Cannabinoids in the Modulation of Oxidative Signaling
Abstract
:1. Introduction
1.1. The Endocannabinoid System
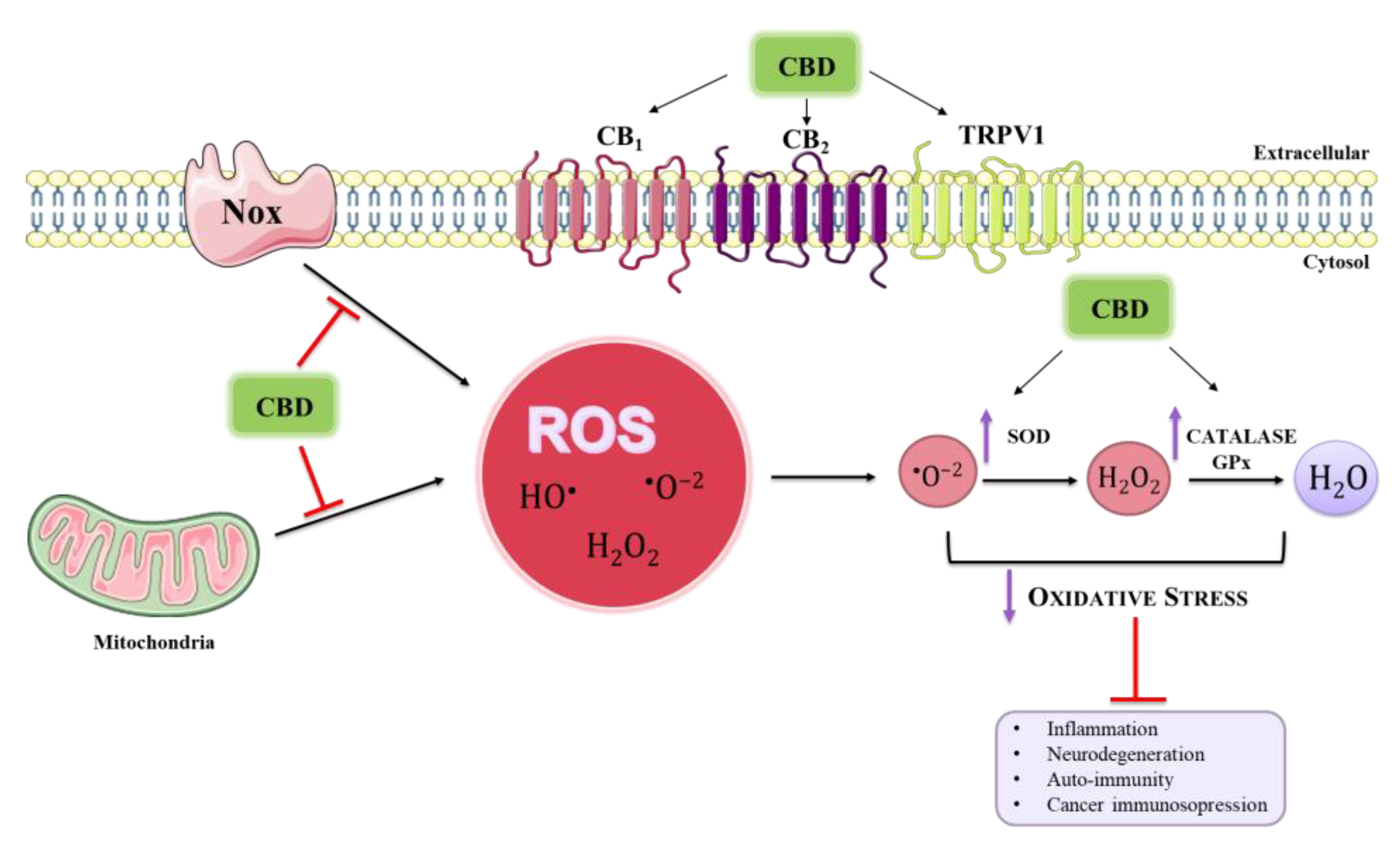
1.2. Oxidative Stress and Reactive Oxygen Species
1.3. Methodology
2. Cannabinoids and ROS Modulation in Neurodegenerative Diseases
3. Cannabinoids and ROS Modulation in Cancer
4. Cannabinoids and ROS Modulation in Inflammatory Diseases
5. Cannabinoids and ROS Modulation in the Immune System
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| 5-HT1A | 5-hydroxytryptamine receptor subtype 1A |
| AD | Alzheimer’s disease |
| Ads | Autoimmune diseases |
| AEA | N-arachidonoylethanolamide/anandamide |
| BBB | Blood brain barrier |
| cAMP | Cyclic adenosine monophosphate |
| CAT | Catalase |
| CB1 | Cannabinoid receptors 1 |
| CB2 | Cannabinoid receptors 2 |
| CBD | Cannabidiol |
| CBG | Cannabigerol |
| CGD | Chronic granulomatous diseases |
| CNS | Central nervous system |
| COX | Cyclooxygenase |
| CRC | Colorectal cancer |
| DAGLα/β | Diacylglycerol lipase-α and -β |
| DAGs | Diacylglycerols |
| EAE | Autoimmune encephalomyelitis |
| ECs | Endocannabinoids |
| ECS | Endocannabinoid system |
| FAAH | Fatty acid amide hydrolase |
| GPCRs | G-protein-coupled receptor |
| GPR55 | G-protein-coupled receptor 55 |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| H2O2 | Hydrogen peroxide |
| HD | Huntington’s disease |
| HO• | Hydroxyl radical |
| IB | Inflammatory bowel disease |
| iNOS | Inducible nitric oxide synthase |
| IS | Immune system |
| LDL | Low-density lipoproteins |
| LPS | Lipopolysaccharide |
| MAGL | Monoacyl glycerol lipase |
| MAPK | Mitogen-activated protein kinase |
| MS | Multiple sclerosis |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| NAPE | N-arachidonoyl phosphatidyl ethanolamine |
| NMDAr | N-methyl-D-aspartate receptor |
| NOX1 | NADPH oxidase 1 |
| NOX2 | NADPH oxidase 2 |
| NOX4 | NADPH oxidase 4 |
| O2 •− | Superoxide anion |
| PD | Parkinson’s disease |
| PI3K | Phosphoinositide 3-kinase |
| PNS | Peripheral nervous system |
| PPARs | Peroxisome proliferator-activated receptors |
| RA | Rheumatoid arthritis |
| Redox | Reduction-oxidation |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SCBs | Synthetic cannabinoids |
| SOD | Superoxide dismutase |
| T1DM | Type 1 diabetes mellitus |
| THC | Delta-9-tetrahydrocannabinol |
| TLR4 | Toll-like receptor 4 |
| TRPV1 | Transient receptor potential cation channel subfamily V member 1 |
| VLDL | Low density lipoprotein |
| XO | Xanthine oxidase |
References
- Pisanti, S.; Bifulco, M. Medical Cannabis: A plurimillennial history of an evergreen. J. Cell. Physiol. 2019, 234, 8342–8351. [Google Scholar] [CrossRef]
- Di Marzo, V.; Bifulco, M.; De Petrocellis, L. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef]
- Braile, M.; Marcella, S.; Marone, G.; Galdiero, M.R.; Varricchi, G.; Loffredo, S. The Interplay between the Immune and the Endocannabinoid Systems in Cancer. Cells 2021, 10, 1282. [Google Scholar] [CrossRef]
