The Functions of Chloroplastic Ascorbate in Vascular Plants and Algae
Abstract
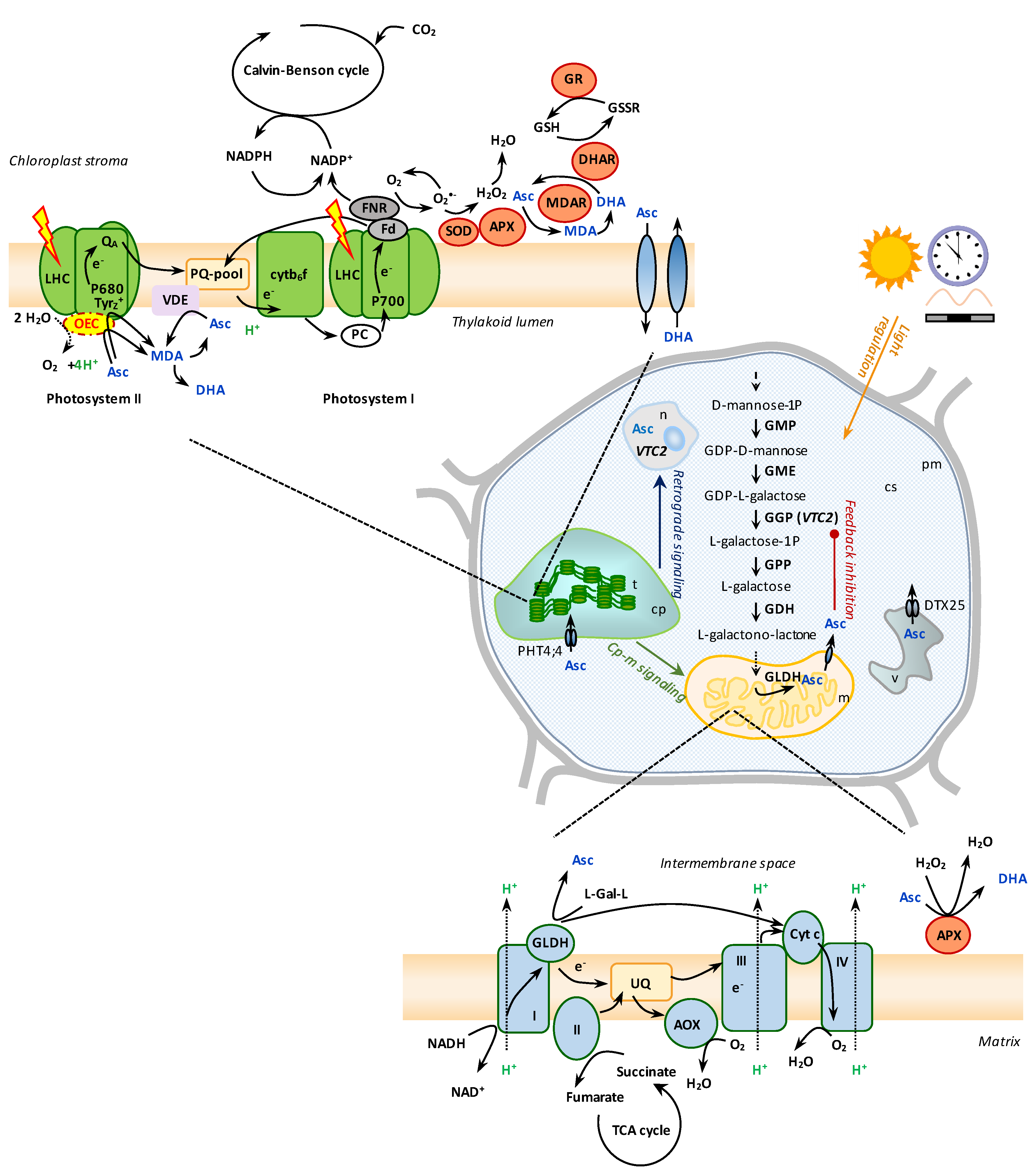
:1. Introduction
2. Regulation of Ascorbate Biosynthesis by Light
3. Interaction between Photosynthetic and Mitochondrial Electron Transport in Relation to Asc Biosynthesis
4. Ascorbate Is an Alternative, ‘Emergency’ Donor to Photosystem II
5. Ascorbate May Impair the Oxygen-Evolving Complex
6. The Role of Ascorbate in Non-Photochemical Quenching
7. Reactive Oxygen Species Management by Ascorbate in the Chloroplast
8. Open Questions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macknight, R.C.; Laing, W.A.; Bulley, S.M.; Broad, R.C.; Johnson, A.A.; Hellens, R.P. Increasing ascorbate levels in crops to enhance human nutrition and plant abiotic stress tolerance. Curr. Opin. Biotechnol. 2017, 44, 153–160. [Google Scholar] [CrossRef]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C content in fruits: Biosynthesis and regulation. Front. Plant Sci. 2019, 9, 2006. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbate biosynthesis and function in photoprotection. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2000, 355, 1455–1464. [Google Scholar] [CrossRef]
- Millar, A.H.; Mittova, V.; Kiddle, G.; Heazlewood, J.L.; Bartoli, C.G.; Theodoulou, F.L.; Foyer, C.H. Control of ascorbate synthesis by respiration and its implications for stress responses. Plant Physiol. 2003, 133, 443–447. [Google Scholar] [CrossRef] [Green Version]
- Conklin, P.L.; Saracco, S.A.; Norris, S.R.; Last, R.L. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 2000, 154, 847–856. [Google Scholar] [CrossRef]
- Fenech, M.; Amorim-Silva, V.; Esteban del Valle, A.; Arnaud, D.; Ruiz-Lopez, N.; Castillo, A.G.; Smirnoff, N.; Botella, M.A. The role of GDP-L-galactose phosphorylase in the control of ascorbate biosynthesis. Plant Physiol. 2021, 185, 1574–1594. [Google Scholar] [CrossRef]
- Laing, W.A.; Martínez-Sánchez, M.; Wright, M.A.; Bulley, S.M.; Brewster, D.; Dare, A.P.; Rassam, M.; Wang, D.; Storey, R.; Macknight, R.C.; et al. An upstream open reading frame is essential for feedback regulation of ascorbate biosynthesis in Arabidopsis. Plant Cell 2015, 27, 772–786. [Google Scholar] [CrossRef] [Green Version]
- Bulley, S.; Laing, W. The regulation of ascorbate biosynthesis. Curr. Opin. Plant Biol. 2016, 33, 15–22. [Google Scholar] [CrossRef]
- Deslous, P.; Bournonville, C.; Decros, G.; Okabe, Y.; Mauxion, J.P.; Jorly, J.; Gadin, S.; Brès, C.; Mori, K.; Ferrand, C.; et al. Overproduction of ascorbic acid impairs pollen fertility in tomato. J. Exp. Bot. 2021, 72, 3091–3107. [Google Scholar] [CrossRef]
- Terzaghi, M.; De Tullio, M.C. The perils of planning strategies to increase vitamin C content in plants: Beyond the hype. Front. Plant Sci. 2022, 13, 1096549. [Google Scholar] [CrossRef]
- Ntagkas, N.; Woltering, E.J.; Marcelis, L.F.M. Light regulates ascorbate in plants: An integrated view on physiology and biochemistry. Environ. Exp. Bot. 2018, 147, 271–280. [Google Scholar] [CrossRef]
- Kavkova, E.I.; Blöchl, C.; Tenhaken, R. The Myo-inositol pathway does not contribute to ascorbic acid synthesis. Plant Biol. 2019, 21, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Kyndt, T.; Hancock, R.D. Vitamin C in plants: Novel concepts, new perspectives, and outstanding issues. Antiox. Redox Signal. 2020, 32, 463–485. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
- Njus, D.; Kelleya, P.M.; Tub, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef]
- Asada, K. The water-water cycle as alternative photon and electron sinks. Philos. Trans. R. Soc. Lond. B 2000, 355, 1419–1431. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Halliwell, B. Purification and properties of dehydroascorbate reductase from spinach leaves. Phytochemistry 1977, 16, 1347–1350. [Google Scholar] [CrossRef]
- Terai, Y.; Ueno, H.; Ogawa, T.; Sawa, Y.; Miyagi, A.; Kawai-Yamada, M.; Ishikawa, T.; Maruta, T. Dehydroascorbate reductases and glutathione set a threshold for high-light-induced ascorbate accumulation. Plant Physiol. 2020, 183, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Green, M.; Fry, S. Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-l-threonate. Nature 2005, 433, 83–87. [Google Scholar] [CrossRef]
- Truffault, V.; Fry, S.C.; Stevens, R.G.; Gautier, H. Ascorbate degradation in tomato leads to accumulation of oxalate, threonate and oxalyl threonate. Plant J. 2017, 89, 996–1008. [Google Scholar] [CrossRef] [Green Version]
- Fry, S.C. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 1998, 332, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Green, M.A.; Fry, S.C. Apoplastic degradation of ascorbate: Novel enzymes and metabolites permeating the plant cell wall. Plant Biosys. 2005, 139, 2–7. [Google Scholar] [CrossRef]
- Kliebenstein, D.J.; Lambrix, V.; Reichelt, M.; Gershenzon, J.; Mitchell-Olds, T. Gene duplication in the diversification of secondary metabolism: Tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 2001, 13, 681–693. [Google Scholar]
- Brisson, L.; El Bakkali-Taheri, N.; Giorgi, M.; Fadel, A.; Kaizer, J.; Réglier, M.; Tron, T.; Ajandouz, E.H.; Simaan, A.J. 1-Aminocyclopropane-1-carboxylic acid oxidase: Insight into cofactor binding from experimental and theoretical studies. J. Biol. Inorg. Chem. 2012, 17, 939–949. [Google Scholar] [CrossRef]
- Mellor, N.; Band, L.R.; Pěnčík, A.; Novák, O.; Rashed, A.; Holman, T.; Wilson, M.H.; Voß, U.; Bishopp, A.; King, J.R.; et al. Dynamic regulation of auxin oxidase and conjugating enzymes AtDAO1 and GH3 modulates auxin homeostasis. Proc. Natl. Acad. Sci. USA 2016, 113, 11022–11027. [Google Scholar] [CrossRef] [Green Version]
- Monfort, A.; Wutz, A. Breathing in epigenetic change with vitamin C. EMBO Rep. 2013, 14, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Young, J.I.; Züchner, S.; Wang, G. Regulation of the epigenome by vitamin C. Annu. Rev. Nutr. 2015, 35, 545–564. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.H.; Chen, G.D.; Hao, F.; Chen, H.; Fang, Z.; Chen, F.-F.; Pang, B.; Yang, Q.-L.; Wei, X.; Fan, Q.-Q.; et al. A vitamin-C-derived DNA modification catalysed by an algal TET homologue. Nature 2019, 569, 581–585. [Google Scholar] [CrossRef]
- Brabson, J.P.; Leesang, T.; Mohammad, S.; Cimmino, L. Epigenetic regulation of genomic stability by Vitamin C. Front. Genet. 2021, 12, 675780. [Google Scholar] [CrossRef]
- Grillet, L.; Ouerdane, L.; Flis, P.; Hoang, M.T.T.; Isaure, M.-P.; Lobinski, R.; Curie, C.; Mari, S. Ascorbate efflux as a new strategy for iron reduction and transport in plants. J. Biol. Chem. 2014, 289, 2515–2525. [Google Scholar] [CrossRef]
- Makavitskaya, M.; Svistunenko, D.; Navaselsky, I.; Hryvusevich, P.; Mackievic, V.; Rabadanova, C.; Tyutereva, E.; Samokhina, V.; Straltsova, D.; Sokolik, A.; et al. Novel roles of ascorbate in plants: Induction of cytosolic Ca2+ signals and efflux from cells via anion channels. J. Exp. Bot. 2018, 69, 3477–3489. [Google Scholar] [CrossRef]
- Lim, B.; Smirnoff, N.; Cobbett, C.S.; Golz, J.F. Ascorbate-deficient vtc2 mutants in Arabidopsis do not exhibit decreased growth. Front. Plant Sci. 2016, 7, 1025. [Google Scholar] [CrossRef] [Green Version]
- Plumb, W.; Townsend, A.J.; Rasool, B.; Alomrani, S.; Razak, N.; Karpinska, B.; Ruban, A.V.; Foyer, C.H. Ascorbate-mediated regulation of growth, photoprotection, and photoinhibition in Arabidopsis thaliana. J. Exp. Bot. 2018, 69, 2823–2835. [Google Scholar] [CrossRef] [Green Version]
- Müller-Moulé, P.; Golan, T.; Niyogi, K.K. Ascorbate-deficient mutants of Arabidopsis grow in high light despite chronic photooxidative stress. Plant Physiol. 2004, 134, 1163–1172. [Google Scholar] [CrossRef] [Green Version]
