Rho GTPase Signaling in Platelet Regulation and Implication for Antiplatelet Therapies
Abstract
1. Introduction
2. Platelet Structure
3. Role of Platelets in Hemostasis and Thrombosis
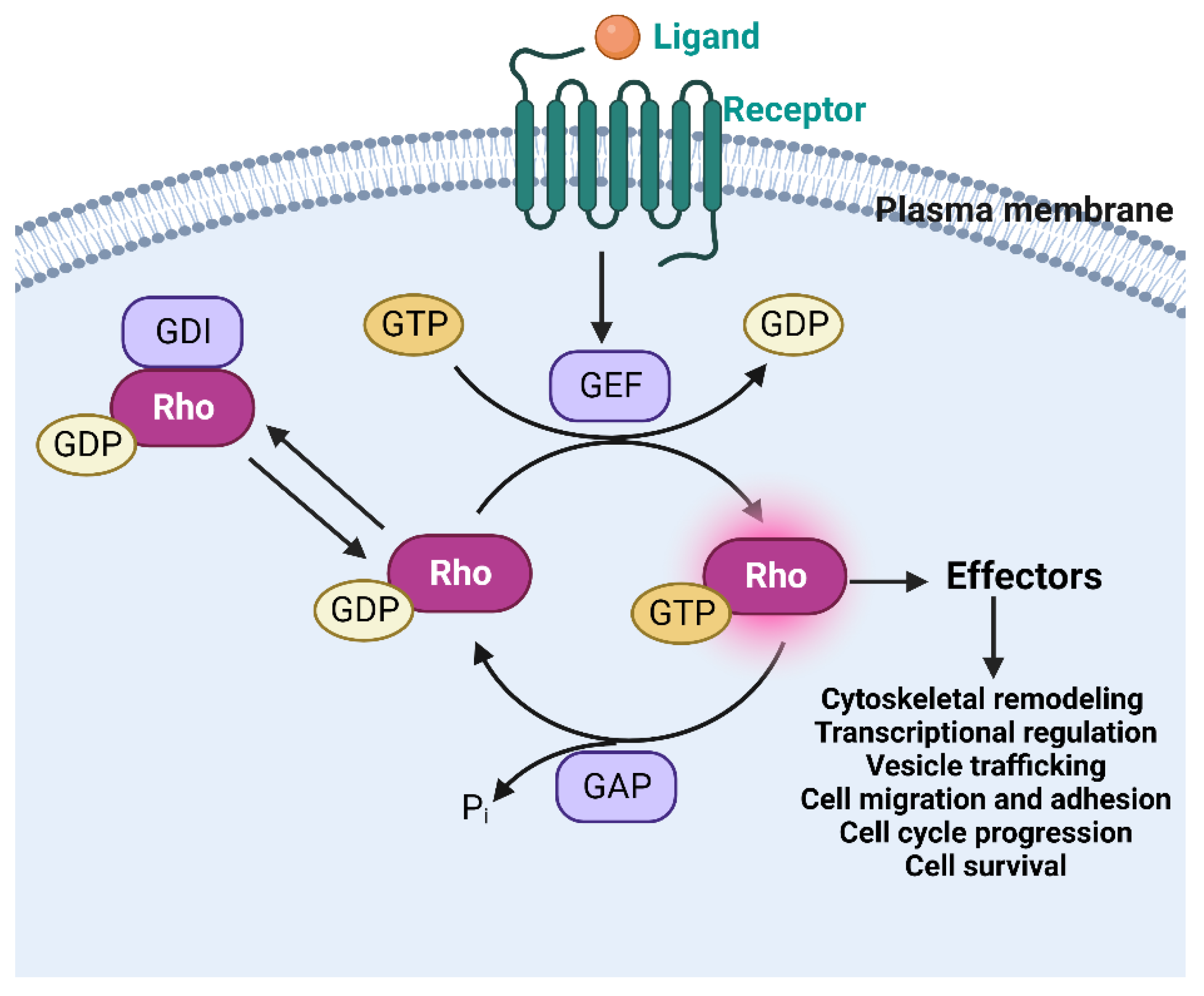
4. Rho GTPases in Platelets
4.1. Role of RhoA in Platelet Function
4.2. Role of Rac1 in Platelet Function
4.3. Role of Cdc42 in Platelet Function
4.4. RhoGTPases and Signaling Crosstalk in Platelet Function
5. Pharmacological Targeting Rho GTPases as an Antiplatelet Approach
6. Future Perspectives and Conclusions
Funding
Conflicts of Interest
References
- Bishop, A.L.; Hall, A. Rho GTPases and their effector proteins. Biochem. J. 2000, 348, 241–255. [Google Scholar] [CrossRef]
- Moon, S.Y.; Zheng, Y. Rho GTPase-activating proteins in cell regulation. Trends Cell Biol. 2003, 13, 13–22. [Google Scholar] [CrossRef]
- Van Aelst, L.; D’Souza-Schorey, C. Rho GTPases and signaling networks. Genes Dev. 1997, 11, 2295–2322. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef]
- Ridley, A.J. Rho family proteins: Coordinating cell responses. Trends Cell Biol. 2001, 11, 471–477. [Google Scholar] [CrossRef]
- Zohn, I.M.; Campbell, S.L.; Khosravi-Far, R.; Rossman, K.L.; Der, C.J. Rho family proteins and Ras transformation: The RHOad less traveled gets congested. Oncogene 1998, 17, 1415–1438. [Google Scholar] [CrossRef]
- Versteeg, H.H.; Heemskerk, J.W.; Levi, M.; Reitsma, P.H. New fundamentals in hemostasis. Physiol. Rev. 2013, 93, 327–358. [Google Scholar] [CrossRef] [PubMed]
- Morii, N.; Teru-uchi, T.; Tominaga, T.; Kumagai, N.; Kozaki, S.; Ushikubi, F.; Narumiya, S. A rho gene product in human blood platelets. II. Effects of the ADP-ribosylation by botulinum C3 ADP-ribosyltransferase on platelet aggregation. J. Biol. Chem. 1992, 267, 20921–20926. [Google Scholar] [CrossRef] [PubMed]
- Aslan, J.E.; McCarty, O.J. Rho GTPases in platelet function. J. Thromb. Haemost. 2013, 11, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.E. The platelet cytoskeleton. Thromb. Haemost. 1993, 70, 884–893. [Google Scholar] [CrossRef]
- Nemoto, Y.; Namba, T.; Teru-uchi, T.; Ushikubi, F.; Morii, N.; Narumiya, S. A rho gene product in human blood platelets. I. Identification of the platelet substrate for botulinum C3 ADP-ribosyltransferase as rhoA protein. J. Biol. Chem. 1992, 267, 20916–20920. [Google Scholar] [CrossRef]
- Klages, B.; Brandt, U.; Simon, M.I.; Schultz, G.; Offermanns, S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J. Cell Biol. 1999, 144, 745–754. [Google Scholar] [CrossRef]
- Flevaris, P.; Stojanovic, A.; Gong, H.; Chishti, A.; Welch, E.; Du, X. A molecular switch that controls cell spreading and retraction. J. Cell Biol. 2007, 179, 553–565. [Google Scholar] [CrossRef]
- Pleines, I.; Hagedorn, I.; Gupta, S.; May, F.; Chakarova, L.; van Hengel, J.; Offermanns, S.; Krohne, G.; Kleinschnitz, C.; Brakebusch, C.; et al. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood 2012, 119, 1054–1063. [Google Scholar] [CrossRef]
- Akbar, H.; Duan, X.; Saleem, S.; Davis, A.K.; Zheng, Y. RhoA and Rac1 GTPases Differentially Regulate Agonist-Receptor Mediated Reactive Oxygen Species Generation in Platelets. PLoS ONE 2016, 11, e0163227. [Google Scholar] [CrossRef]
- McCarty, O.J.; Larson, M.K.; Auger, J.M.; Kalia, N.; Atkinson, B.T.; Pearce, A.C.; Ruf, S.; Henderson, R.B.; Tybulewicz, V.L.; Machesky, L.M.; et al. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J. Biol. Chem. 2005, 280, 39474–39484. [Google Scholar] [CrossRef]