- Chayasirisobhon, S. The Role of Cannabidiol in Neurological Disorders. Perm. J. 2021, 25, 156. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature reviews. Neurology 2020, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid System: A Target for Cancer Treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [Green Version]
- De Petrocellis, L.; Melck, D.; Palmisano, A.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation. Proc. Natl. Acad. Sci. USA 1998, 95, 8375–8380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisanti, S.; Picardi, P.; D’Alessandro, A.; Laezza, C.; Bifulco, M. The endocannabinoid signaling system in cancer. Trends Pharmacol. Sci. 2013, 34, 273–282. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Avilia, G.; Bifulco, M.; Laezza, C. Cannabinoids: Therapeutic Use in Clinical Practice. Int. J. Mol. Sci. 2022, 23, 3344. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Borgonetti, V.; Brighenti, V.; Biagi, M.; Benvenuti, S.; Corsi, L. Cannabis sativa L. and Nonpsychoactive Cannabinoids: Their Chemistry and Role against Oxidative Stress, Inflammation, and Cancer. BioMed Res. Int. 2018, 1691428. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Ni, K.; Deng, H.; Chen, X. Dancing with reactive oxygen species generation and elimination in nanotheranostics for disease treatment. Adv. Drug Deliv. Rev. 2020, 158, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Hackett, B.; O’Driscoll, D.N.; Sun, M.C.; Downer, E.J. Cannabidiol modulation of oxidative stress and signalling. Neuronal Signal. 2021, 5, NS20200080. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Malheiro, R.F.; Carmo, H.; Carvalho, F.; Silva, J. Cannabinoid-mediated targeting of mitochondria on the modulation of mitochondrial function and dynamics. Pharmacol. Res. 2022, 187, 106603. [Google Scholar] [CrossRef]
- Dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef]
- Sacks, D.; Baxter, B.; Campbell, B.C.V.; Carpenter, J.S.; Cognard, C.; Dippel, D.; Eesa, M.; Fischer, U.; Hausegger, K.; Hirsch, J.A.; et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2018, 13, 612–632. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef]
- Negre-Salvayre, A.; Guerby, P.; Gayral, S.; Laffargue, M.; Salvayre, R. Role of reactive oxygen species in atherosclerosis: Lessons from murine genetic models. Free Radic. Biol. Med. 2020, 149, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Cocetta, V.; Governa, P.; Borgonetti, V.; Tinazzi, M.; Peron, G.; Catanzaro, D.; Berretta, M.; Biagi, M.; Manetti, F.; Dall’Acqua, S.; et al. Cannabidiol Isolated from Cannabis sativa L. Protects Intestinal Barrier from In Vitro Inflammation and Oxidative Stress. Front. Pharmacol. 2021, 12, 641210. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Shekher, A.; Puneet Narula, A.S.; Abrahamse, H.; Gupta, S.C. Cannabis and its constituents for cancer: History, biogenesis, chemistry and pharmacological activities. Pharmacol. Res. 2021, 163, 105302. [Google Scholar] [CrossRef]