- Höller, S.; Ueda, Y.; Wu, L.; Wang, Y.; Hajirezaei, M.-R.; Ghaffari, M.-R.; von Wirén, N.; Frei, M. Ascorbate biosynthesis and its involvement in stress tolerance and plant development in rice (Oryza sativa L.). Plant Mol. Biol. 2015, 88, 545–560. [Google Scholar] [CrossRef]
- Rosado-Souza, L.; Fernie, A.R.; Aarabi, F. Ascorbate and thiamin: Metabolic modulators in plant acclimation responses. Plants 2020, 9, 101. [Google Scholar] [CrossRef] [Green Version]
- Broad, R.C.; Bonneau, J.P.; Hellens, R.P.; Johnson, A.A.T. Manipulation of ascorbate biosynthetic, recycling, and regulatory pathways for improved abiotic stress tolerance in plants. Int. J. Mol. Sci. 2020, 21, 1790. [Google Scholar] [CrossRef] [Green Version]
- Xiao, M.; Li, Z.; Zhu, L.; Wang, J.; Zhang, B.; Zheng, F.; Zhao, B.; Zhang, H.; Wang, Y.; Zhang, Z. The multiple roles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator. Front. Plant Sci. 2021, 12, 598173. [Google Scholar] [CrossRef]
- Maruta, T. How does light facilitate vitamin C biosynthesis in leaves? Biosci. Biotechnol. Biochem. 2022, 86, 1173–1182. [Google Scholar] [CrossRef]
- Kiyota, M.; Numayama, N.; Goto, K. Circadian rhythms of the l-ascorbic acid level in Euglena and spinach. J. Photochem. Photobiol. B Biol. 2006, 84, 197–203. [Google Scholar] [CrossRef]
- Dowdle, J.; Ishikawa, T.; Gatzek, S.; Rolinski, S.; Smirnoff, N. Two genes in Arabidopsis thaliana encoding GDP-l-galactose phosphorylase are required for ascorbate biosynthesis and seedling viability. Plant J. 2007, 52, 673–689. [Google Scholar] [CrossRef]
- Maruta, T.; Yonemitsu, M.; Yabuta, Y.; Tamoi, M.; Ishikawa, T.; Shigeoka, S. Arabidopsis Phosphomannose Isomerase 1, but not Phosphomannose Isomerase 2, is essential for ascorbic acid biosynthesis. J. Biol. Chem. 2008, 283, 28842–28851. [Google Scholar] [CrossRef] [Green Version]
- Müller-Moulé, P.; Conklin, P.L.; Niyogi, K.K. Ascorbate deficiency can limit violaxanthin de-epoxidase activity in vivo. Plant Physiol. 2002, 128, 970–977. [Google Scholar] [CrossRef] [Green Version]
- Müller-Moulé, P.; Havaux, M.; Niyogi, K.K. Zeaxanthin deficiency enhances the high light sensitivity of an ascorbate-deficient mutant of Arabidopsis. Plant Physiol. 2003, 133, 748–760. [Google Scholar] [CrossRef] [Green Version]
- Conklin, P.L.; Williams, E.H.; Last, R.L. Ozone-induced expression of stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc. Natl. Acad. Sci. USA 1996, 93, 9970–9974. [Google Scholar] [CrossRef] [Green Version]
- Bellini, E.; De Tullio, M.C. Ascorbic acid and ozone: Novel perspectives to explain an elusive relationship. Plants 2019, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- Kakan, X.; Yu, Y.; Li, S.; Li, X.; Huang, R.; Wang, J. Ascorbic acid modulation by ABI4 transcriptional repression of VTC2 in the salt tolerance of Arabidopsis. BMC Plant Biol. 2021, 21, 112. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Zhang, R.; Huang, R. The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012, 71, 273–287. [Google Scholar] [CrossRef]
- Zhang, H.; Xiang, Y.; He, N.; Liu, X.; Liu, H.; Fang, L.; Zhang, F.; Sun, X.; Zhang, D.; Li, X.; et al. Enhanced vitamin C production mediated by an ABA-induced PTP-like nucleotidase improves plant drought tolerance in Arabidopsis and maize. Mol. Plant 2020, 13, 760–776. [Google Scholar] [CrossRef]
- Iwagami, T.; Ogawa, T.; Ishikawa, T.; Maruta, T. Activation of ascorbate metabolism by nitrogen starvation and its physiological impacts in Arabidopsis thaliana. Biosci. Biotechnol. Biochem. 2022, 86, 476–489. [Google Scholar] [CrossRef]
- Yabuta, Y.; Maruta, T.; Nakamura, A.; Mieda, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Conversion of L-galactono-1,4-lactone to L-ascorbate is regulated by the photosynthetic electron transport chain in Arabidopsis. Biosci. Biotechnol. Biochem. 2008, 72, 2598–2607. [Google Scholar] [CrossRef] [Green Version]