- Akbar, H.; Kim, J.; Funk, K.; Cancelas, J.A.; Shang, X.; Chen, L.; Johnson, J.F.; Williams, D.A.; Zheng, Y. Genetic and pharmacologic evidence that Rac1 GTPase is involved in regulation of platelet secretion and aggregation. J. Thromb. Haemost. 2007, 5, 1747–1755. [Google Scholar] [CrossRef]
- Flevaris, P.; Li, Z.; Zhang, G.; Zheng, Y.; Liu, J.; Du, X. Two distinct roles of mitogen-activated protein kinases in platelets and a novel Rac1-MAPK-dependent integrin outside-in retractile signaling pathway. Blood 2009, 113, 893–901. [Google Scholar] [CrossRef]
- Pula, G.; Poole, A.W. Critical roles for the actin cytoskeleton and cdc42 in regulating platelet integrin α 2 β 1. Platelets 2008, 19, 199–210. [Google Scholar] [CrossRef]
- Akbar, H.; Shang, X.; Perveen, R.; Berryman, M.; Funk, K.; Johnson, J.F.; Tandon, N.N.; Zheng, Y. Gene targeting implicates Cdc42 GTPase in GPVI and non-GPVI mediated platelet filopodia formation, secretion and aggregation. PLoS ONE 2011, 6, e22117. [Google Scholar] [CrossRef]
- Duan, X.; Perveen, R.; Dandamudi, A.; Adili, R.; Johnson, J.; Funk, K.; Berryman, M.; Davis, A.K.; Holinstat, M.; Zheng, Y.; et al. Pharmacologic targeting of Cdc42 GTPase by a small molecule Cdc42 activity-specific inhibitor prevents platelet activation and thrombosis. Sci. Rep. 2021, 11, 13170. [Google Scholar] [CrossRef] [PubMed]
- Aslan, J.E. Platelet Rho GTPase regulation in physiology and disease. Platelets 2019, 30, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.S.; Casari, C.; Wu, C.; Piatt, R.; Pasala, S.; Campbell, R.A.; Poe, K.O.; Ghalloussi, D.; Lee, R.H.; Rotty, J.D.; et al. Deletion of the Arp2/3 complex in megakaryocytes leads to microthrombocytopenia in mice. Blood Adv. 2017, 1, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Pleines, I.; Cherpokova, D.; Bender, M. Rho GTPases and their downstream effectors in megakaryocyte biology. Platelets 2019, 30, 9–16. [Google Scholar] [CrossRef]
- Pleines, I.; Dutting, S.; Cherpokova, D.; Eckly, A.; Meyer, I.; Morowski, M.; Krohne, G.; Schulze, H.; Gachet, C.; Debili, N.; et al. Defective tubulin organization and proplatelet formation in murine megakaryocytes lacking Rac1 and Cdc. Blood 2013, 122, 3178–3187. [Google Scholar] [CrossRef]
- Brewer, D.B. Max Schultze and the living, moving, phagocytosing leucocytes: 1865. Med. Hist. 1994, 38, 91–101. [Google Scholar] [CrossRef]
- Brewer, D.B. Max Schultze (1865), G. Bizzozero (1882) and the discovery of the platelet. Br. J. Haematol. 2006, 133, 251–258. [Google Scholar] [CrossRef]
- de Gaetano, G.; Cerletti, C. Platelet adhesion and aggregation and fibrin formation in flowing blood: A historical contribution by Giulio Bizzozero. Platelets 2002, 13, 85–89. [Google Scholar] [CrossRef]
- Tocantins, L.M. The mammalian blood platelet in health and disease. Medicine 1938, 17, 155. [Google Scholar] [CrossRef]
- Josefsson, E.C.; Vainchenker, W.; James, C. Regulation of Platelet Production and Life Span: Role of Bcl-xL and Potential Implications for Human Platelet Diseases. Int. J. Mol. Sci. 2020, 21, 7591. [Google Scholar] [CrossRef]
- White, J.G. Platelet stucture. In Platelets, 3rd ed.; Michelson, A.D., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2013; pp. 117–144. [Google Scholar]
- Britten, M.W.; Lümers, L.; Tominaga, K.; Peters, J.; Dirkmann, D. Glycocalyx components affect platelet function, whole blood coagulation, and fibrinolysis: An in vitro study suggesting a link to trauma-induced coagulopathy. BMC Anesthesiol. 2021, 21, 83. [Google Scholar] [CrossRef]
- White, J.G.; Clawson, C.C. The surface-connected canalicular system of blood platelets--a fenestrated membrane system. Am. J. Pathol. 1980, 101, 353–364. [Google Scholar]
- Saarikangas, J.; Zhao, H.; Lappalainen, P. Regulation of the actin cytoskeleton-plasma membrane interplay by phosphoinositides. Physiol. Rev. 2010, 90, 259–289. [Google Scholar] [CrossRef]
- Gremmel, T.; Frelinger, A.L., 3rd; Michelson, A.D. Platelet Physiology. Semin. Thromb. Hemost. 2016, 42, 191–204. [Google Scholar]
- Escolar, G.; Krumwiede, M.; White, J.G. Organization of the actin cytoskeleton of resting and activated platelets in suspension. Am. J. Pathol. 1986, 123, 86–94. [Google Scholar]
- Whiteheart, S.W. Fueling Platelets: Where Does the Glucose Come From? Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1592–1594. [Google Scholar] [CrossRef]
- White, J.G. Platelet glycosomes. Platelets 1999, 10, 242–246. [Google Scholar] [CrossRef]
- White, J.G. Medich giant platelet disorder: A unique alpha granule deficiency I. Structural abnormalities. Platelets 2004, 15, 345–353. [Google Scholar] [CrossRef]
- White, J.G. Electron dense chains and clusters in human platelets. Platelets 2002, 13, 317–325. [Google Scholar] [CrossRef]
- Blair, P.; Flaumenhaft, R. Platelet alpha-granules: Basic biology and clinical correlates. Blood Rev. 2009, 23, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Berger, G.; Masse, J.M.; Cramer, E.M. Alpha-granule membrane mirrors the platelet plasma membrane and contains the glycoproteins Ib, IX, and V. Blood 1996, 87, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, L.H.; Duchez, A.-C.; Cloutier, N.; Soulet, D.; Martin, N.; Bollinger, J.; Paré, A.; Rousseau, M.; Naika, G.S.; Lévesque, T. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood J. Am. Soc. Hematol. 2014, 124, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Frojmovic, M.M.; Wong, T.; White, J.G. Platelet plasma membrane is equally distributed between surface and osmotically-evaginable surface-connecting membrane, independent of size, subpopulation or species. Nouv. Rev. Fr. Hematol. 1992, 34, 99–110. [Google Scholar] [PubMed]
- Gerrard, J.M.; White, J.G.; Rao, G.H.; Townsend, D. Localization of platelet prostaglandin production in the platelet dense tubular system. Am. J. Pathol. 1976, 83, 283–298. [Google Scholar]
- White, J.G. Platelet membrane interactions. Platelets 1999, 10, 368–381. [Google Scholar] [CrossRef]
- Gale, A.J. Continuing education course #2: Current understanding of hemostasis. Toxicol. Pathol. 2011, 39, 273–280. [Google Scholar]
- Periayah, M.H.; Halim, A.S.; Mat Saad, A.Z. Mechanism Action of Platelets and Crucial Blood Coagulation Pathways in Hemostasis. Int. J. Hematol. Oncol. Stem. Cell Res. 2017, 11, 319–327. [Google Scholar]
- Massberg, S.; Gawaz, M.; Grüner, S.; Schulte, V.; Konrad, I.; Zohlnhöfer, D.; Heinzmann, U.; Nieswandt, B. A crucial role of glycoprotein VI for platelet recruitment to the injured arterial wall in vivo. J. Exp. Med. 2003, 197, 41–49. [Google Scholar] [CrossRef]
- Nieswandt, B.; Brakebusch, C.; Bergmeier, W.; Schulte, V.; Bouvard, D.; Mokhtari-Nejad, R.; Lindhout, T.; Heemskerk, J.W.; Zirngibl, H.; Fässler, R. Glycoprotein VI but not α2β1 integrin is essential for platelet interaction with collagen. EMBO J. 2001, 20, 2120–2130. [Google Scholar] [CrossRef]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling During Platelet Adhesion and Activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef]
- Gachet, C. ADP receptors of platelets and their inhibition. Thromb. Haemost. 2001, 86, 222–232. [Google Scholar] [CrossRef]
- Cattaneo, M. Platelet P2 receptors: Old and new targets for antithrombotic drugs. Expert Rev. Cardiovasc. Ther. 2007, 5, 45–55. [Google Scholar] [CrossRef]
- Kahn, M.L.; Nakanishi-Matsui, M.; Shapiro, M.J.; Ishihara, H.; Coughlin, S.R. Protease-activated receptors 1 and 4 mediate activation of human platelets by thrombin. J. Clin. Invest. 1999, 103, 879–887. [Google Scholar] [CrossRef]
- Hoyer, D.; Hannon, J.P.; Martin, G.R. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav. 2002, 71, 533–554. [Google Scholar] [CrossRef]
- Vanags, D.; Rodgers, S.; Duncan, E.; Lloyd, J.; Bochner, F. Potentiation of ADP-induced aggregation in human platelet-rich plasma by 5-hydroxytryptamine and adrenaline. Br. J. Pharmacol. 1992, 106, 917–923. [Google Scholar] [CrossRef]
- Hirata, M.; Hayashi, Y.; Ushikubi, F.; Yokota, Y.; Kageyama, R.; Nakanishi, S.; Narumiya, S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature 1991, 349, 617–620. [Google Scholar] [CrossRef]
- Shattil, S.J. Signaling through platelet integrin αIIbβ3: Inside-out, outside-in, and sideways. Thromb. Haemost. 1999, 82, 318–325. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Shattil, S.J.; Newman, P.J. Integrins: Dynamic scaffolds for adhesion and signaling in platelets. Blood 2004, 104, 1606–1615. [Google Scholar] [CrossRef]
- Furie, B.; Furie, B.C. Mechanisms of Thrombus Formation. N. Engl. J. Med. 2008, 359, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Packham, M.A. Role of platelets in thrombosis and hemostasis. Can. J. Physiol. Pharmacol. 1994, 72, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, A.M.; Raskob, G.E. Global Burden of Thrombosis: Epidemiologic Aspects. Circ. Res. 2016, 118, 1340–1347. [Google Scholar] [CrossRef]
- Koupenova, M.; Kehrel, B.E.; Corkrey, H.A.; Freedman, J.E. Thrombosis and platelets: An update. Eur. Heart J. 2016, 38, 785–791. [Google Scholar] [CrossRef]
- Chernysh, I.N.; Nagaswami, C.; Kosolapova, S.; Peshkova, A.D.; Cuker, A.; Cines, D.B.; Cambor, C.L.; Litvinov, R.I.; Weisel, J.W. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci. Rep. 2020, 10, 5112. [Google Scholar] [CrossRef]
- Kesieme, E.; Kesieme, C.; Jebbin, N.; Irekpita, E.; Dongo, A. Deep vein thrombosis: A clinical review. J. Blood Med. 2011, 2, 59–69. [Google Scholar] [CrossRef]
- Mackman, N.; Bergmeier, W.; Stouffer, G.A.; Weitz, J.I. Therapeutic strategies for thrombosis: New targets and approaches. Nat. Rev. Drug Discov. 2020, 19, 333–352. [Google Scholar] [CrossRef]
- Goggs, R.; Williams, C.M.; Mellor, H.; Poole, A.W. Platelet Rho GTPases-a focus on novel players, roles and relationships. Biochem. J. 2015, 466, 431–442. [Google Scholar] [CrossRef]
- Kim, S.; Dangelmaier, C.; Bhavanasi, D.; Meng, S.; Wang, H.; Goldfinger, L.E.; Kunapuli, S.P. Rhog Regulates GPVI/FcRγ-Mediated Platelet Activation and Thrombus Formation. Blood 2013, 122, 1060. [Google Scholar] [CrossRef]
- Goggs, R.; Harper, M.T.; Pope, R.J.; Savage, J.S.; Williams, C.M.; Mundell, S.J.; Heesom, K.J.; Bass, M.; Mellor, H.; Poole, A.W. RhoG protein regulates platelet granule secretion and thrombus formation in mice. J. Biol. Chem. 2013, 288, 34217–34229. [Google Scholar] [CrossRef]
- Burkhart, J.M.; Vaudel, M.; Gambaryan, S.; Radau, S.; Walter, U.; Martens, L.; Geiger, J.; Sickmann, A.; Zahedi, R.P. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood 2012, 120, e73–e82. [Google Scholar] [CrossRef]
- Huang, J.S.; Dong, L.; Kozasa, T.; Le Breton, G.C. Signaling through G(alpha)13 switch region I is essential for protease-activated receptor 1-mediated human platelet shape change, aggregation, and secretion. J. Biol. Chem. 2007, 282, 10210–10222. [Google Scholar] [CrossRef]
- Arthur, W.T.; Petch, L.A.; Burridge, K. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 2000, 10, 719–722. [Google Scholar] [CrossRef]
- Gong, H.; Shen, B.; Flevaris, P.; Chow, C.; Lam, S.C.; Voyno-Yasenetskaya, T.A.; Kozasa, T.; Du, X. G protein subunit Galpha13 binds to integrin alphaIIbbeta3 and mediates integrin “outside-in” signaling. Science 2010, 327, 340–343. [Google Scholar] [CrossRef]
- Schoenwaelder, S.M.; Hughan, S.C.; Boniface, K.; Fernando, S.; Holdsworth, M.; Thompson, P.E.; Salem, H.H.; Jackson, S.P. RhoA sustains integrin alpha IIbbeta 3 adhesion contacts under high shear. J. Biol. Chem. 2002, 277, 14738–14746. [Google Scholar] [CrossRef]
- Hechler, B.; Léon, C.; Vial, C.; Vigne, P.; Frelin, C.; Cazenave, J.-P.; Gachet, C. The P2Y1 Receptor Is Necessary for Adenosine 5′-Diphosphate–Induced Platelet Aggregation. Blood 1998, 92, 152–159. [Google Scholar] [CrossRef]
- Gu, Y.; Filippi, M.D.; Cancelas, J.A.; Siefring, J.E.; Williams, E.P.; Jasti, A.C.; Harris, C.E.; Lee, A.W.; Prabhakar, R.; Atkinson, S.J.; et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 2003, 302, 445–449. [Google Scholar] [CrossRef]
- Falet, H.; Hoffmeister, K.M.; Neujahr, R.; Italiano, J.E., Jr.; Stossel, T.P.; Southwick, F.S.; Hartwig, J.H. Importance of free actin filament barbed ends for Arp2/3 complex function in platelets and fibroblasts. Proc. Natl. Acad. Sci. USA 2002, 99, 16782–16787. [Google Scholar] [CrossRef]
- Liao, F.; Shin, H.S.; Rhee, S.G. Tyrosine phosphorylation of phospholipase C-gamma 1 induced by cross-linking of the high-affinity or low-affinity Fc receptor for IgG in U937 cells. Proc. Natl. Acad. Sci. USA 1992, 89, 3659–3663. [Google Scholar] [CrossRef]
- Lind, S.E.; Janmey, P.A.; Chaponnier, C.; Herbert, T.J.; Stossel, T.P. Reversible binding of actin to gelsolin and profilin in human platelet extracts. J. Cell Biol. 1987, 105, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Biyasheva, A.; Svitkina, T.; Kunda, P.; Baum, B.; Borisy, G. Cascade pathway of filopodia formation downstream of SCAR. J. Cell Sci. 2004, 117 Pt 6, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Yamaguchi, H.; Suetsugu, S.; Takenawa, T. IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 2000, 408, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeister, K.M.; Falet, H.; Toker, A.; Barkalow, K.L.; Stossel, T.P.; Hartwig, J.H. Mechanisms of cold-induced platelet actin assembly. J. Biol. Chem. 2001, 276, 24751–24759. [Google Scholar] [CrossRef]
- Piechulek, T.; Rehlen, T.; Walliser, C.; Vatter, P.; Moepps, B.; Gierschik, P. Isozyme-specific stimulation of phospholipase C-gamma2 by Rac GTPases. J. Biol. Chem. 2005, 280, 38923–38931. [Google Scholar] [CrossRef]
- Welch, H.C.; Coadwell, W.J.; Stephens, L.R.; Hawkins, P.T. Phosphoinositide 3-kinase-dependent activation of Rac. FEBS Lett. 2003, 546, 93–97. [Google Scholar] [CrossRef]
- Jackson, S.P.; Schoenwaelder, S.M.; Goncalves, I.; Nesbitt, W.S.; Yap, C.L.; Wright, C.E.; Kenche, V.; Anderson, K.E.; Dopheide, S.M.; Yuan, Y.; et al. PI 3-kinase p110beta: A new target for antithrombotic therapy. Nat. Med. 2005, 11, 507–514. [Google Scholar] [CrossRef]
- Sells, M.A.; Knaus, U.G.; Bagrodia, S.; Ambrose, D.M.; Bokoch, G.M.; Chernoff, J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr. Biol. 1997, 7, 202–210. [Google Scholar] [CrossRef]
- Akbar, H.; Cancelas, J.; Williams, D.A.; Zheng, J.; Zheng, Y. Rational design and applications of a Rac GTPase-specific small molecule inhibitor. Methods Enzymol. 2006, 406, 554–565. [Google Scholar]
- Akbar, H.; Duan, X.; Piatt, R.; Saleem, S.; Davis, A.K.; Tandon, N.N.; Bergmeier, W.; Zheng, Y. Small molecule targeting the Rac1-NOX2 interaction prevents collagen-related peptide and thrombin-induced reactive oxygen species generation and platelet activation. J. Thromb. Haemost. 2018, 16, 2083–2096. [Google Scholar] [CrossRef]
- Pleines, I.; Elvers, M.; Strehl, A.; Pozgajova, M.; Varga-Szabo, D.; May, F.; Chrostek-Grashoff, A.; Brakebusch, C.; Nieswandt, B. Rac1 is essential for phospholipase C-gamma2 activation in platelets. Pflugers. Arch. 2009, 457, 1173–1185. [Google Scholar] [CrossRef]
- Stefanini, L.; Boulaftali, Y.; Ouellette, T.D.; Holinstat, M.; Désiré, L.; Leblond, B.; Andre, P.; Conley, P.B.; Bergmeier, W. Rap1-Rac1 circuits potentiate platelet activation. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 434–441. [Google Scholar] [CrossRef]
- Kozma, R.; Ahmed, S.; Best, A.; Lim, L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol. Cell Biol. 1995, 15, 1942–1952. [Google Scholar] [CrossRef]
- Yang, L.; Wang, L.; Zheng, Y. Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts. Mol. Biol. Cell 2006, 17, 4675–4685. [Google Scholar] [CrossRef]
- Dash, D.; Aepfelbacher, M.; Siess, W. Integrin αIIbβ3-mediated translocation of CDC42Hs to the cytoskeleton in stimulated human platelets. J. Biol. Chem. 1995, 270, 17321–17326. [Google Scholar] [CrossRef]
- Svitkina, T.M.; Borisy, G.G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 1999, 145, 1009–1026. [Google Scholar] [CrossRef]
- Welch, M.D.; Mullins, R.D. Cellular control of actin nucleation. Annu. Rev. Cell Dev. Biol. 2002, 18, 247–288. [Google Scholar] [CrossRef]
- Faix, J.; Rottner, K. The making of filopodia. Curr. Opin. Cell Biol. 2006, 18, 18–25. [Google Scholar] [CrossRef]
- Bear, J.E.; Svitkina, T.M.; Krause, M.; Schafer, D.A.; Loureiro, J.J.; Strasser, G.A.; Maly, I.V.; Chaga, O.Y.; Cooper, J.A.; Borisy, G.G. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 2002, 109, 509–521. [Google Scholar] [CrossRef]
- Krugmann, S.; Jordens, I.; Gevaert, K.; Driessens, M.; Vandekerckhove, J.; Hall, A. Cdc42 induces filopodia by promoting the formation of an IRSp53: Mena complex. Curr. Biol. 2001, 11, 1645–1655. [Google Scholar] [CrossRef]
- Pleines, I.; Eckly, A.; Elvers, M.; Hagedorn, I.; Eliautou, S.; Bender, M.; Wu, X.; Lanza, F.; Gachet, C.; Brakebusch, C.; et al. Multiple alterations of platelet functions dominated by increased secretion in mice lacking Cdc42 in platelets. Blood 2010, 115, 3364–3373. [Google Scholar] [CrossRef] [PubMed]
- Ngo, A.T.P.; Parra-Izquierdo, I.; Aslan, J.E.; McCarty, O.J.T. Rho GTPase regulation of reactive oxygen species generation and signalling in platelet function and disease. Small GTPases 2021, 12, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Akbar, H.; Shang, X.; Perveen, R.; Funk, K.; Berryman, M.; Zheng, Y. Specific Pharmacologic Targeting of Rho GTPases Rac1, Cdc42 and RhoA Reveals Their Differential and Critical Roles in Regulation of Platelet Activation. Blood 2010, 116, 2019. [Google Scholar] [CrossRef]
- Aslan, J.E.; Baker, S.M.; Loren, C.P.; Haley, K.M.; Itakura, A.; Pang, J.; Greenberg, D.L.; David, L.L.; Manser, E.; Chernoff, J.; et al. The PAK system links Rho GTPase signaling to thrombin-mediated platelet activation. Am. J. Physiol.-Cell Physiol. 2013, 305, C519–C528. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Y. Approaches of targeting Rho GTPases in cancer drug discovery. Expert. Opin. Drug Discov. 2015, 10, 991–1010. [Google Scholar] [CrossRef]
- Gray, J.L.; von Delft, F.; Brennan, P.E. Targeting the Small GTPase Superfamily through Their Regulatory Proteins. Angew. Chem. Int. Ed. 2020, 59, 6342–6366. [Google Scholar] [CrossRef]