- Acharya, N.; Penukonda, S.; Shcheglova, T.; Hagymasi, A.T.; Basu, S.; Srivastava, P.K. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc. Natl. Acad. Sci. USA 2017, 114, 5005–5010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, S.S.; Hayes, J.P.; Fiore, N.T.; Moalem-Taylor, G. The cannabinoid system and microglia in health and disease. Neuropharmacology 2021, 190, 108555. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Mesa, X.M.; Moreno Vergara, A.F.; Contreras Bolaños, L.A.; Guevara Moriones, N.; Mejía Piñeros, A.L.; Santander González, S.P. Therapeutic Prospects of Cannabinoids in the Immunomodulation of Prevalent Autoimmune Diseases. Cannabis Cannabinoid Res. 2021, 6, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Juknat, A.; Gao, F.; Kaushansky, N.; Coppola, G.; Vogel, Z. Pathways and gene networks mediating the regulatory effects of cannabidiol, a nonpsychoactive cannabinoid, in autoimmune T cells. J. Neuroinflamm. 2016, 13, 136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangel-López, E.; Colín-González, A.L.; Paz-Loyola, A.L.; Pinzón, E.; Torres, I.; Serratos, I.N.; Castellanos, P.; Wajner, M.; Souza, D.O.; Santamaría, A. Cannabinoid receptor agonists reduce the short-term mitochondrial dysfunction and oxidative stress linked to excitotoxicity in the rat brain. Neuroscience 2015, 285, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Yousaf, M.; Chang, D.; Liu, Y.; Liu, T.; Zhou, X. Neuroprotection of Cannabidiol, Its Synthetic Derivatives and Combination Preparations against Microglia-Mediated Neuroinflammation in Neurological Disorders. Molecules 2022, 27, 4961. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Jin, S.; Lu, Y.; Peng, Y.; Zhao, L.; Wang, X. Cannabidiol protects against Alzheimer’s disease in C. elegans via ROS scavenging activity of its phenolic hydroxyl groups. Eur. J. Pharmacol. 2022, 919, 174829. [Google Scholar] [CrossRef]
- Harvey, B.S.; Ohlsson, K.S.; Mååg, J.L.; Musgrave, I.F.; Smid, S.D. Contrasting protective effects of cannabinoids against oxidative stress and amyloid-β evoked neurotoxicity in vitro. Neurotoxicology 2012, 33, 138–146. [Google Scholar] [CrossRef]
- Elmazoglu, Z.; Rangel-López, E.; Medina-Campos, O.N.; Pedraza-Chaverri, J.; Túnez, I.; Aschner, M.; Santamaría, A.; Karasu, Ç. Cannabinoid-profiled agents improve cell survival via reduction of oxidative stress and inflammation, and Nrf2 activation in a toxic model combining hyperglycemia+Aβ1-42 peptide in rat hippocampal neurons. Neurochem. Int. 2020, 140, 104817. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Kolishetti, N.; Arias, A.Y.; Vashist, A.; Nair, M. Cannabidiol for neurodegenerative disorders: A comprehensive review. Front. Pharmacol. 2022, 13, 989717. [Google Scholar] [CrossRef] [PubMed]
- García, S.; Martín Giménez, V.M.; Mocayar Marón, F.J.; Reiter, R.J.; Manucha, W. Melatonin and cannabinoids: Mitochondrial-targeted molecules that may reduce inflammaging in neurodegenerative diseases. Histol. Histopathol. 2020, 35, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, C.; García-Martín, A.; Rolland, A.; DeMesa, J.; Muñoz, E. Cannabidiol and Other Cannabinoids in Demyelinating Diseases. Int. J. Mol. Sci. 2021, 22, 2992. [Google Scholar] [CrossRef]