- Bulley, S.M.; Cooney, J.M.; Laing, W. Elevating ascorbate in Arabidopsis stimulates the production of abscisic acid, phaseic acid, and to a lesser extent auxin (IAA) and jasmonates, resulting in increased expression of DHAR1 and multiple transcription factors associated with abiotic stress tolerance. Int. J. Mol. Sci. 2021, 22, 6743. [Google Scholar]
- Tamaoki, M.; Mukai, F.; Asai, N.; Nakajima, N.; Kubo, A.; Aono, M.; Saji, H. Light-controlled expression of a gene encoding L-galactono-γ-lactone dehydrogenase which affects ascorbate pool size in Arabidopsis thaliana. Plant Sci. 2003, 164, 1111–1117. [Google Scholar] [CrossRef]
- Yabuta, Y.; Mieda, T.; Rapolu, M.; Nakamura, A.; Motoki, T.; Maruta, T.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Light regulation of ascorbate biosynthesis is dependent on the photosynthetic electron transport chain but independent of sugars in Arabidopsis. J. Exp. Bot. 2007, 58, 2661–2671. [Google Scholar] [CrossRef] [Green Version]
- Massot, C.; Stevens, R.; Genard, M.; Longuenesse, J.-J.; Gautier, H. Light affects ascorbate content and ascorbate-related gene expression in tomato leaves more than in fruits. Planta 2012, 235, 153–163. [Google Scholar] [CrossRef]
- Pallanca, J.E.; Smirnoff, N. Ascorbic acid metabolism in pea seedlings. A comparison of D-glucosone, L-sorbosone, and L-galactono-1,4-lactone as ascorbate precursors. Plant Physiol. 1999, 120, 453–461. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, F.; Kato, M.; Hyodo, H.; Ikoma, Y.; Sugiura, M.; Yano, M. Effect of sucrose on ascorbate level and expression of genes involved in the ascorbate biosynthesis and recycling pathway in harvested broccoli florets. J. Exp. Bot. 2005, 56, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Badejo, A.A.; Wada, K.; Gao, Y.; Maruta, T.; Sawa, Y.; Shigeoka, S.; Ishikawa, T. Translocation and the alternative D-galacturonate pathway contribute to increasing the ascorbate level in ripening tomato fruits together with the D-mannose/L-galactose pathway. J. Exp. Bot. 2012, 63, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, K.; Nakane, T.; Kume, S.; Shiomi, Y.; Maruta, T.; Ishikawa, T.; Shigeoka, S. Transient expression analysis revealed the importance of VTC2 expression level in light/dark regulation of ascorbate biosynthesis in Arabidopsis. Biosci. Biotechnol. Biochem. 2014, 78, 60–66. [Google Scholar] [CrossRef]
- Tanaka, H.; Maruta, T.; Tamoi, M.; Yabuta, Y.; Yoshimura, K.; Ishikawa, T.; Shigeoka, S. Transcriptional control of vitamin C defective 2 and tocopherol cyclase genes by light and plastid-derived signals: The partial involvement of GENOMES UNCOUPLED 1. Plant Sci. 2015, 231, 20–29. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Gusmaroli, G.; Terzaghi, W.; Lau, O.S.; Yanagawa, Y.; Zhang, Y.; Li, J.; Lee, J.H.; Zhu, D.; et al. Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 2010, 22, 108–123. [Google Scholar] [CrossRef]
- Nezames, C.D.; Deng, X.W. The COP9 signalosome: Its regulation of cullin-based E3 ubiquitin ligases and role in photomorphogenesis. Plant Physiol. 2012, 160, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yu, Y.; Zhang, Z.; Quan, R.; Zhang, H.; Ma, L.; Deng, X.W.; Huang, R. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell 2013, 25, 625–636. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Lorence, A.; Gruszewski, H.A.; Chevone, B.I.; Nessler, C.L. AMR1, an Arabidopsis gene that coordinately and negatively regulates the mannose/L-galactose ascorbic acid biosynthetic pathway. Plant Physiol. 2009, 150, 942–950. [Google Scholar] [CrossRef] [Green Version]
- Hu, T.; Ye, J.; Tao, P.; Li, H.; Zhang, J.; Zhang, Y.; Ye, Z. The tomato HD-Zip I transcription factor SlHZ24 modulates ascorbate accumulation through positive regulation of the D-mannose/L-galactose pathway. Plant J. 2016, 85, 16–29. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liang, D.; Li, M.; Ma, F. Light and abiotic stresses regulate the expression of GDP-L-galactose phosphorylase and levels of ascorbic acid in two kiwifruit genotypes via light-responsive and stress-inducible cis-elements in their promoters. Planta 2013, 238, 535–547. [Google Scholar] [CrossRef]
- Fukunaga, K.; Fujikawa, Y.; Esaka, M. Light regulation of ascorbic acid biosynthesis in rice via light responsive cis-elements in genes encoding ascorbic acid biosynthetic enzymes. Biosci. Biotechnol. Biochem. 2010, 74, 888–891. [Google Scholar] [CrossRef]
- Conklin, P.L.; DePaolo, D.; Wintle, B.; Schatz, C.; Buckenmeyer, G. Identification of Arabidopsis VTC3 as a putative and unique dual function protein kinase::protein phosphatase involved in the regulation of the ascorbic acid pool in plants. J. Exp. Bot. 2013, 64, 2793–2804. [Google Scholar] [CrossRef] [Green Version]
- Leferink, N.G.; van Duijn, E.; Barendregt, A.; Heck, A.J.R.; van Berkel, W.J.H. Galactono-lactone dehydrogenase requires a redox-sensitive thiol for optimal production of vitamin C. Plant Physiol. 2009, 150, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Paciolla, C.; Tommasi, F. The ascorbate system in two bryophytes: Brachythecium velutinum and Marchantia polymorpha. Biol. Plant. 2003, 47, 387–393. [Google Scholar] [CrossRef]
- Sodeyama, T.; Nishikawa, H.; Harai, K.; Takeshima, D.; Sawa, Y.; Maruta, T.; Ishikawa, T. The D-mannose/L-galactose pathway is the dominant ascorbate biosynthetic route in the moss Physcomitrium patens. Plant J. 2021, 107, 1724–1738. [Google Scholar] [CrossRef]
- Urzica, E.I.; Adler, L.N.; Page, M.D.; Linster, C.L.; Arbing, M.A.; Casero, D.; Pellegrini, M.; Merchant, S.S.; Clarke, S.G. Impact of oxidative stress on ascorbate biosynthesis in Chlamydomonas via regulation of the VTC2 gene encoding a GDP-L-galactose phosphorylase. J. Biol. Chem. 2012, 287, 14234–14245. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Meireles, A.; Neupert, J.; Zsigmond, L.; Rosado-Souza, L.; Kovács, L.; Nagy, V.; Galambos, A.; Fernie, A.R.; Bock, R.; Tóth, S.Z. Regulation of ascorbate biosynthesis in green algae has evolved to enable rapid stress-induced response via the VTC2 gene encoding GDP-l-galactose phosphorylase. New Phytol. 2017, 214, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Vidal-Meireles, A.; Tóth, D.; Kovács, L.; Neupert, J.; Tóth, S.Z. Ascorbate deficiency does not limit non-photochemical quenching in Chlamydomonas reinhardtii. Plant Physiol. 2020, 182, 597–611. [Google Scholar] [CrossRef] [Green Version]
- Nagy, V.; Vidal-Meireles, A.; Tengölics, R.; Rákhely, G.; Garab, G.; Kovács, L.; Tóth, S.Z. Ascorbate accumulation during sulphur deprivation and its effects on photosystem II activity and H2 production of the green alga Chlamydomonas reinhardtii. Plant. Cell Environ. 2016, 39, 1460–1472. [Google Scholar] [CrossRef] [Green Version]
- Nagy, V.; Vidal-Meireles, A.; Podmaniczki, A.; Szentmihályi, K.; Rákhely, G.; Zsigmond, L.; Kovács, L.; Tóth, S.Z. The mechanism of photosystem-II inactivation during sulphur deprivation-induced H2 production in Chlamydomonas reinhardtii. Plant J. 2018, 94, 548–561. [Google Scholar] [CrossRef] [Green Version]
- Tóth, S.Z.; Nagy, V.; Puthur, J.T.; Kovács, L.; Garab, G. The physiological role of ascorbate as photosystem II electron donor: Protection against photoinactivation in heat-stressed leaves. Plant Physiol. 2011, 156, 382–392. [Google Scholar] [CrossRef] [Green Version]
- Senn, M.E.; Gergoff Grozeff, G.E.; Alegre, M.L.; Barrile, F.; De Tullio, M.C.; Bartoli, C.G. Effect of mitochondrial ascorbic acid synthesis on photosynthesis. Plant Physiol. Biochem. 2016, 104, 29–35. [Google Scholar] [CrossRef]
