Non-Surgical Periodontal Treatment Impact on Subgingival Microbiome and Intra-Oral Halitosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Eligibility Criteria
2.3. Variables
2.3.1. Sociodemographic and Medical Questionnaires
2.3.2. Periodontal Assessment
2.3.3. Halitosis Assessment
2.3.4. Treatment Protocol
2.3.5. Subgingival Microbiome Sequencing
2.4. Measurement Reliability and Reproducibility
2.5. Statistical Analysis
3. Results
3.1. Impact of Periodontal Treatment on Subgingival Microbiome
3.2. Correlation between Subgingival Microbiome Abundance and VSC before and after Periodontal Treatment
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dye, B.A. Global Periodontal Disease Epidemiology. Periodontol. 2000 2012, 58, 10–25. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Periodontal Microbial Ecology. Periodontol. 2000 2005, 38, 135–187. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Paster, B.J.; Boches, S.K.; Galvin, J.L.; Ericson, R.E.; Lau, C.N.; Levanos, V.A.; Sahasrabudhe, A.; Dewhirst, F.E. Bacterial Diversity in Human Subgingival Plaque. J. Bacteriol. 2001, 183, 3770–3783. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Kinney, J.S.; Morelli, T.; Braun, T.; Ramseier, C.A.; Herr, A.E.; Sugai, J.V.; Shelburne, C.E.; Rayburn, L.A.; Singh, A.K.; Giannobile, W.V. Saliva/Pathogen Biomarker Signatures and Periodontal Disease Progression. J. Dent. Res. 2011, 90, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Liljestrand, J.M.; Gursoy, U.K.; Hyvärinen, K.; Sorsa, T.; Suominen, A.L.; Könönen, E.; Pussinen, P.J. Combining Salivary Pathogen and Serum Antibody Levels Improves Their Diagnostic Ability in Detection of Periodontitis. J. Periodontol. 2014, 85, 123–131. [Google Scholar] [CrossRef]
- Botelho, J.; Mascarenhas, P.; Viana, J.; Proença, L.; Orlandi, M.; Leira, Y.; Chambrone, L.; Mendes, J.J.; Machado, V. An umbrella review of the evidence linking oral health and systemic noncommunicable diseases. Nat. Commun. 2022, 13, 7614. [Google Scholar] [CrossRef]
- Calil, C.; Liberato, F.L.; Pereira, A.C.; de Castro Meneghim, M.; Goodson, J.M.; Groppo, F.C. The Relationship between Volatile Sulphur Compounds, Tongue Coating and Periodontal Disease. Int. J. Dent. Hyg. 2009, 7, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Yaegaki, K.; Sanada, K. Biochemical and Clinical Factors Influencing Oral Malodor in Periodontal Patients. J. Periodontol. 1992, 63, 783–789. [Google Scholar] [CrossRef]