- Pagano, C.; Navarra, G.; Coppola, L.; Bifulco, M.; Laezza, C. Molecular Mechanism of Cannabinoids in Cancer Progression. Int. J. Mol. Sci. 2021, 22, 3680. [Google Scholar] [CrossRef] [PubMed]
- Senga, S.S.; Grose, R.P. Hallmarks of cancer-the new testament. Open Biol. 2021, 11, 200358. [Google Scholar] [CrossRef]
- Dariš, B.; Tancer Verboten, M.; Knez, Ž.; Ferk, P. Cannabinoids in cancer treatment: Therapeutic potential and legislation. Bosn. J. Basic Med. Sci. 2019, 19, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisanti, S.; Malfitano, A.M.; Grimaldi, C.; Santoro, A.; Gazzerro, P.; Laezza, C.; Bifulco, M. Use of cannabinoid receptor agonists in cancer therapy as palliative and curative agents. Best Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Kisková, T.; Mungenast, F.; Suváková, M.; Jäger, W.; Thalhammer, T. Future Aspects for Cannabinoids in Breast Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Semlali, A.; Beji, S.; Ajala, I.; Rouabhia, M. Effects of tetrahydrocannabinols on human oral cancer cell proliferation, apoptosis, autophagy, oxidative stress, and DNA damage. Arch. Oral Biol. 2021, 129, 105200. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ma, J.; Lu, W. The Significance of Mitochondrial Dysfunction in Cancer. Int. J. Mol. Sci. 2020, 21, 5598. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, A.; Zhu, M.; Su, Q.; Zhu, Z.; Yang, T.; Chen, Y.; Peng, X.; Zhang, Y. Targeting mitochondrial dysfunctions in pancreatic cancer evokes new therapeutic opportunities. Crit. Rev. Oncol./Hematol. 2022, 180, 103858. [Google Scholar] [CrossRef]
- Sun, D.; Li, X.; Nie, S.; Liu, J.; Wang, S. Disorders of cancer metabolism: The therapeutic potential of cannabinoids. Biomed. Pharmacother. 2023, 157, 113993. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Berliner, J.A.; Navab, M.; Fogelman, A.M.; Frank, J.S.; Demer, L.L.; Edwards, P.A.; Watson, A.D.; Lusis, A.J. Atherosclerosis: Basic mechanisms. Oxidation, inflammation, and genetics. Circulation 1995, 91, 2488–2496. [Google Scholar] [CrossRef]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef] [Green Version]
- Singla, S.; Sachdeva, R.; Mehta, J.L. Cannabinoids and atherosclerotic coronary heart disease. Clin. Cardiol. 2012, 35, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Van Eenige, R.; Ying, Z.; Tambyrajah, L.; Pronk, A.C.M.; Blomberg, N.; Giera, M.; Wang, Y.; Coskun, T.; van der Stelt, M.; Rensen, P.C.N.; et al. Cannabinoid type 1 receptor inverse agonism attenuates dyslipidemia and atherosclerosis in APOE∗3-Leiden.CETP mice. J. Lipid Res. 2021, 62, 100070. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, C.; Garcia-Martin, A.; DeMesa, J.; Muñoz, E. Cannabinoids in Metabolic Syndrome and Cardiac Fibrosis. Curr. Hypertens. Rep. 2020, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- Barutta, F.; Bruno, G.; Mastrocola, R.; Bellini, S.; Gruden, G. The role of cannabinoid signaling in acute and chronic kidney diseases. Kidney Int. 2018, 94, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Udi, S.; Hinden, L.; Ahmad, M.; Drori, A.; Iyer, M.R.; Cinar, R.; Herman-Edelstein, M.; Tam, J. Dual inhibition of cannabinoid CB1 receptor and inducible NOS attenuates obesity-induced chronic kidney disease. Br. J. Pharmacol. 