- Chen, Z.; Gallie, D.R. The ascorbic acid redox state controls guard cell signaling and stomatal movement. Plant Cell 2004, 16, 1143–1162. [Google Scholar] [CrossRef] [Green Version]
- Kerchev, P.I.; Pellny, T.K.; Vivancos, P.D.; Kiddle, G.; Hedden, P.; Driscoll, S.; Vanacker, H.; Verrier, P.; Hancock, R.D.; Foyer, C.H. The transcription factor ABI4 Is required for the ascorbic acid-dependent regulation of growth and regulation of jasmonate-dependent defense signaling pathways in Arabidopsis. Plant Cell 2011, 23, 3319–3334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapson, L.W.; Isherwood, F.A.; Chen, Y.T. Biological synthesis of L-ascorbic acid: The conversion of L-galactono-γ-lactone into L-ascorbic acid by plant mitochondria. Biochem. J. 1954, 56, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Leferink, N.G.; van den Berg, W.A.; van Berkel, W.J. L-Galactono-γ-lactone dehydrogenase from Arabidopsis thaliana, a flavoprotein involved in vitamin C biosynthesis. FEBS J. 2008, 275, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Meyer, E.H.; Letts, J.A.; Maldonado, M. Structural insights into the assembly and the function of the plant oxidative phosphorylation system. New Phytol. 2022, 235, 1315–1329. [Google Scholar] [CrossRef]
- Sazanov, L.A. A giant molecular proton pump: Structure and mechanism of respiratory complex I. Nat. Rev. Mol. Cell Biol. 2015, 16, 375–388. [Google Scholar] [CrossRef]
- Schimmeyer, J.; Bock, R.; Meyer, E.H. L-Galactono-1,4-lactone dehydrogenase is an assembly factor of the membrane arm of mitochondrial complex I in Arabidopsis. Plant Mol. Biol. 2016, 90, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, C.G.; Pastori, G.M.; Foyer, C.H. Ascorbate biosynthesis in mitochondria is linked to the electron transport chain between complexes III and IV. Plant Physiol. 2000, 123, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Welchen, E.; Gonzalez, D.H. Cytochrome c, a hub linking energy, redox, stress and signaling pathways in mitochondria and other cell compartments. Physiol. Plant. 2016, 157, 310–321. [Google Scholar] [CrossRef]
- Welchen, E.; Hildebrand, T.; Lewejohann, D.; Gonzalez, D.H.; Braun, H.P. Lack of cytochrome c in Arabidopsis decreases stability of Complex IV and modifies redox metabolism without affecting Complexes I and III. Biochim. Biophys. Acta 2012, 1817, 990–1001. [Google Scholar] [CrossRef] [Green Version]
- Bartoli, C.G.; Yu, J.; Gómez, F.; Fernández, L.; McIntosh, L.; Foyer, C.H. Inter-relationships between light and respiration in the control of ascorbic acid synthesis and accumulation in Arabidopsis thaliana leaves. J. Exp. Bot. 2006, 57, 1621–1631. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Sulpice, R.; Gibon, Y.; Fernie, A.R. The enigmatic contribution of mitochondrial function in photosynthesis. J. Exp. Bot. 2008, 59, 1675–1684. [Google Scholar] [CrossRef] [Green Version]
- Nunes-Nesi, A.; Carrari, F.; Lytovchenko, A.; Smith, A.M.; Loureiro, M.E.; Ratcliffe, R.G.; Sweetlove, L.J.; Fernie, A.R. Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol. 2005, 137, 611–622. [Google Scholar] [CrossRef]
- Tomaz, T.; Bagard, M.; Pracharoenwattana, I.; Linden, P.; Lee, C.P.; Carroll, A.J.; Stroher, E.; Smith, S.M.; Gardestrom, P.; Millar, A.H. Mitochondrial malate dehydrogenase lowers leaf respiration and alters photorespiration and plant growth in Arabidopsis. Plant Physiol. 2010, 154, 1143–1157. [Google Scholar] [CrossRef] [Green Version]
- Gebicki, J.M.; Nauser, T.; Domazou, A.; Steinmann, D.; Bounds, P.L.; Koppenol, W.H. Reduction of protein radicals by GSH and ascorbate: Potential biological significance. Amino Acids 2010, 39, 1131–1137. [Google Scholar] [CrossRef]