- Aktories, K.; Schmidt, G.; Just, I. Rho GTPases as targets of bacterial protein toxins. Biol. Chem. 2000, 381, 421–426. [Google Scholar] [CrossRef]
- Aktories, K.; Barth, H.; Just, I. Clostridium Botulinum C3 Exoenzyme and C3-Like Transferases. In Bacterial Protein Toxins; Aktories, K., Just, I., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 207–233. [Google Scholar]
- Just, I.; Selzer, J.; Wilm, M.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature 1995, 375, 500–503. [Google Scholar] [CrossRef]
- Just, I.; Wilm, M.; Selzer, J.; Rex, G.; von Eichel-Streiber, C.; Mann, M.; Aktories, K. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J. Biol. Chem. 1995, 270, 13932–13936. [Google Scholar] [CrossRef]
- Huzoor, A.; Wang, W.; Kornhauser, R.; Volker, C.; Stock, J.B. Protein prenylcysteine analog inhibits agonist-receptor-mediated signal transduction in human platelets. Proc. Natl. Acad. Sci. USA 1993, 90, 868–872. [Google Scholar] [CrossRef]
- Cox, A.D.; Der, C.J. Ras history: The saga continues. Small GTPases 2010, 1, 2–27. [Google Scholar] [CrossRef] [PubMed]
- Baines, A.T.; Xu, D.; Der, C.J. Inhibition of Ras for cancer treatment: The search continues. Future Med. Chem. 2011, 3, 1787–1808. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Marchioni, F.; Sipes, N.; Evelyn, C.R.; Jerabek-Willemsen, M.; Duhr, S.; Seibel, W.; Wortman, M.; Zheng, Y. Rational design of small molecule inhibitors targeting RhoA subfamily Rho GTPases. Chem. Biol. 2012, 19, 699–710. [Google Scholar] [CrossRef]
- Evelyn, C.R.; Ferng, T.; Rojas, R.J.; Larsen, M.J.; Sondek, J.; Neubig, R.R. High-throughput screening for small-molecule inhibitors of LARG-stimulated RhoA nucleotide binding via a novel fluorescence polarization assay. J. Biomol. Screen 2009, 14, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Marchioni, F.; Evelyn, C.R.; Sipes, N.; Zhou, X.; Seibel, W.; Wortman, M.; Zheng, Y. Small-molecule inhibitors targeting G-protein-coupled Rho guanine nucleotide exchange factors. Proc. Natl. Acad. Sci. USA 2013, 110, 3155–3160. [Google Scholar] [CrossRef]
- Nagumo, H.; Sasaki, Y.; Ono, Y.; Okamoto, H.; Seto, M.; Takuwa, Y. Rho kinase inhibitor HA-1077 prevents Rho-mediated myosin phosphatase inhibition in smooth muscle cells. Am. J. Physiol. Cell Physiol. 2000, 278, C57–C65. [Google Scholar] [CrossRef]
- Uehata, M.; Ishizaki, T.; Satoh, H.; Ono, T.; Kawahara, T.; Morishita, T.; Tamakawa, H.; Yamagami, K.; Inui, J.; Maekawa, M.; et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997, 389, 990–994. [Google Scholar] [CrossRef]
- Pandey, D.; Goyal, P.; Bamburg, J.R.; Siess, W. Regulation of LIM-kinase 1 and cofilin in thrombin-stimulated platelets. Blood 2006, 107, 575–583. [Google Scholar] [CrossRef]
- Paul, B.Z.; Kim, S.; Dangelmaier, C.; Nagaswami, C.; Jin, J.; Hartwig, J.H.; Weisel, J.W.; Daniel, J.L.; Kunapuli, S.P. Dynamic regulation of microtubule coils in ADP-induced platelet shape change by p160ROCK (Rho-kinase). Platelets 2003, 14, 159–169. [Google Scholar] [CrossRef]
- Gao, Y.; Dickerson, J.B.; Guo, F.; Zheng, J.; Zheng, Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. USA 2004, 101, 7618–7623. [Google Scholar] [CrossRef]
- Dwivedi, S.; Pandey, D.; Khandoga, A.L.; Brandl, R.; Siess, W. Rac1-mediated signaling plays a central role in secretion-dependent platelet aggregation in human blood stimulated by atherosclerotic plaque. J. Transl. Med. 2010, 8, 128. [Google Scholar] [CrossRef]