2020, 177, 110–127. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, P.; Rajesh, M.; Pan, H.; Patel, V.; Mukhopadhyay, B.; Bátkai, S.; Gao, B.; Haskó, G.; Pacher, P. Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free. Radic. Biol. Med. 2010, 48, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borrelli, F.; Fasolino, I.; Romano, B.; Capasso, R.; Maiello, F.; Coppola, D.; Orlando, P.; Battista, G.; Pagano, E.; Di Marzo, V.; et al. Beneficial effect of the non-psychotropic plant cannabinoid cannabigerol on experimental inflammatory bowel disease. Biochem. Pharmacol. 2013, 85, 1306–1316. [Google Scholar] [CrossRef]
- Bayram, S.; Parlar, A.; Arslan, S.O. The curative effect of cannabinoid 2 receptor agonist on functional failure and disruptive inflammation caused by intestinal ischemia and reperfusion. Fundam. Clin. Pharmacol. 2020, 34, 80–90. [Google Scholar] [CrossRef]
- Lin, X.; Huang, T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free. Radic. Res. 2016, 50, 585–595. [Google Scholar] [CrossRef]
- Dobrică, E.C.; Cozma, M.A.; Găman, M.A.; Voiculescu, V.M.; Găman, A.M. The Involvement of Oxidative Stress in Psoriasis: A Systematic Review. Antioxidants 2022, 11, 282. [Google Scholar] [CrossRef]
- Norooznezhad, A.H.; Norooznezhad, F. Cannabinoids: Possible agents for treatment of psoriasis via suppression of angiogenesis and inflammation. Med. Hypotheses 2017, 99, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, P.; Garley, M.; Wroński, A.; Jabłońska, E.; Skrzydlewska, E. Cannabidiol Modifies the Formation of NETs in Neutrophils of Psoriatic Patients. Int. J. Mol. Sci. 2020, 21, 6795. [Google Scholar] [CrossRef] [PubMed]
- Ambrożewicz, E.; Wójcik, P.; Wroński, A.; Łuczaj, W.; Jastrząb, A.; Žarković, N.; Skrzydlewska, E. Pathophysiological Alterations of Redox Signaling and Endocannabinoid System in Granulocytes and Plasma of Psoriatic Patients. Cells 2018, 7, 159. [Google Scholar] [CrossRef] [Green Version]
- Parlar, A.; Arslan, S.O.; Yumrutas, O.; Elibol, E.; Yalcin, A.; Uckardes, F.; Aydin, H.; Dogan, M.F.; Kayhan Kustepe, E.; Ozer, M.K. Effects of cannabinoid receptor 2 synthetic agonist, AM1241, on bleomycin induced pulmonary fibrosis. Biotech. Histochem. Off. Publ. Biol. Stain. Comm. 2021, 96, 48–59. [Google Scholar] [CrossRef]
- Omari, S.A.; Adams, M.J.; Geraghty, D.P. TRPV1 Channels in Immune Cells and Hematological Malignancies. Adv. Pharmacol. 2017, 79, 173–198. [Google Scholar] [CrossRef] [PubMed]
- Almogi-Hazan, O.; Or, R. Cannabis, the Endocannabinoid System and Immunity-the Journey from the Bedside to the Bench and Back. Int. J. Mol. Sci. 2020, 21, 4448. [Google Scholar] [CrossRef]
- Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. [Google Scholar] [CrossRef]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.W.; Kim, Y.; Kang, J.H.; Kang, S.S.; Ahn, Y.K.; Park, C.S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef] [Green Version]