- Katoh, S.; San Pietro, A. Ascorbate-supported NADP photoreduction by heated Euglena chloroplasts. Arch. Biochem. Biophys. 1967, 122, 144–152. [Google Scholar] [CrossRef]
- Yamashita, T.; Butler, W.L. Photoreduction and photophosphorylation with Tris-washed chloroplasts. Plant Physiol. 1968, 43, 1978–1986. [Google Scholar] [CrossRef] [Green Version]
- Mano, J.; Hideg, É.; Asada, K. Ascorbate in thylakoid lumen functions as an alternative electron donor to photosystem II and photosystem I. Arch. Biochem. Biophys. 2004, 429, 71–80. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Schansker, G.; Garab, G.; Strasser, R.J. Photosynthetic electron transport activity in heat-treated barley leaves: The role of internal alternative electron donors to photosystem II. Biochim. Biophys. Acta 2007, 1767, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Tóth, S.Z.; Puthur, J.T.; Nagy, V.; Garab, G. Experimental evidence for ascorbate-dependent electron transport in leaves with inactive oxygen-evolving complexes. Plant Physiol. 2009, 149, 1568–1578. [Google Scholar] [CrossRef]
- Yamane, Y.; Kashino, Y.; Koike, H.; Satoh, K. Effects of high temperatures on the photosynthetic systems in spinach: Oxygen-evolving activities, fluorescence characteristics and the denaturation process. Photosynth. Res. 1998, 57, 51–59. [Google Scholar] [CrossRef]
- Barra, M.; Haumann, M.; Dau, H. Specific loss of the extrinsic 18 kDa protein from photosystem II upon heating to 47 °C causes inactivation of oxygen evolution likely due to Ca release from the Mn-complex. Photosynth. Res. 2005, 84, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Lelandais, M.A. A comparison of the relative rates of transport of ascorbate and glucose across the thylakoid, chloroplast and plasmalemma membranes of pea leaf mesophyll cells. J. Plant Physiol. 1996, 148, 391–398. [Google Scholar] [CrossRef]
- Chen, G.X.; Blubaugh, D.J.; Homann, P.H.; Golbeck, J.H.; Cheniae, G.M. Superoxide contributes to the rapid inactivation of specific secondary donors of the photosystem II reaction center during photodamage of manganese-depleted photosystem II membranes. Biochemistry 1995, 34, 2317–2332. [Google Scholar] [CrossRef] [PubMed]
- Spetea, C.; Hideg, E.; Vass, I. Low pH accelerates light-induced damage of photosystem II by enhancing the probability of the donor-side mechanism of photoinhibition. Biochim. Biophys. Acta 1997, 1318, 275–283. [Google Scholar] [CrossRef] [Green Version]
- Blubaugh, D.J.; Cheniae, G.M. Kinetics of photoinhibition in hydroxylamine-extracted photosystem II membranes: Relevance to photoactivation and sites of electron donation. Biochemistry 1990, 29, 5109–5118. [Google Scholar] [CrossRef]
- Jegerschöld, C.; Styring, S. Spectroscopic characterization of intermediate steps involved in donor-side-induced photoinhibition of photosystem II. Biochemistry 1996, 35, 7794–7801. [Google Scholar] [CrossRef]
- Lorence, A.; Chevone, B.I.; Mendes, P.; Nessler, C.L. myo-Inositol oxygenase offers a possible entry point into plant ascorbate biosynthesis. Plant Physiol. 2004, 134, 1200–1205. [Google Scholar] [CrossRef] [Green Version]
- Tóth, S.Z.; Schansker, G.; Garab, G. The physiological roles and metabolism of ascorbate in chloroplasts. Physiol. Plant. 2013, 148, 161–175. [Google Scholar] [CrossRef]
- Ivanov, B.N.; Asada, K.; Kramer, D.; Edwards, G. Characterization of photosynthetic electron transport in bundle sheath cells of maize. I. Ascorbate effectively stimulates cyclic electron flow around PSI. Planta 2005, 20, 572–581. [Google Scholar] [CrossRef]
- Ivanov, B.; Asada, K.; Edwards, G.E. Analysis of donors of electrons to photosystem I and cyclic electron flow by redox kinetics of P700 in chloroplasts of isolated bundle sheath strands of maize. Photosynth. Res. 2007, 92, 65–74. [Google Scholar] [CrossRef]
- Ivanov, B.N. Role of ascorbic acid in photosynthesis. Biochemistry 2014, 79, 282–289. [Google Scholar] [CrossRef]