- Buset, S.L.; Walter, C.; Friedmann, A.; Weiger, R.; Borgnakke, W.S.; Zitzmann, N.U. Are Periodontal Diseases Really Silent? A Systematic Review of Their Effect on Quality of Life. J. Clin. Periodontol. 2016, 43, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.; Fraser, H.M.; McCracken, G.I.; Stone, K.M.; John, M.T.; Preshaw, P.M. Impact of Periodontitis on Oral Health-Related Quality of Life. J. Dent. 2013, 41, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Rayman, S.; Almas, K. Halitosis among Racially Diverse Populations: An Update. Int. J. Dent. Hyg. 2008, 6, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Delanghe, G.; Ghyselen, J.; van Steenberghe, D.; Feenstra, L. Multidisciplinary Breath-Odour Clinic. Lancet 1997, 350, 187. [Google Scholar] [CrossRef] [PubMed]
- Tonzetich, J. Production and Origin of Oral Malodor: A Review of Mechanisms and Methods of Analysis. J. Periodontol. 1977, 48, 13–20. [Google Scholar] [CrossRef]
- Bolepalli, A.C.; Munireddy, C.; Peruka, S.; Polepalle, T.; Choudary Alluri, L.S.; Mishaeel, S. Determining the Association between Oral Malodor and Periodontal Disease: A Case Control Study. J. Int. Soc. Prev. Community Dent. 2015, 5, 413–418. [Google Scholar] [CrossRef]
- Milella, L. The Negative Effects of Volatile Sulphur Compounds. J. Vet. Dent. 2015, 32, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Farnum, A.; Parnas, M.; Hoque Apu, E.; Cox, E.; Lefevre, N.; Contag, C.H.; Saha, D. Harnessing Insect Olfactory Neural Circuits for Detecting and Discriminating Human Cancers. Biosens. Bioelectron. 2023, 219, 114814. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.P.V.; Bennet, S.; Cotton, S.L.; Goodson, J.M.; Kent, R.; Haffajee, A.D.; Socransky, S.S.; Hasturk, H.; Van Dyke, T.E.; Dewhirst, F.E.; et al. Impact of Periodontal Therapy on the Subgingival Microbiota of Severe Periodontitis: Comparison Between Good Responders and Individuals with Refractory Periodontitis Using the Human Oral Microbe Identification Microarray. J. Periodontol. 2012, 83, 1279–1287. [Google Scholar] [CrossRef]
- Yaegaki, K.; Coil, J.M. Examination, Classification, and Treatment of Halitosis; Clinical Perspectives. J. Can. Dent. Assoc. 2000, 66, 257–261. [Google Scholar] [PubMed]
- Kostelc, J.G.; Preti, G.; Zelson, P.R.; Brauner, L.; Baehni, P. Oral Odors in Early Experimental Gingivitis. J. Periodontal. Res. 1984, 19, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.; Tonzetich, J. Effect of Hydrogen Sulfide and Methyl Mercaptan on the Permeability of Oral Mucosa. J. Dent. Res. 1984, 63, 994–997. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The Plaque Control Record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef] [PubMed]
- Hamp, S.-E.; Nyman, S.; Lindhe, J. Periodontal Treatment of Multi Rooted Teeth. Results after 5 Years. J. Clin. Periodontol. 1975, 2, 126–135. [Google Scholar] [CrossRef]
- Lindhe, J.; Nyman, S. The Role of Occlusion in Periodontal Disease and the Biological Rationale for Splinting in Treatment of Periodontitis. Oral Sci. Rev. 1977, 10, 11–43. [Google Scholar] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and Grading of Periodontitis: Framework and Proposal of a New Classification and Case Definition. J. Periodontol. 2018, 89, S159–S172. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, A.; Riggio, M.; Rolph, H.; Bagg, J.; Hodge, P. Clinical Examination of Subjects with Halitosis. Oral Dis. 2007, 13, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Matos, F.; Aranha, A.; Borges, Á.; Pedro, F.; Raslan, S.; Hamida, F.; Veiga, K.; Porto, A. Can Different Stages of Leprosy Treatment Influence the Profile of Oral Health? Oral Status in Leprosy. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e376–e383. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-Filtering Vastly Improves Diversity Estimates from Illumina Amplicon Sequencing. Nat. Methods 2013, 10, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E.; et al. Chimeric 16S RRNA Sequence Formation and Detection in Sanger and 454-Pyrosequenced PCR Amplicons. Genome Res. 2011, 21, 494–504. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S RRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of RRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Greenman, J. Halitology (Breath Odour: Aetiopathogenesis and Management). Oral Dis. 2012, 18, 333–345. [Google Scholar] [CrossRef]
- Morita, M.; Wang, H.L. Relationship between Sulcular Sulfide Level and Oral Malodor in Subjects with Periodontal Disease. J. Periodontol. 2001, 72, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Setoguchi, T.; Machigashira, M.; Yamamoto, M.; Yotsumoto, Y.; Yoshimori, M.; Izumi, Y.; Yaegaki, K. The Effects of Methyl Mercaptan on Epithelial Cell Growth and Proliferation. Int. Dent. J. 2002, 52 (Suppl. 3), 241–246. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W. Fusobacterium Nucleatum: A Commensal-Turned Pathogen. Curr. Opin. Microbiol. 2015, 23, 141–147. [Google Scholar] [CrossRef]
- Field, C.A.; Gidley, M.D.; Preshaw, P.M.; Jakubovics, N. Investigation and Quantification of Key Periodontal Pathogens in Patients with Type 2 Diabetes. J. Periodontal. Res. 2012, 47, 470–478. [Google Scholar] [CrossRef]
- Moore, W.E.; Moore, L.V. The Bacteria of Periodontal Diseases. Periodontol. 2000 1994, 5, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Griffen, A.L.; Beall, C.J.; Campbell, J.H.; Firestone, N.D.; Kumar, P.S.; Yang, Z.K.; Podar, M.; Leys, E.J. Distinct and Complex Bacterial Profiles in Human Periodontitis and Health Revealed by 16S Pyrosequencing. ISME J. 2012, 6, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Loozen, G.; Ozcelik, O.; Boon, N.; De Mol, A.; Schoen, C.; Quirynen, M.; Teughels, W. Inter-Bacterial Correlations in Subgingival Biofilms: A Large-Scale Survey. J. Clin. Periodontol. 2014, 41, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.-Y.; Zhang, Q.; Li, J.-L.; Yang, S.-H.; Shi, Q. Progression of Periodontal Inflammation in Adolescents Is Associated with Increased Number of PorphyromonasGingivalis, Prevotella Intermedia, TannerellaForsythensis, and Fusobacterium Nucleatum. Int. J. Paediatr. Dent. 2014, 24, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Riep, B.; Edesi-Neuss, L.; Claessen, F.; Skarabis, H.; Ehmke, B.; Flemmig, T.F.; Bernimoulin, J.-P.; Göbel, U.B.; Moter, A. Are Putative Periodontal Pathogens Reliable Diagnostic Markers? J. Clin. Microbiol. 2009, 47, 1705–1711. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, T.; Suzuki, N.; Nakano, Y.; Yasui, M.; Yoneda, M.; Shimazaki, Y.; Hirofuji, T.; Yamashita, Y. Discrimination of the Oral Microbiota Associated with High Hydrogen Sulfide and Methyl Mercaptan Production. Sci. Rep. 2012, 2, 215. [Google Scholar] [CrossRef]
- Hsu, T.; Gemmell, M.R.; Franzosa, E.A.; Berry, S.; Mukhopadhya, I.; Hansen, R.; Michaud, M.; Nielsen, H.; Miller, W.G.; Nielsen, H.; et al. Comparative Genomics and Genome Biology of Campylobacter Showae. Emerg. Microbes Infect. 2019, 8, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Seerangaiyan, K.; van Winkelhoff, A.J.; Harmsen, H.J.M.; Rossen, J.W.A.; Winkel, E.G. The Tongue Microbiome in Healthy Subjects and Patients with Intra-Oral Halitosis. J. Breath Res. 2017, 11, 036010. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Cordero, S.; Hoffman, H.; Stahl, S.S. Occurrence of Staphylococcus in Periodontal Pockets of Diabetic and Nondiabetic Adults. J. Periodontol. 1979, 50, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Leadbetter, E.R.; Holt, S.C.; Socransky, S.S. Capnocytophaga: New Genus of Gram-Negative Gliding Bacteria. I. General Characteristics, Taxonomic Considerations and Significance. Arch. Microbiol. 1979, 122, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Spratt, D.A.; Greenman, J.; Schaffer, A.G. Capnocytophaga Gingivalis: Effects of Glucose Concentration on Growth and Hydrolytic Enzyme Production. Microbiology 1996, 142 Pt 8, 2161–2164. [Google Scholar] [CrossRef]
- Fujimura, M.; Calenic, B.; Yaegaki, K.; Murata, T.; Ii, H.; Imai, T.; Sato, T.; Izumi, Y. Oral Malodorous Compound Activates Mitochondrial Pathway Inducing Apoptosis in Human Gingival Fibroblasts. Clin. Oral Investig. 2010, 14, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. Evidence That Hydrogen Sulfide Is a Genotoxic Agent. Mol. Cancer Res. 2006, 4, 9–14. [Google Scholar] [CrossRef] [PubMed]
- YalçınYeler, D.; Aydın, M.; Hocaoğlu, P.T.; Koraltan, M.; Özdemir, H.; Kotil, T.; Gül, M. Ultrastructural Changes in Epithelial Cells of Rats Exposed to Low Concentration of Hydrogen Sulfide for 50 Days. Ultrastruct. Pathol. 2016, 40, 351–357. [Google Scholar] [CrossRef]
- Karpiński, T.M. Role of Oral Microbiota in Cancer Development. Microorganisms 2019, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Bian, H.; Li, X.; Wu, H.; Bi, Q.; Yan, Y.; Wang, Y. Hydrogen Sulfide Promotes Cell Proliferation of Oral Cancer through Activation of the COX2/AKT/ERK1/2 Axis. Oncol. Rep. 2016, 35, 2825–2832. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, G.; Gattuso, G.; Pedullà, E.; Rapisarda, E.; Nicolosi, D.; Salmeri, M. Association of Oral Dysbiosis with Oral Cancer Development. Oncol. Lett. 2020, 19, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
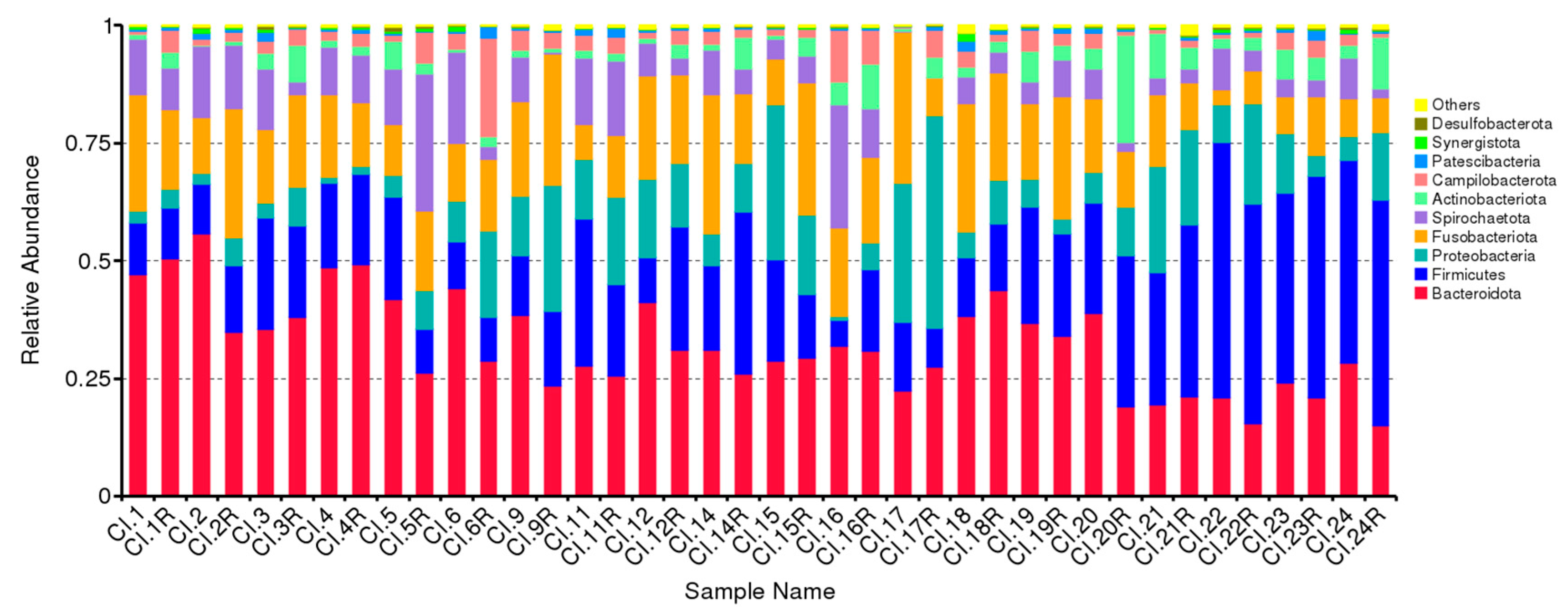
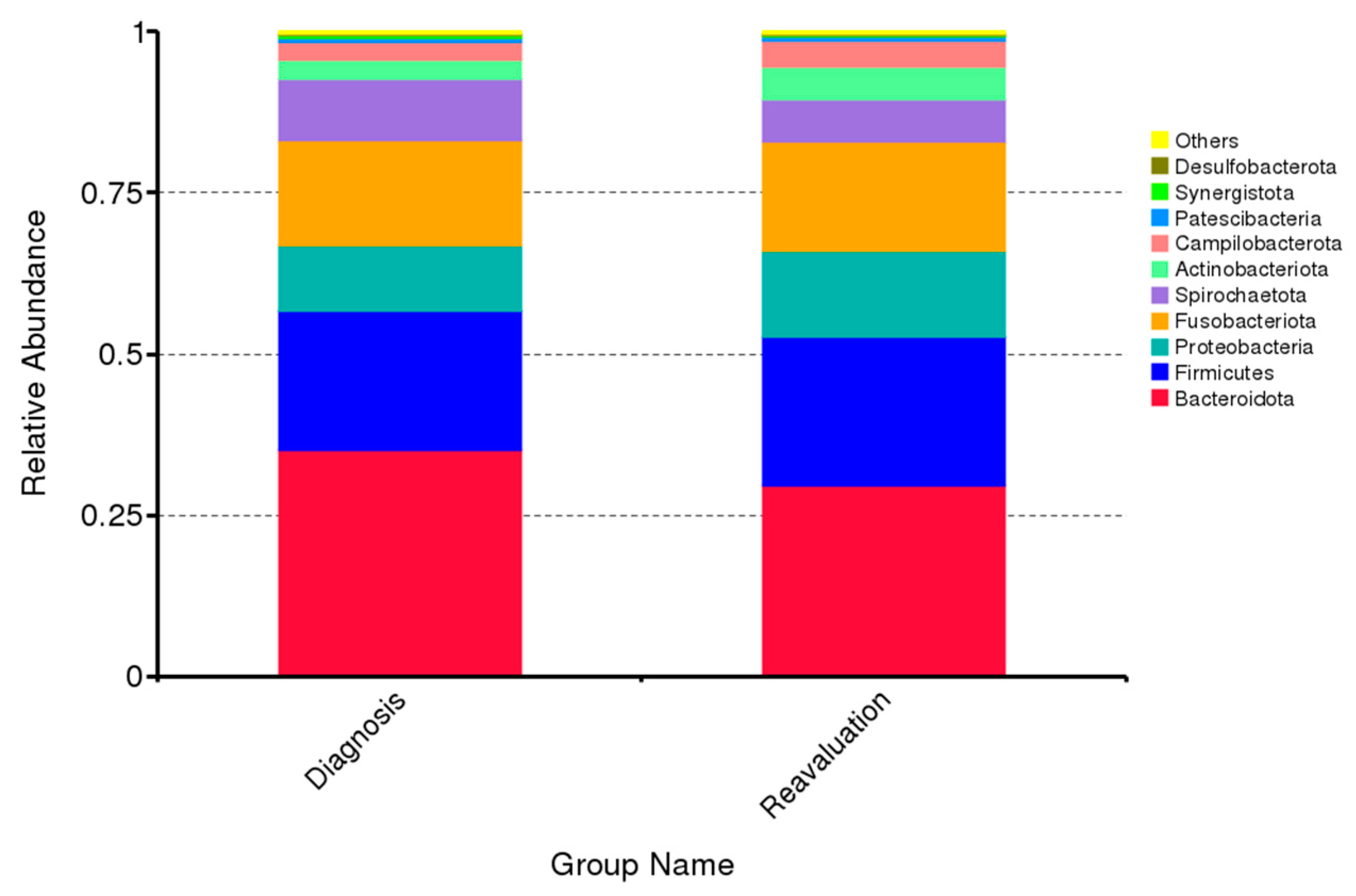
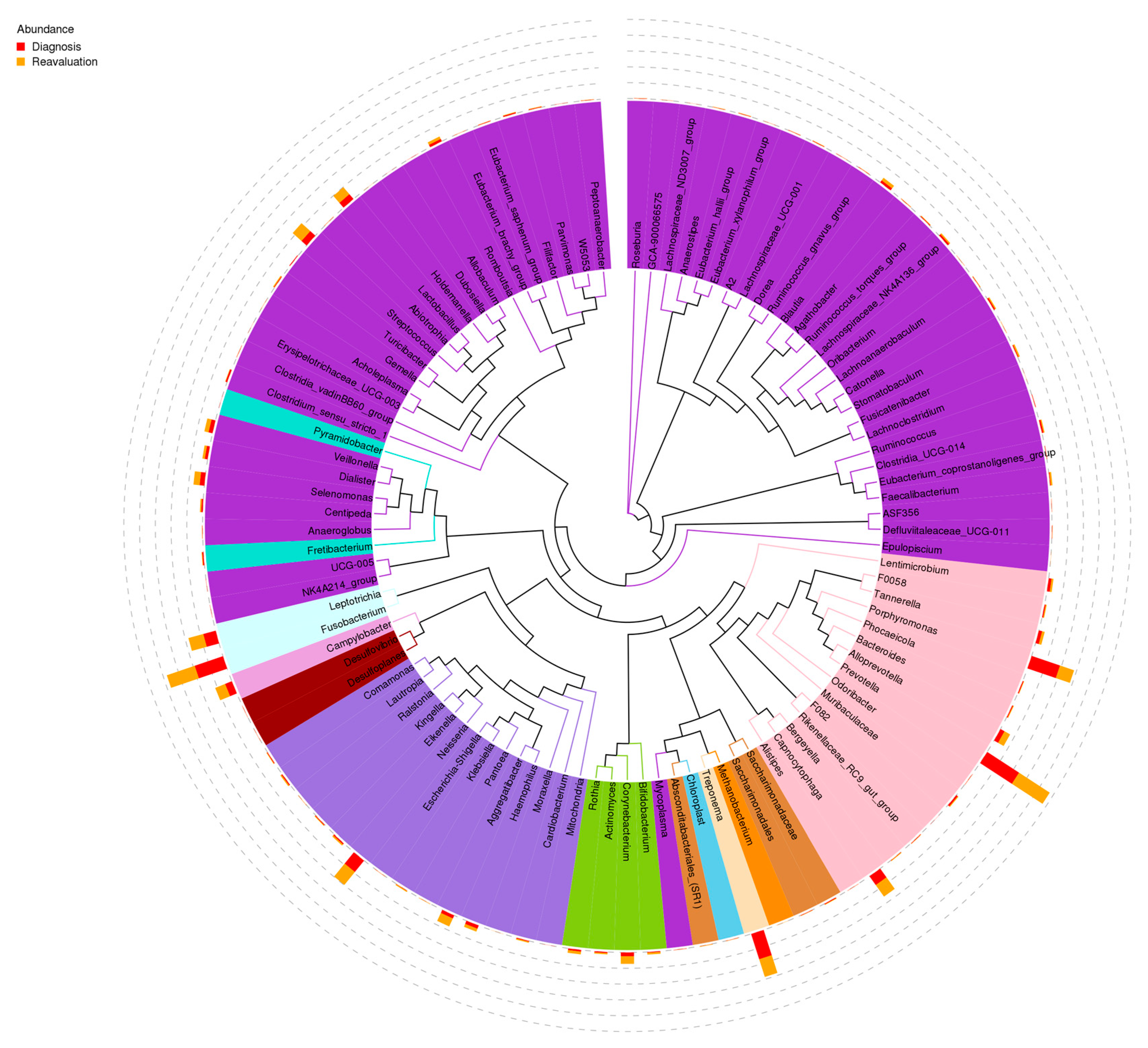
| Variable | |
|---|---|
| Age, mean (SD) (min-max) | 56.6 (10.3) (41–80) |
| Females, % (n) | 70.0 (14) |
| Systemic conditions, % (n) | |
| Hypertension | 20.0 (4) |
| Arthritis | 5.0 (1) |
| Toothbrushing per day, % (n) | |
| 1 | 15.0 (3) |
| 2 | 85.0 (17) |
| Oral hygiene habits, % (n) | |
| Manual brush | 90.0 (18) |
| Tongue scraper | 0.0 (0) |
| Mouthwash | 65.0 (13) |
| Interdental flossing | 20.0 (4) |
| Interdental brush | 25.0 (5) |
| Denture | 15.0 (3) |
| Smoking habits, % (n) | |
| Never | 65.0 (13) |
| Active | 35.0 (7) |
| Alcohol consumption, % (n) | 60.0 (12) |
| Periodontitis staging and grading, % (n) | |
| II-C | 5.0 (1) |
| III-B | 35.0 (7) |
| III-C | 5.0 (1) |
| IV-B | 10.0 (2) |
| IV-C | 45.0 (9) |
| Generalized periodontitis | 95.0 (19) |
| Variable | Baseline | Follow-Up | p-Value * |
|---|---|---|---|
| VSCs, mean (SD) (min-max) | 115.2 (113.7) | 58.0 (52.3) | 0.007 |
| Winkler Index, median (min-max) | 3.0 (0–8) | 2.0 (0.8) | 0.231 |
| OHIP-14, mean (SD) | |||
| Total score | 18.7 (11.4) | 17.8 (11.4) | 0.588 |
| Functional limitation | 1.9 (1.7) | 2.2 (1.7) | 0.277 |
| Physical pain | 3.5 (2.0) | 3.4 (2.0) | 0.937 |
| Psychological discomfort | 4.4 (2.5) | 4.1 (2.2) | 0.546 |
| Physical disability | 3.6 (2.4) | 3.2 (2.1) | 0.382 |
| Psychological disability | 2.7 (2.3) | 2.2 (2.2) | 0.253 |
| Social disability | 1.9 (2.3) | 1.8 (2.4) | 0.774 |
| Handicap | 1.9 (1.9) | 1.9 (2.4) | 0.863 |
| Mean CAL, mean (SD) | 3.3 (0.8) | 2.6 (0.6) | 0.457 |
| Mean PPD, mean (SD) | 4.0 (1.7) | 3.8 (1.6) | 0.406 |
| Distribution of PPD > 5 mm, % (n) | 18.8 (15.9) | 4.8 (10.2) | 0.368 |
| Distribution of CAL > 7 mm, % (n) | 14.5 (20.3) | 10.8 (18.9) | 0.194 |
| Complex | Bacteria | VSC Levels | |||||
|---|---|---|---|---|---|---|---|
| ΔB-FW | p-Value | B | p-Value | FW | p-Value | ||
| Red | P. gingivalis | 0.044 | 0.855 | −0.160 | 0.500 | −0.261 | 0.267 |
| T. forsythia | −0.254 | 0.281 | 0.207 | 0.382 | −0.056 | 0.816 | |
| T. denticola | 0.013 | 0.957 | −0.006 | 0.980 | 0.013 | 0.960 | |
| Orange | F. nucleatum | 0.047 | 0.844 | 0.166 | 0.483 | 0.456 | 0.044 |
| C. showae | −0.744 | 0.149 | 0.824 | 0.086+ | 0.976 | 0.004 | |
| Green | C. gingivalis | 0.062 | 0.795 | 0.149 | 0.530 | 0.449 | 0.047 |
| C.sputigena | −0.451 | 0.046 | 0.281 | 0.229 | −0.287 | 0.219 | |
| P. intermedia | 0.130 | 0.584 | −0.120 | 0.613 | −0.002 | 0.994 | |
| P. nigrescens | 0.342 | 0.141 | −0.247 | 0.293 | 0.143 | 0.548 | |
| F. periodonticum | 0.014 | 0.955 | 0.112 | 0.638 | 0.271 | 0.248 | |
| - | P. micra | 0.094 | 0.694 | −0.138 | 0.563 | −0.112 | 0.637 |
| C. ochracea | 0.099 | 0.678 | −0.042 | 0.860 | 0.106 | 0.657 | |
| A. actinomycetemcomitans | −0.232 | 0.520 | −0.023 | 0.949 | −0.265 | 0.460 | |
| A. israelii | −0.200 | 0.427 | 0.109 | 0.666 | −0.163 | 0.517 | |
| Actinomyces gerencseriae | 0.363 | 0.139 | −0.184 | 0.465 | 0.326 | 0.187 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izidoro, C.; Botelho, J.; Machado, V.; Reis, A.M.; Proença, L.; Barroso, H.; Alves, R.; Mendes, J.J. Non-Surgical Periodontal Treatment Impact on Subgingival Microbiome and Intra-Oral Halitosis. Int. J. Mol. Sci. 2023, 24, 2518. https://doi.org/10.3390/ijms24032518
Izidoro C, Botelho J, Machado V, Reis AM, Proença L, Barroso H, Alves R, Mendes JJ. Non-Surgical Periodontal Treatment Impact on Subgingival Microbiome and Intra-Oral Halitosis. International Journal of Molecular Sciences. 2023; 24(3):2518. https://doi.org/10.3390/ijms24032518
Chicago/Turabian StyleIzidoro, Catarina, João Botelho, Vanessa Machado, Ana Mafalda Reis, Luís Proença, Helena Barroso, Ricardo Alves, and José João Mendes. 2023. "Non-Surgical Periodontal Treatment Impact on Subgingival Microbiome and Intra-Oral Halitosis" International Journal of Molecular Sciences 24, no. 3: 2518. https://doi.org/10.3390/ijms24032518
APA StyleIzidoro, C., Botelho, J., Machado, V., Reis, A. M., Proença, L., Barroso, H., Alves, R., & Mendes, J. J. (2023). Non-Surgical Periodontal Treatment Impact on Subgingival Microbiome and Intra-Oral Halitosis. International Journal of Molecular Sciences, 24(3), 2518. https://doi.org/10.3390/ijms24032518