- Balenga, N.A.; Aflaki, E.; Kargl, J.; Platzer, W.; Schröder, R.; Blättermann, S.; Kostenis, E.; Brown, A.J.; Heinemann, A.; Waldhoer, M. GPR55 regulates cannabinoid 2 receptor-mediated responses in human neutrophils. Cell Res. 2011, 21, 1452–1469. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Arif, M.; Varga, Z.V.; Mátyás, C.; Paloczi, J.; Lehocki, A.; Haskó, G.; Pacher, P. Cannabinoid receptor 2 activation alleviates diabetes-induced cardiac dysfunction, inflammation, oxidative stress, and fibrosis. GeroScience 2022, 44, 1727–1741. [Google Scholar] [CrossRef]
- Matouk, A.I.; Taye, A.; El-Moselhy, M.A.; Heeba, G.H.; Abdel-Rahman, A.A. Abnormal cannabidiol confers cardioprotection in diabetic rats independent of glycemic control. Eur. J. Pharmacol. 2018, 820, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Vella, R.K.; Jackson, D.J.; Fenning, A.S. Δ9-Tetrahydrocannabinol Prevents Cardiovascular Dysfunction in STZ-Diabetic Wistar-Kyoto Rats. BioMed Res. Int. 2017, 2017, 7974149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Fan, D.; Cao, X.; Ye, Q.; Wang, Q.; Zhang, M.; Xiao, C. The Role of Reactive Oxygen Species in the Rheumatoid Arthritis-Associated Synovial Microenvironment. Antioxidants 2022, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wu, H.; Du, Y. The roles of small-molecule inflammatory mediators in rheumatoid arthritis. Scand. J. Immunol. 2021, 93, e12982. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, S.G.; Naidu, P.S.; Cravatt, B.F.; Dudley, D.T.; Lichtman, A.H. Fatty acid amide hydrolase blockade attenuates the development of collagen-induced arthritis and related thermal hyperalgesia in mice. Pharmacol. Biochem. Behav. 2011, 99, 718–725. [Google Scholar] [CrossRef] [Green Version]
- Lowin, T.; Straub, R.H. Cannabinoid-based drugs targeting CB1 and TRPV1, the sympathetic nervous system, and arthritis. Arthritis Res. Ther. 2015, 17, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gui, H.; Liu, X.; Liu, L.R.; Su, D.F.; Dai, S.M. Activation of cannabinoid receptor 2 attenuates synovitis and joint distruction in collagen-induced arthritis. Immunobiology 2015, 220, 817–822. [Google Scholar] [CrossRef]
- Logashina, Y.A.; Palikova, Y.A.; Palikov, V.A.; Kazakov, V.A.; Smolskaya, S.V.; Dyachenko, I.A.; Tarasova, N.V.; Andreev, Y.A. Anti-Inflammatory and Analgesic Effects of TRPV1 Polypeptide Modulator APHC3 in Models of Osteo- and Rheumatoid Arthritis. Mar. Drugs 2021, 19, 39. [Google Scholar] [CrossRef]
- Szabó, A.; Helyes, Z.; Sándor, K.; Bite, A.; Pintér, E.; Németh, J.; Bánvölgyi, A.; Bölcskei, K.; Elekes, K.; Szolcsányi, J. Role of transient receptor potential vanilloid 1 receptors in adjuvant-induced chronic arthritis: In vivo study using gene-deficient mice. J. Pharmacol. Exp. Ther. 2005, 314, 111–119. [Google Scholar] [CrossRef]
- Pegoretti, V.; Swanson, K.A.; Bethea, J.R.; Probert, L.; Eisel, U.L.M.; Fischer, R. Inflammation and Oxidative Stress in Multiple Sclerosis: Consequences for Therapy Development. Oxidative Med. Cell. Longev. 2020, 2020, 7191080. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol Attenuates Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis Through Induction of Myeloid-Derived Suppressor Cells. Front. Immunol. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Kummari, E.; Sherman, J.; Yang, E.J.; Dhital, S.; Gilfeather, C.; Yray, G.; Morgan, T.; Kaplan, B.L.F. CBD Suppression of EAE Is Correlated with Early Inhibition of Splenic IFN-γ + CD8+ T Cells and Modest Inhibition of Neuroinflammation. J. Neuroimmune Pharmacol. Off. J. Soc. NeuroImmune Pharmacol. 2021, 16, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, Z.; Zhang, J. Pathomechanisms of Oxidative Stress in Inflammatory Bowel Disease and Potential Antioxidant Therapies. Oxidative Med. Cell. Longev. 2017, 2017, 4535194. [Google Scholar] [CrossRef] [Green Version]
- Massa, F.; Marsicano, G.; Hermann, H.; Cannich, A.; Monory, K.; Cravatt, B.F.; Ferri, G.L.; Sibaev, A.; Storr, M.; Lutz, B. The endogenous cannabinoid system protects against colonic inflammation. J. Clin. Investig. 2004, 113, 1202–1209. [Google Scholar] [CrossRef] [Green Version]
- Storr, M.A.; Keenan, C.M.; Emmerdinger, D.; Zhang, H.; Yüce, B.; Sibaev, A.; Massa, F.; Buckley, N.E.; Lutz, B.; Göke, B.; et al. Targeting endocannabinoid degradation protects against experimental colitis in mice: Involvement of CB1 and CB2 receptors. J. Mol. Med. 2008, 86, 925–936. [Google Scholar] [CrossRef]


| CANNABINOIDS | DISEASES | EFFECTS |
|---|---|---|
| THC, CBD | Neurodegenerative: AD, HD, epilepsy, MS, PD | ↓ ROS production [18] ↓ proinflammatory cytokines secretion [18] ↓ NFkB signaling [18] ↑ CB1-CB2 [19] |
| THC, CBD, AEA, 2-AG | Cancer | ↓ ROS production [20] ↑ GSH [20] |
| CBD, CBG, ECs | Inflammatory | ↓ ROS production [21] ↓ inflammation [21] ↓ oxidative/nitrosative stress [22] Alteration of XO, NOX1 and NOX4 [23] |
| THC, CBD, ECs | Immune system | ↓ ROS production [24] ↓ neutrophil degranulation [24] ↑ CX3CR1hi macrophages [25,26] ↓ lipid peroxidation [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, C.; Savarese, B.; Coppola, L.; Navarra, G.; Avilia, G.; Laezza, C.; Bifulco, M. Cannabinoids in the Modulation of Oxidative Signaling. Int. J. Mol. Sci. 2023, 24, 2513. https://doi.org/10.3390/ijms24032513
Pagano C, Savarese B, Coppola L, Navarra G, Avilia G, Laezza C, Bifulco M. Cannabinoids in the Modulation of Oxidative Signaling. International Journal of Molecular Sciences. 2023; 24(3):2513. https://doi.org/10.3390/ijms24032513
Chicago/Turabian StylePagano, Cristina, Beatrice Savarese, Laura Coppola, Giovanna Navarra, Giorgio Avilia, Chiara Laezza, and Maurizio Bifulco. 2023. "Cannabinoids in the Modulation of Oxidative Signaling" International Journal of Molecular Sciences 24, no. 3: 2513. https://doi.org/10.3390/ijms24032513
APA StylePagano, C., Savarese, B., Coppola, L., Navarra, G., Avilia, G., Laezza, C., & Bifulco, M. (2023). Cannabinoids in the Modulation of Oxidative Signaling. International Journal of Molecular Sciences, 24(3), 2513. https://doi.org/10.3390/ijms24032513