- Trubitsin, B.V.; Mamedov, M.D.; Semenov, A.Y.; Tikhonov, A.N. Interaction of ascorbate with photosystem I. Photosynth. Res. 2014, 122, 215–231. [Google Scholar] [CrossRef]
- Ivanov, B.N.; Sacksteder, C.A.; Kramer, D.M.; Edwards, G.E. Light-Induced ascorbate-dependent electron transport and membrane energization in chloroplasts of bundle sheath cells of the C4 plant maize. Arch. Biochem. Biophys. 2001, 385, 145–153. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Lőrincz, T.; Szarka, A. Concentration does matter: The beneficial and potentially harmful effects of ascorbate in humans and plants. Antioxid. Redox Signal. 2018, 29, 1516–1533. [Google Scholar] [CrossRef] [Green Version]
- Zechmann, B.; Stumpe, M.; Mauch, F. Immunocytochemical determination of the subcellular distribution of ascorbate in plants. Planta 2011, 233, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Razeghifard, M.R.; Klughammer, C.; Pace, R.J. Electron paramagnetic resonance kinetic studies of the S states in spinach thylakoids. Biochemistry 1997, 36, 86–92. [Google Scholar] [CrossRef]
- Godaux, D.; Bailleul, B.; Berne, N.; Cardol, P. Induction of photosynthetic carbon fixation in anoxia relies on hydrogenase activity and Proton-Gradient Regulation-Like1-mediated cyclic electron flow in Chlamydomonas reinhardtii. Plant Physiol. 2015, 168, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.P.; Kim, K.-R.; Choi, S.P.; Han, S.J.; Kim, M.S.; Sim, S.J. Repeated production of hydrogen by sulfate re-addition in sulfur deprived culture of Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 2010, 35, 13387–13391. [Google Scholar] [CrossRef]
- Tamura, N.; Inoue, H.; Inoue, Y. Inactivation of the water-oxidizing complex by exogenous reductants in PSII membranes depleted of extrinsic proteins. Plant Cell Physiol. 1990, 31, 469–477. [Google Scholar]
- Anderson, J.M.; Chow, W.S.; De Las Rivas, J. Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: The grana enigma. Photosynth. Res. 2008, 98, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Ifuku, K. Localization and functional characterization of the extrinsic subunits of photosystem II: An update. Biosci. Biotechnol. Biochem. 2015, 79, 1223–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagao, R.; Suzuki, T.; Okumura, A.; Niikura, A.; Iwai, M.; Dohmae, N.; Tomo, T.; Shen, J.-R.; Ikeuchi, M.; Enami, I. Topological analysis of the extrinsic PsbO, PsbP and PsbQ proteins in a green algal PSII complex by cross-linking with a water-soluble carbodiimide. Plant Cell Physiol. 2010, 51, 718–727. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyeva, Y.; Suorsa, M.; Rossi, F.; Pavesi, A.; Kater, M.M.; Antonacci, A.; Tadini, L.; Pribil, M.; Schneider, A.; Wanner, G.; et al. Arabidopsis plants lacking PsbQ and PsbR subunits of the oxygen-evolving complex show altered PSII super-complex organization and short-term adaptive mechanisms. Plant J. 2013, 75, 671–684. [Google Scholar] [CrossRef]
- Gest, N.; Gautier, H.; Stevens, R. Ascorbate as seen through plant evolution: The rise of a successful molecule? J. Exp. Bot. 2013, 64, 33–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Podmaniczki, A.; Nagy, V.; Vidal-Meireles, A.; Tóth, D.; Patai, R.; Kovács, L.; Tóth, S.Z. Ascorbate inactivates the oxygen-evolving complex in prolonged darkness. Physiol. Plant. 2021, 171, 232–245. [Google Scholar] [CrossRef]
- Kirchhoff, H.; Hall, C.; Wood, M.; Herbstová, M.; Tsabari, O.; Nevo, R.; Charuvi, D.; Shimoni, E.; Reich, Z. Dynamic control of protein diffusion within the granal thylakoid lumen. Proc. Natl. Acad. Sci. USA 2011, 108, 20248–20253. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.L.S.; Lima-Melo, Y.; Carvalho, F.E.L.; Feitosa, A.G.S.; Lima Neto, M.C.; Caverzan, A.; Margis-Pinheiro, M.; