; Fleischer, T.; Halvorsen, A.R.; Daunay, A.; Busato, F.; Solberg, S.; Jørgensen, L.; Kure, E.; Edvardsen, H.; Børresen-Dale, A.-L.; et al. Genome-wide DNA methylation analyses in lung adenocarcinomas: Association with EGFR, KRAS and TP53 mutation status, gene expression and prognosis. Mol. Oncol. 2016, 10, 330–343. [Google Scholar] [PubMed]
- Wang, J.; Duan, Y.; Meng, Q.-H.; Gong, R.; Guo, C.; Zhao, Y.; Zhang, Y. Integrated analysis of DNA methylation profiling and gene expression profiling identifies novel markers in lung cancer in Xuanwei, China. PLoS ONE 2018, 13, e0203155. [Google Scholar]
- Ooki, A.; Maleki, Z.; Tsay, J.-C.J.; Goparaju, C.; Brait, M.; Turaga, N.; Nam, H.-S.; Rom, W.N.; Pass, H.I.; Sidransky, D.; et al. A Panel of Novel Detection and Prognostic Methylated DNA Markers in Primary Non–Small Cell Lung Cancer and Serum DNA. Clin. Cancer Res. 2017, 23, 7141–7152. [Google Scholar]
- Yang, Z.; Qi, W.; Sun, L.; Zhou, H.; Zhou, B.; Hu, Y. DNA methylation analysis of selected genes for the detection of early-stage lung cancer using circulating cell-free DNA. Adv. Clin. Exp. Med. Off. Organ Wroc. Med. Univ. 2019, 28, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Ramírez, C.; Cañadas-Garre, M.; Robles, A.I.; Molina, M.; Faus-Dáder, M.J.; Calleja-Hernández, M. Liquid biopsy in early stage lung cancer. Transl. Lung Cancer Res. 2016, 5, 517. [Google Scholar] [CrossRef] [Green Version]
- Hulbert, A.; Jusue-Torres, I.; Stark, A.; Chen, C.; Rodgers, K.; Lee, B.; Griffin, C.; Yang, A.; Huang, P.; Wrangle, J.; et al. Early Detection of Lung Cancer Using DNA Promoter Hypermethylation in Plasma and SputumEpigenetic Lung Cancer Screening. Clin. Cancer Res. 2017, 23, 1998–2005. [Google Scholar]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; CCGA Consortium. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [PubMed]
- Klein, E.; Richards, D.; Cohn, A.; Tummala, M.; Lapham, R.; Cosgrove, D.; Chung, G.; Clement, J.; Gao, J.; Hunkapiller, N.; et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann. Oncol. 2021, 32, 1167–1177. [Google Scholar] [PubMed]
- Neal, R.D.; Johnson, P.; Clarke, C.A.; Hamilton, S.A.; Zhang, N.; Kumar, H.; Swanton, C.; Sasieni, P. Cell-Free DNA–Based Multi-Cancer Early Detection Test in an Asymptomatic Screening Population (NHS-Galleri): Design of a Pragmatic, Prospective Randomised Controlled Trial. Cancers 2022, 14, 4818. [Google Scholar] [PubMed]
- Poggiana, C.; Rossi, E.; Zamarchi, R. Possible role of circulating tumor cells in early detection of lung cancer. J. Thorac. Dis. 2020, 12, 3821. [Google Scholar] [CrossRef]
- Santarpia, M.; Liguori, A.; Karachaliou, N.; Gonzalez-Cao, M.; Daffinà, M.G.; D’Aveni, A.; Marabello, G.; Altavilla, G.; Rosell, R. Osimertinib in the treatment of non-small-cell lung cancer: Design, development and place in therapy. Lung Cancer Targets Ther. 2017, 8, 109. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Shi, J.; Schmidt, B.; Liu, Q.; Shi, G.; Xu, X.; Liu, C.; Gao, Z.; Guo, T.; Shan, B. Circulating Tumor Cells as a Biomarker to Assist Molecular Diagnosis for Early Stage Non-Small Cell Lung Cancer. Cancer Manag. Res. 2020, 12, 841. [Google Scholar] [CrossRef]
- Correnti, M.; Raggi, C. Stem-like plasticity and heterogeneity of circulating tumor cells: Current status and prospect challenges in liver cancer. Oncotarget 2017, 8, 7094. [Google Scholar]
- Zhang, Y.; Wang, Y.; Du, Z.; Wu, M.; Zhang, G. Detection of micrometastases in lung cancer with magnetic nanoparticles and quantum dots. Int. J. Nanomed. 2012, 7, 2315. [Google Scholar] [CrossRef] [Green Version]
- Tu, Q.; Wu, X.; Le Rhun, E.; Blonski, M.; Wittwer, B.; Taillandier, L.; Bittencourt, M.D.C.; Faure, G.C. CellSearch® technology applied to the detection and quantification of tumor cells in CSF of patients with lung cancer leptomeningeal metastasis. Lung Cancer 2015, 90, 352–357. [Google Scholar]
- Hofman, V.; Long, E.; Ilie, M.; Bonnetaud, C.; Vignaud, J.M.; Fléjou, J.F.; Lantuejoul, S.; Piaton, E.; Mourad, N.; Butori, C.; et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology 2012, 23, 30–38. [Google Scholar] [CrossRef]
- Turetta, M.; Bulfoni, M.; Brisotto, G.; Fasola, G.; Zanello, A.; Biscontin, E.; Mariuzzi, L.; Steffan, A.; Di Loreto, C.; Cesselli, D.; et al. Assessment of the Mutational Status of NSCLC Using Hypermetabolic Circulating Tumor Cells. Cancers 2018, 10, 270. [Google Scholar] [PubMed] [Green Version]
- Reclusa, P.; Taverna, S.; Pucci, M.; Durendez, E.; Calabuig, S.; Manca, P.; Serrano, M.J.; Sober, L.; Pauwels, P.; Russo, A.; et al. Exosomes as diagnostic and predictive biomarkers in lung cancer. J. Thorac. Dis. 2017, 9 (Suppl. S13), S1373–S1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanni, I.; Alama, A.; Grossi, F.; Dal Bello, M.G.; Coco, S. Exosomes: A new horizon in lung cancer. Drug Discov. Today 2017, 22, 927–936. [Google Scholar] [PubMed]
- Momen-Heravi, F.; Balaj, L.; Alian, S.; Mantel, P.-Y.; Halleck, A.E.; Trachtenberg, A.J.; Soria, C.E.; Oquin, S.; Bonebreak, C.M.; Saracoglu, E.; et al. Current methods for the isolation of extracellular vesicles. Biol. Chem. 2013, 394, 1253–1262. [Google Scholar]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3, 23430. [Google Scholar]
- Qu, X.; Li, Q.; Yang, J.; Zhao, H.; Wang, F.; Zhang, F.; Zhang, S.; Zhang, H.; Wang, R.; Wang, Q.; et al. Double-Stranded DNA in Exosomes of Malignant Pleural Effusions as a Novel DNA Source for EGFR Mutation Detection in Lung Adenocarcinoma. Front. Oncol. 2019, 9, 931. [Google Scholar]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 14, 531–548. [Google Scholar]
- Rodríguez, M.; Silva, J.; López-Alfonso, A.; López-Muñiz, M.B.; Peña, C.; Domínguez, G.; García, J.M.; López-Gónzalez, A.; Méndez, M.; Provencio, M.; et al. Different exosome cargo from plasma/bronchoalveolar lavage in non-small-cell lung cancer. Genes Chromosom. Cancer 2014, 53, 713–724. [Google Scholar]
- Rabinowits, G.; Gerçel-Taylor, C.; Day, J.M.; Taylor, D.D.; Kloecker, G.H. Exosomal MicroRNA: A Diagnostic Marker for Lung Cancer. Clin. Lung Cancer 2009, 10, 42–46. [Google Scholar] [CrossRef]
- Tomasetti, M.; Lee, W.; Santarelli, L.; Neuzil, J. Exosome-derived microRNAs in cancer metabolism: Possible implications in cancer diagnostics and therapy. Exp. Mol. Med. 2017, 49, e285. [Google Scholar]
- Galka-Marciniak, P.; Urbanek-Trzeciak, M.O.; Nawrocka, P.M.; Dutkiewicz, A.; Giefing, M.; Lewandowska, M.A.; Kozlowski, P. Somatic Mutations in miRNA Genes in Lung Cancer—Potential Functional Consequences of Non-Coding Sequence Variants. Cancers 2019, 11, 793. [Google Scholar] [PubMed] [Green Version]
- Almeida, M.I.; Reis, R.M.; Calin, G.A. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2011, 717, 1–8. [Google Scholar]
- Mitchell, P.S.; Parkin, R.K.; Kroh, E.M.; Fritz, B.R.; Wyman, S.K.; Pogosova-Agadjanyan, E.L.; Peterson, A.; Noteboom, J.; O’Briant, K.C.; Allen, A.; et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 2008, 105, 10513–10518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puerta-Gil, P.; García-Baquero, R.; Jia, A.Y.; Ocaña, S.; Alvarez-Múgica, M.; Alvarez-Ossorio, J.L.; Cordon-Cardo, C.; Cava, F.; Sánchez-Carbayo, M. miR-143, miR-222, and miR-452 Are Useful as Tumor Stratification and Noninvasive Diagnostic Biomarkers for Bladder Cancer. Am. J. Pathol. 2012, 180, 1808–1815. [Google Scholar] [CrossRef]
- Zhi, F.; Cao, X.; Xie, X.; Wang, B.; Dong, W.; Gu, W.; Ling, Y.; Wang, R.; Yang, Y.; Liu, Y. Identification of Circulating MicroRNAs as Potential Biomarkers for Detecting Acute Myeloid Leukemia. PLoS ONE 2013, 8, e56718. [Google Scholar]
- Reis, P.P.; Drigo, S.A.; Carvalho, R.F.; Lapa, R.M.L.; Felix, T.F.; Patel, D.; Cheng, D.; Pintilie, M.; Liu, G.; Tsao, M.-S. Circulating miR-16-5p, miR-92a-3p, and miR-451a in Plasma from Lung Cancer Patients: Potential Application in Early Detection and a Regulatory Role in Tumorigenesis Pathways. Cancers 2020, 12, 2071. [Google Scholar] [CrossRef]
- Wozniak, M.B.; Scelo, G.; Muller, D.C.; Mukeria, A.; Zaridze, D.; Brennan, P. Circulating MicroRNAs as Non-Invasive Biomarkers for Early Detection of Non-Small-Cell Lung Cancer. PLoS ONE 2015, 10, e0125026. [Google Scholar]
- Huang, J.; Wu, J.; Li, Y.; Li, X.; Yang, T.; Yang, Q.; Jiang, Y. Deregulation of Serum MicroRNA Expression Is Associated with Cigarette Smoking and Lung Cancer. BioMed Res. Int. 2014, 2014, 364316. [Google Scholar] [CrossRef] [Green Version]
- Zhao, K.; Cheng, J.; Chen, B.; Liu, Q.; Xu, D.; Zhang, Y. Circulating microRNA-34 family low expression correlates with poor prognosis in patients with non-small cell lung cancer. J. Thorac. Dis. 2017, 9, 3735. [Google Scholar] [CrossRef] [Green Version]
- Arnaiz, E.; Sole, C.; Manterola, L.; Iparraguirre, L.; Otaegui, D.; Lawrie, C.H. (Eds.) CircRNAs and cancer: Biomarkers and master regulators. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Suzuki, H.; Zuo, Y.; Wang, J.; Zhang, M.Q.; Malhotra, A.; Mayeda, A. Characterization of RNase R-digested cellular RNA source that consists of lariat and circular RNAs from pre-mRNA splicing. Nucleic Acids Res. 2006, 34, e63. [Google Scholar] [CrossRef] [Green Version]
- Vincent, H.A.; Deutscher, M.P. Substrate Recognition and Catalysis by the Exoribonuclease RNase R. J. Biol. Chem. 2006, 281, 29769–29775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salzman, J.; Chen, R.E.; Olsen, M.N.; Wang, P.L.; Brown, P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013, 9, e1003777. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sharpless, N.E. Detecting and characterizing circular RNAs. Nat. Biotechnol. 2014, 32, 453–461. [Google Scholar] [CrossRef]
- De Fraipont, F.; Gazzeri, S.; Cho, W.C.; Eymin, B. Circular RNAs and RNA splice variants as biomarkers for prognosis and therapeutic response in the liquid biopsies of lung cancer patients. Front. Genet. 2019, 10, 390. [Google Scholar] [PubMed] [Green Version]
- Pedraz-Valdunciel, C.; Giannoukakos, S.; Potie, N.; Giménez-Capitán, A.; Huang, C.; Hackenberg, M.; Fernandez-Hilario, A.; Bracht, J.; Filipska, M.; Aldeguer, E.; et al. Digital multiplexed analysis of circular RNAs in FFPE and fresh non-small cell lung cancer specimens. Mol. Oncol. 2022, 16, 2367–2383. [Google Scholar] [CrossRef]
- Chen, K.-Z.; Lou, F.; Yang, F.; Zhang, J.-B.; Ye, H.; Chen, W.; Guan, T.; Zhao, M.-Y.; Su, X.-X.; Shi, R.; et al. Circulating Tumor DNA Detection in Early-Stage Non-Small Cell Lung Cancer Patients by Targeted Sequencing. Sci. Rep. 2016, 6, 31985. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Wang, X.; Wei, S.; Chen, Y.; Chen, Y.; Fan, X.; Han, S.; Wu, G. hsa_circ_0013958: A circular RNA and potential novel biomarker for lung adenocarcinoma. FEBS J. 2017, 284, 2170–2182. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, H.; Jing, W.; Luo, P.; Qiu, S.; Liu, X.; Zhu, M.; Liang, C.; Yu, M.; Tu, J. The Circular RNA hsa_circ_0001445 Regulates the Proliferation and Migration of Hepatocellular Carcinoma and May Serve as a Diagnostic Biomarker. Dis. Markers 2018, 2018, 3073467. [Google Scholar] [CrossRef] [Green Version]
- Hang, D.; Zhou, J.; Qin, N.; Zhou, W.; Ma, H.; Jin, G.; Hu, Z.; Dai, J.; Shen, H. A novel plasma circular RNA circFARSA is a potential biomarker for non-small cell lung cancer. Cancer Med. 2018, 7, 2783–2791. [Google Scholar] [CrossRef]
- Yu, L.; Li, K.; Zhang, X. Next-generation metabolomics in lung cancer diagnosis, treatment and precision medicine: Mini review. Oncotarget 2017, 8, 115774. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Misra, B.B. Challenges and Opportunities in Cancer Metabolomics. Proteomics 2019, 19, e1900042. [Google Scholar] [CrossRef] [PubMed]
- Molina, R.; Filella, X.; Augé, J.; Fuentes, R.; Bover, I.; Rifa, J.; Moreno, V.; Canals, E.; Viñolas, N.; Marquez, A.; et al. Tumor Markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in Patients with Non-Small Cell Lung Cancer as an Aid in Histological Diagnosis and Prognosis. Tumor Biol. 2003, 24, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Barlési, F.; Gimenez, C.; Torre, J.-P.; Doddoli, C.; Mancini, J.; Greillier, L.; Roux, F.; Kleisbauer, J.-P. Prognostic value of combination of Cyfra 21-1, CEA and NSE in patients with advanced non-small cell lung cancer. Respir. Med. 2004, 98, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Pang, W.W.; Abdul-Rahman, P.S.; Wan-Ibrahim, W.I.; Hashim, O.H. Can the acute-phase reactant proteins be used as cancer biomarkers? Int. J. Biol. Markers 2010, 25, 1–11. [Google Scholar] [CrossRef]
- Kang, S.-M.; Sung, H.-J.; Ahn, J.-M.; Park, J.-Y.; Lee, S.-Y.; Park, C.-S.; Cho, J.-Y. The Haptoglobin β chain as a supportive biomarker for human lung cancers. Mol. Biosyst. 2011, 7, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.-J.; Ahn, J.-M.; Yoon, Y.-H.; Rhim, T.-Y.; Park, C.-S.; Park, J.-Y.; Lee, S.-Y.; Kim, J.-W.; Cho, J.-Y. Identification and Validation of SAA as a Potential Lung Cancer Biomarker and its Involvement in Metastatic Pathogenesis of Lung Cancer. J. Proteome Res. 2011, 10, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Jensen, L.; Whitehead, A.S. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem. J. 1998, 334, 489–503. [Google Scholar] [CrossRef]
- De Petris, L.; Orre, L.M.; Kanter, L.; Pernemalm, M.; Koyi, H.; Lewensohn, R.; Lehtiö, J. Tumor expression of S100A6 correlates with survival of patients with stage I non-small-cell lung cancer. Lung Cancer 2009, 63, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Pujol, J.L.; Grenier, J.; Daurès, J.P.; Daver, A.; Pujol, H.; Michel, F.B. Serum fragment of cytokeratin subunit 19 measured by CYFRA 21-1 immunoradiometric assay as a marker of lung cancer. Cancer Res. 1993, 53, 61–66. [Google Scholar]
- Bergqvist, M.; Brattström, D.; Hesselius, P.; Wiklund, B.; Silen, A.; Wagenius, G.; Brodin, O. Cytokeratin 8 and 18 fragments measured in serum and their relation to survival in patients with non-small cell lung cancer. Anticancer Res. 1999, 19, 1833–1836. [Google Scholar]
- Xu, B.J.; Gonzalez, A.L.; Kikuchi, T.; Yanagisawa, K.; Massion, P.P.; Wu, H.; Mason, S.E.; Olson, S.J.; Shyr, Y.; Carbone, D.P.; et al. MALDI-MS derived prognostic protein markers for resected non-small cell lung cancer. Proteom. Clin. Appl. 2008, 2, 1508–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamay, T.N.; Zamay, G.S.; Kolovskaya, O.S.; Zukov, R.A.; Petrova, M.M.; Gargaun, A.; Berezovski, M.V.; Kichkailo, A.S. Current and Prospective Protein Biomarkers of Lung Cancer. Cancers 2017, 9, 155. [Google Scholar] [CrossRef] [Green Version]
- Alcala, K.; Guida, F.; Johansson, M.; Johansson, M.; Robbins, H.A.; Smith-Byrne, K.; Stevens, V.; Zahed, H. Lung Cancer Cohort Consortium The Blood Proteome of Imminent Lung Cancer Diagnosis. medRxiv 2022. [Google Scholar] [CrossRef]
- Mehan, M.R.; Williams, S.A.; Siegfried, J.M.; Bigbee, W.L.; Weissfeld, J.L.; Wilson, D.O.; Pass, H.I.; Rom, W.N.; Muley, T.; Meister, M.; et al. Validation of a blood protein signature for non-small cell lung cancer. Clin. Proteom. 2014, 11, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyle, P.; Chapman, C.J.; Holdenrieder, S.; Murray, A.; Robertson, C.; Wood, W.C.; Maddison, P.; Healey, G.; Fairley, G.H.; Barnes, A.C.; et al. Clinical validation of an autoantibody test for lung cancer. Ann. Oncol. 2011, 22, 383–389. [Google Scholar] [CrossRef]
- Tan, E.M. Autoantibodies as reporters identifying aberrant cellular mechanisms in tumorigenesis. J. Clin. Investig. 2001, 108, 1411–1415. [Google Scholar] [CrossRef]
- Houghton, A.N. Cancer antigens: Immune recognition of self and altered self. J. Exp. Med. 1994, 180, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Tan, E.M.; Zhang, J. Autoantibodies to tumor-associated antigens: Reporters from the immune system. Immunol. Rev. 2008, 222, 328–340. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Tan, E.M. Autoantibodies to tumor-associated antigens as diagnostic biomarkers in hepatocellular carcinoma and other solid tumors. Expert Rev. Mol. Diagn. 2010, 10, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Veronesi, G.; Bianchi, F.; Infante, M.; Alloisio, M. The challenge of small lung nodules identified in CT screening: Can biomarkers assist diagnosis? Biomark. Med. 2016, 10, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Tsay, J.-C.J.; Li, J.; Yie, T.-A.; Munger, J.S.; Pass, H.; Rom, W.N.; Zhang, Y.; Tan, E.M.; Zhang, J.-Y. Autoantibodies against tumor-associated antigens in the early detection of lung cancer. Lung Cancer 2016, 99, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Burotto, M.; Thomas, A.; Subramaniam, D.; Giaccone, G.; Rajan, A. Biomarkers in Early-Stage Non–Small-Cell Lung Cancer: Current Concepts and Future Directions. J. Thorac. Oncol. 2014, 9, 1609–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Werner, S.; Tao, S.; Zörnig, I.; Brenner, H. Blood autoantibodies against tumor-associated antigens as biomarkers in early detection of colorectal cancer. Cancer Lett. 2014, 346, 178–187. [Google Scholar] [CrossRef]
- Lubin, R.; Zalcman, G.; Bouchet, L.; Trédaniel, J.; Legros, Y.; Cazals, D.; Hirsch, A.; Soussi, T. Serum p53 antibodies as early markers of lung cancer. Nat. Med. 1995, 1, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Chapman, C.; Healey, G.; Peek, L.; Parsons, G.; Baldwin, D.; Barnes, A.; Sewell, H.; Fritsche, H.; Robertson, J. Technical validation of an autoantibody test for lung cancer. Ann. Oncol. 2010, 21, 1687–1693. [Google Scholar] [CrossRef]
- Farlow, E.C.; Patel, K.; Basu, S.; Lee, B.S.; Kim, A.W.; Coon, J.S.; Faber, L.P.; Bonomi, P.; Liptay, M.J.; Borgia, J.A. Development of a Multiplexed Tumor-Associated Autoantibody-Based Blood Test for the Detection of Non–Small Cell Lung CancerEarly Detection Serum Test for NSCLC. Clin. Cancer Res. 2010, 16, 3452–3462. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Chang, W.; Zhao, J.; Yu, Y.; Tan, X.; Su, T.; Zhao, L.; Huang, S.; Liu, S.; Cao, G. Development of Autoantibody Signatures as Novel Diagnostic Biomarkers of Non–Small Cell Lung CancerNew Serum Diagnostic Biomarkers for NSCLC. Clin. Cancer Res. 2010, 16, 3760–3768. [Google Scholar] [CrossRef] [Green Version]
- Lam, S.; Boyle, P.; Healey, G.F.; Maddison, P.; Peek, L.; Murray, A.; Chapman, C.J.; Allen, J.; Wood, W.C.; Sewell, H.F.; et al. EarlyCDT-Lung: An Immunobiomarker Test as an Aid to Early Detection of Lung Cancer. Cancer Prev. Res. 2011, 4, 1126–1134. [Google Scholar] [CrossRef]
- Wang, T.; Liu, H.; Pei, L.; Wang, K.; Song, C.; Wang, P.; Ye, H.; Zhang, J.; Ji, Z.; Ouyang, S.; et al. Screening of tumor-associated antigens based on Oncomine database and evaluation of diagnostic value of autoantibodies in lung cancer. Clin. Immunol. 2020, 210, 108262. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, Y.; Liu, M.; Si, Q.; Wang, T.; Pei, L.; Wang, P.; Ye, H.; Shi, J.; Wang, X.; et al. A panel of autoantibodies against tumor-associated antigens in the early immunodiagnosis of lung cancer. Immunobiology 2020, 225, 151848. [Google Scholar] [CrossRef]
- Li, R.-Y.; Liang, Z.-Y. Circulating tumor DNA in lung cancer: Real-time monitoring of disease evolution and treatment response. Chin. Med. J. 2020, 133, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xiao, Y.; Zhao, J.; Chen, M.; Xu, Y.; Zhong, W.; Xing, J.; Wang, M. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology 2016, 21, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-M.; Krebs, M.G.; Lancashire, L.; Sloane, R.; Backen, A.; Swain, R.K.; Priest, L.J.C.; Greystoke, A.; Zhou, C.; Morris, K.; et al. Clinical Significance and Molecular Characteristics of Circulating Tumor Cells and Circulating Tumor Microemboli in Patients With Small-Cell Lung Cancer. J. Clin. Oncol. 2012, 30, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Kim, E.Y.; Lee, S.H.; Kwon, D.S.; Kim, A.; Chang, Y.S. Presence of mEGFR ctDNA predicts a poor clinical outcome in lung adenocarcinoma. Thorac. Cancer 2019, 10, 2267–2273. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.Y.; Lee, J.S.; Kim, I.A.; Kim, H.J.; Kim, W.S.; Lee, K.Y. Extracellular vesicle-based EGFR genotyping in bronchoalveolar lavage fluid from treatment-naive non-small cell lung cancer patients. Transl. Lung Cancer Res. 2019, 8, 1051. [Google Scholar] [CrossRef] [PubMed]
- Michaelidou, K.; Koutoulaki, C.; Mavridis, K.; Vorrias, E.; Papadaki, M.A.; Koutsopoulos, A.V.; Mavroudis, D.; Agelaki, S. Detection of KRAS G12/G13 Mutations in Cell Free-DNA by Droplet Digital PCR, Offers Prognostic Information for Patients with Advanced Non-Small Cell Lung Cancer. Cells 2020, 9, 2514. [Google Scholar] [CrossRef]
- Wen, S.W.C.; Andersen, R.F.; Hansen, T.F.; Nyhus, C.H.; Hager, H.; Hilberg, O.; Jakobsen, A. The prognostic impact of circulating homeobox A9 methylated DNA in advanced non-small cell lung cancer. Transl. Lung Cancer Res. 2021, 10, 855. [Google Scholar] [CrossRef]
- Pavan, A.; Bragadin, A.B.; Calvetti, L.; Ferro, A.; Zulato, E.; Attili, I.; Nardo, G.; Maso, A.D.; Frega, S.; Menin, A.G.; et al. Role of next generation sequencing-based liquid biopsy in advanced non-small cell lung cancer patients treated with immune checkpoint inhibitors: Impact of STK11, KRAS and TP53 mutations and co-mutations on outcome. Transl. Lung Cancer Res. 2021, 10, 202. [Google Scholar] [CrossRef]
- Suzuki, M.; Yoshino, I. Aberrant methylation in non-small cell lung cancer. Surg. Today 2010, 40, 602–607. [Google Scholar] [CrossRef]
- Brock, M.V.; Hooker, C.M.; Ota-Machida, E.; Han, Y.; Guo, M.; Ames, S.; Glöckner, S.; Piantadosi, S.; Gabrielson, E.; Pridham, G.; et al. DNA Methylation Markers and Early Recurrence in Stage I Lung Cancer. N. Engl. J. Med. 2008, 358, 1118–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khandelwal, A.; Seam, R.K.; Gupta, M.; Rana, M.K.; Prakash, H.; Vasquez, K.M.; Jain, A. Circulating micro RNA-590-5p functions as a liquid biopsy marker in non-small cell lung cancer. Cancer Sci. 2020, 111, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Boeri, M.; Verri, C.; Conte, D.; Roz, L.; Modena, P.; Facchinetti, F.; Calabrò, E.; Croce, C.M.; Pastorino, U.; Sozzi, G. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 3713–3718. [Google Scholar] [CrossRef] [Green Version]
- Tian, F.; Shen, Y.; Chen, Z.; Li, R.; Lu, J.; Ge, Q. Aberrant miR-181b-5p and miR-486-5p expression in serum and tissue of non-small cell lung cancer. Gene 2016, 591, 338–343. [Google Scholar] [CrossRef]
- Wang, Z.X.; Bian, H.B.; Wang, J.R.; Cheng, Z.X.; Wang, K.M.; De, W. Prognostic significance of serum miRNA-21 expression in human non-small cell lung cancer. J. Surg. Oncol. 2011, 104, 847–851. [Google Scholar] [CrossRef]
- Bianconi, F.; Palumbo, I.; Spanu, A.; Nuvoli, S.; Fravolini, M.L.; Palumbo, B. PET/CT Radiomics in Lung Cancer: An Overview. Appl. Sci. 2020, 10, 1718. [Google Scholar] [CrossRef] [Green Version]
- Yoneda, K.; Imanishi, N.; Ichiki, Y.; Tanaka, F. A liquid biopsy in primary lung cancer. Surg. Today 2019, 49, 1–14. [Google Scholar] [CrossRef]
- Kessler, M.D.; Pawar, N.R.; Martin, S.S.; Antalis, T.M.; O’Connor, T.D. Improving Cancer Detection and Treatment with Liquid Biopsies and ptDNA. Trends Cancer 2018, 4, 643–654. [Google Scholar] [CrossRef]
- Sorensen, B.S.; Wu, L.; Wei, W.; Tsai, J.; Weber, B.; Nexo, E.; Meldgaard, P. Monitoring of epidermal growth factor receptor tyrosine kinase inhibitor-sensitizing and resistance mutations in the plasma DNA of patients with advanced non–small cell lung cancer during treatment with erlotinib. Cancer 2014, 120, 3896–3901. [Google Scholar] [CrossRef]
- Reijans, M.; Gestel, S.; Haes, E.; Vandesteene, C.; Gomez, J.C.; Gouedard, C.; Patera, S.; Murray, S.; Maertens, G. Feasibility study of a ctEGFR prototype assay on the fully automated Idylla™ platform. Ann. Oncol. 2019, 30, v577. [Google Scholar] [CrossRef]
- Heeke, S.; Benzaquen, J.; Hofman, V.; Ilié, M.; Allegra, M.; Long-Mira, E.; Lassalle, S.; Tanga, V.; Salacroup, C.; Bonnetaud, C.; et al. Critical Assessment in Routine Clinical Practice of Liquid Biopsy for EGFR Status Testing in Non–Small-Cell Lung Cancer: A Single-Laboratory Experience (LPCE, Nice, France). Clin. Lung Cancer 2020, 21, 56–65.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H. Treatments for EGFR-mutant non-small cell lung cancer (NSCLC): The road to a success, paved with failures. Pharmacol. Ther. 2017, 174, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Cabanero, M.; Tsao, M.S. Circulating Tumour DNA in EGFR-Mutant Non-Small-Cell Lung Cancer. Curr. Oncol. 2018, 25, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Ding, P.; Becker, T.; Bray, V.; Chua, W.; Ma, Y.; Lynch, D.; Po, J.; Luk, A.; Caixeiro, N.; de Souza, P.; et al. The predictive and prognostic significance of liquid biopsy in advanced epidermal growth factor receptor-mutated non-small cell lung cancer: A prospective study. Lung Cancer 2019, 134, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Provencio, M.; Torrente, M.; Calvo, V.; Pérez-Callejo, D.; Gutiérrez, L.; Franco, F.; Pérez-Barrios, C.; Barquín, M.; Royuela, A.; García-García, F.; et al. Prognostic value of quantitative ctDNA levels in non small cell lung cancer patients. Oncotarget 2018, 9, 488. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Callejo, D.; Torrente, M.; Romero, A.; Provencio, M. Clinical and Investigational Applications of Liquid Biopsy in Non-Small Cell Lung Cancer. J. Tumor 2016, 4, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Nicolazzo, C.; Raimondi, C.; Mancini, M.; Caponnetto, S.; Gradilone, A.; Gandini, O.; Mastromartino, M.; Del Bene, G.; Prete, A.A.; Longo, F.; et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor Nivolumab. Sci. Rep. 2016, 6, 31726. [Google Scholar] [CrossRef]
- Boffa, D.J.; Graf, R.P.; Salazar, M.C.; Hoag, J.; Lu, D.; Krupa, R.; Louw, J.; Dugan, L.; Wang, Y.; Landers, M.; et al. Cellular Expression of PD-L1 in the Peripheral Blood of Lung Cancer Patients is Associated with Worse Survival. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Ilié, M.; Szafer-Glusman, E.; Hofman, V.; Chamorey, E.; Lalvée, S.; Selva, E.; Leroy, S.; Marquette, C.-H.; Kowanetz, M.; Hedge, P.; et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann. Oncol. 2018, 29, 193–199. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non–small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Goodman, A.M.; Kato, S.; Bazhenova, L.; Patel, S.P.; Frampton, G.M.; Miller, V.; Stephens, P.J.; Daniels, G.A.; Kurzrock, R. Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse CancersTMB Predicts Response to Immunotherapy in Diverse Cancers. Mol. Cancer Ther. 2017, 16, 2598–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singal, G.; Miller, P.; Agarwala, V.; Li, G.; Kaushik, G.; Backenroth, D.; Gossai, A.; Frampton, G.M.; Torres, A.Z.; Lehnert, E.M.; et al. Association of Patient Characteristics and Tumor Genomics With Clinical Outcomes Among Patients With Non–Small Cell Lung Cancer Using a Clinicogenomic Database. JAMA 2019, 321, 1391–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef]
- Zill, O.A.; Banks, K.C.; Fairclough, S.R.; Mortimer, S.A.; Vowles, J.V.; Mokhtari, R.; Gandara, D.R.; Mack, P.C.; Odegaard, J.I.; Nagy, R.J.; et al. The Landscape of Actionable Genomic Alterations in Cell-Free Circulating Tumor DNA from 21,807 Advanced Cancer PatientsSomatic Genomic Landscape of Circulating Tumor DNA. Clin. Cancer Res. 2018, 24, 3528–3538. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.T.; Yeap, B.Y.; Mino-Kenudson, M.; Digumarthy, S.R.; Costa, D.B.; Heist, R.S.; Solomon, B.; Stubbs, H.; Admane, S.; McDermott, U.; et al. Clinical Features and Outcome of Patients With Non–Small-Cell Lung Cancer Who Harbor EML4-ALK. J. Clin. Oncol. 2009, 27, 4247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson, R.J.A.; Karachaliou, N.; Berenguer, J.; Gimenez-Capitan, A.; Schellen, P.; Teixido, C.; Tannous, J.; Kuiper, J.L.; Drees, E.; Grabowska, M.; et al. Rearranged EML4-ALK fusion transcripts sequester in circulating blood platelets and enable blood-based crizotinib response monitoring in non-small-cell lung cancer. Oncotarget 2016, 7, 1066. [Google Scholar] [CrossRef] [Green Version]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef]
- Hochhegger, B.; Camargo, S.; Teles, G.B.D.S.; Chate, R.C.; Szarf, G.; Guimarães, M.D.; Gross, J.L.; Barbosa, P.N.V.P.; Chiarantano, R.S.; Reis, R.M.; et al. Challenges of Implementing Lung Cancer Screening in a Developing Country: Results of the Second Brazilian Early Lung Cancer Screening Trial (BRELT2). JCO Glob. Oncol. 2022, 8, e2100257. [Google Scholar] [CrossRef]
- Chiarantano, R.S.; Vazquez, F.L.; Franco, A.; Ferreira, L.C.; da Costa, M.C.; Talarico, T.; Oliveira, N.; Miziara, J.E.; Mauad, E.C.; da Silva, E.C.; et al. Implementation of an Integrated Lung Cancer Prevention and Screening Program Using a Mobile Computed Tomography (CT) Unit in Brazil. Cancer Control 2022, 29, 10732748221121385. [Google Scholar] [CrossRef]
- Blandin Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phallen, J.; Sausen, M.; Adleff, V.; Leal, A.; Hruban, C.; White, J.; Anagnostou, V.; Fiksel, J.; Cristiano, S.; Papp, E.; et al. Direct detection of early-stage cancers using circulating tumor DNA. Sci. Transl. Med. 2017, 9, eaan2415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Ramnath, N.; Nagrath, S. Current status of CTCs as liquid biopsy in lung cancer and future directions. Front. Oncol. 2015, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Foss, K.M.; Sima, C.; Ugolini, D.; Neri, M.; Allen, K.E.; Weiss, G.J. miR-1254 and miR-574-5p: Serum-Based microRNA Biomarkers for Early-Stage Non-small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 482–488. [Google Scholar] [CrossRef] [Green Version]
- Pastorino, U.; Boeri, M.; Sestini, S.; Sabia, F.; Milanese, G.; Silva, M.; Suatoni, P.; Verri, C.; Cantarutti, A.; Sverzellati, N.; et al. Baseline computed tomography screening and blood microRNA predict lung cancer risk and define adequate intervals in the BioMILD trial. Ann. Oncol. 2022, 33, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gaur, V.; Mir, I.A.; Saikia, J.; Kumar, S. MicroRNA-3692-3p is overexpressed in lung tumors but may not serve as a prognostic biomarker in lung cancer patients. Mol. Biol. Rep. 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Cao, D.; Hu, X.; Mo, W.; Yao, X.; Mo, J.; Wang, H. Circular RNAs Hsa_circ_101555 and Hsa_circ_008068 as Diagnostic Biomarkers for Early-Stage Lung Adenocarcinoma. Int. J. Gen. Med. 2022, 15, 5579. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, S.; Peng, C.; Liu, Y.; Huang, Y.; Ju, S. Circulating circular RNA hsa_circ_0023179 acts as a diagnostic biomarker for non-small-cell lung cancer detection. J. Cancer Res. Clin. Oncol. 2022, 1–12. [Google Scholar] [CrossRef]
- Robbins, H.A.; Alcala, K.; Moez, E.K.; Guida, F.; Thomas, S.; Zahed, H.; Warkentin, M.T.; Smith-Byrne, K.; Brhane, Y.; Muller, D.; et al. Design and methodological considerations for biomarker discovery and validation in the Integrative Analysis of Lung Cancer Etiology and Risk (INTEGRAL) Program. Ann. Epidemiol. 2023, 77, 1–12. [Google Scholar] [CrossRef]
- Lennon, A.M.; Buchanan, A.H.; Kinde, I.; Warren, A.; Honushefsky, A.; Cohain, A.T.; Ledbetter, D.H.; Sanfilippo, F.; Sheridan, K.; Rosica, D.; et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science 2020, 369, eabb9601. [Google Scholar] [CrossRef]
- LaRose, T.L.; Meheus, F.; Brennan, P.; Johansson, M.; Robbins, H.A. Assessment of Biomarker Testing for Lung Cancer Screening Eligibility. JAMA Netw. Open 2020, 3, e200409. [Google Scholar] [CrossRef] [Green Version]
- Vachani, A.; Carroll, N.M.; Simoff, M.J.; Neslund-Dudas, C.; Honda, S.; Greenlee, R.T.; Rendle, K.A.; Burnett-Hartman, A.; Ritzwoller, D.P. Stage Migration and Lung Cancer Incidence After Initiation of Low-Dose Computed Tomography Screening. J. Thorac. Oncol. 2022, 17, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Fatumo, S.; Chikowore, T.; Choudhury, A.; Ayub, M.; Martin, A.R.; Kuchenbaecker, K. A roadmap to increase diversity in genomic studies. Nat. Med. 2022, 28, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Saulsberry, L.; Olopade, O.I. Precision oncology: Directing genomics and pharmacogenomics toward reducing cancer inequities. Cancer Cell 2021, 39, 730–733. [Google Scholar] [CrossRef] [PubMed]


| Clinical Application | Biofluids | Methodologies | Reference | |
|---|---|---|---|---|
| ctDNA/cfDNA | Diagnosis, Tumor burden, Treatment response, Prognosis. | Peripheral blood, Sputum. | qPCR, dPCR, ddPCR, ARMS, BEAMing | [32,33,34,35] |
| CTCs | Diagnosis, Tumor burden, Prognosis. | Peripheral blood | RT-qPCR, Ep-CAM, NGS | [36,37,38] |
| Extracellular vesicles (exosomes) | Diagnosis, Prognosis. | Peripheral blood | ultracentrifugation, exosomes immunoprecipitation, immune beads precipitation | [39] |
| miRNAs | Diagnosis, Disease, Progression. | Plasma, Serum, Sputum. | RT-qPCR | [40] |
| DNA Methylation biomarkers | Diagnosis, Disease progression. | Plasma | Immunoprecipitation, methyl-sensitive restriction enzymes, sodium bisulfite conversion, q-PCR, and Next-Generation Techniques | [41,42] |
| Metabolites/Proteins | Progression, Predictive, Diagnosis, Prognosis. | Serum | HRMAS MRS UPLC–MS and immunoradiometric assay | [43,44,45,46] |
| Autoantibodies tumor-associated antigens | Diagnosis, Predictive. | Serum | ELISA | [47,48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casagrande, G.M.S.; Silva, M.d.O.; Reis, R.M.; Leal, L.F. Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs. Int. J. Mol. Sci. 2023, 24, 2505. https://doi.org/10.3390/ijms24032505
Casagrande GMS, Silva MdO, Reis RM, Leal LF. Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs. International Journal of Molecular Sciences. 2023; 24(3):2505. https://doi.org/10.3390/ijms24032505
Chicago/Turabian StyleCasagrande, Giovanna Maria Stanfoca, Marcela de Oliveira Silva, Rui Manuel Reis, and Letícia Ferro Leal. 2023. "Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs" International Journal of Molecular Sciences 24, no. 3: 2505. https://doi.org/10.3390/ijms24032505
APA StyleCasagrande, G. M. S., Silva, M. d. O., Reis, R. M., & Leal, L. F. (2023). Liquid Biopsy for Lung Cancer: Up-to-Date and Perspectives for Screening Programs. International Journal of Molecular Sciences, 24(3), 2505. https://doi.org/10.3390/ijms24032505