- Montalvo-Ortiz, B.L.; Castillo-Pichardo, L.; Hernández, E.; Humphries-Bickley, T.; De la Mota-Peynado, A.; Cubano, L.A.; Vlaar, C.P.; Dharmawardhane, S. Characterization of EHop-016, novel small molecule inhibitor of Rac GTPase. J. Biol. Chem. 2012, 287, 13228–13238. [Google Scholar] [CrossRef]
- Shutes, A.; Onesto, C.; Picard, V.; Leblond, B.; Schweighoffer, F.; Der, C.J. Specificity and Mechanism of Action of EHT 1864, a Novel Small Molecule Inhibitor of Rac Family Small GTPases *. J. Biol. Chem. 2007, 282, 35666–35678. [Google Scholar] [CrossRef]
- Florian, M.C.; Dörr, K.; Niebel, A.; Daria, D.; Schrezenmeier, H.; Rojewski, M.; Filippi, M.D.; Hasenberg, A.; Gunzer, M.; Scharffetter-Kochanek, K.; et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem. Cell 2012, 10, 520–530. [Google Scholar] [CrossRef]
- Liu, W.; Du, W.; Shang, X.; Wang, L.; Evelyn, C.; Florian, M.C.; Ryan, A.R.; Rayes, A.; Zhao, X.; Setchell, K.; et al. Rational identification of a Cdc42 inhibitor presents a new regimen for long-term hematopoietic stem cell mobilization. Leukemia 2019, 33, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Pelish, H.E.; Peterson, J.R.; Salvarezza, S.B.; Rodriguez-Boulan, E.; Chen, J.L.; Stamnes, M.; Macia, E.; Feng, Y.; Shair, M.D.; Kirchhausen, T. Secramine inhibits Cdc42-dependent functions in cells and Cdc42 activation in vitro. Nat. Chem. Biol. 2006, 2, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.C.; Chang, H.H.; Lin, C.T.; Lo, S.J. The integrin alpha6beta1 modulation of PI3K and Cdc42 activities induces dynamic filopodium formation in human platelets. J. Biomed. Sci. 2005, 12, 881–898. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Kenney, S.R.; Phillips, G.K.; Simpson, D.; Schroeder, C.E.; Nöth, J.; Romero, E.; Swanson, S.; Waller, A.; Strouse, J.J.; et al. Characterization of a Cdc42 protein inhibitor and its use as a molecular probe. J. Biol. Chem. 2013, 288, 8531–8543. [Google Scholar] [CrossRef]
- Surviladze, Z.; Waller, A.; Strouse, J.J.; Bologa, C.; Ursu, O.; Salas, V.; Parkinson, J.F.; Phillips, G.K.; Romero, E.; Wandinger-Ness, A.; et al. A Potent and Selective Inhibitor of Cdc42 GTPase. In Probe Reports from the NIH Molecular Libraries Program; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2010. [Google Scholar]
- Deacon, S.W.; Beeser, A.; Fukui, J.A.; Rennefahrt, U.E.; Myers, C.; Chernoff, J.; Peterson, J.R. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 2008, 15, 322–331. [Google Scholar] [CrossRef]
- Viaud, J.; Peterson, J.R. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol. Cancer Ther. 2009, 8, 2559–2565. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dandamudi, A.; Akbar, H.; Cancelas, J.; Zheng, Y. Rho GTPase Signaling in Platelet Regulation and Implication for Antiplatelet Therapies. Int. J. Mol. Sci. 2023, 24, 2519. https://doi.org/10.3390/ijms24032519
Dandamudi A, Akbar H, Cancelas J, Zheng Y. Rho GTPase Signaling in Platelet Regulation and Implication for Antiplatelet Therapies. International Journal of Molecular Sciences. 2023; 24(3):2519. https://doi.org/10.3390/ijms24032519
Chicago/Turabian StyleDandamudi, Akhila, Huzoor Akbar, Jose Cancelas, and Yi Zheng. 2023. "Rho GTPase Signaling in Platelet Regulation and Implication for Antiplatelet Therapies" International Journal of Molecular Sciences 24, no. 3: 2519. https://doi.org/10.3390/ijms24032519
APA StyleDandamudi, A., Akbar, H., Cancelas, J., & Zheng, Y. (2023). Rho GTPase Signaling in Platelet Regulation and Implication for Antiplatelet Therapies. International Journal of Molecular Sciences, 24(3), 2519. https://doi.org/10.3390/ijms24032519




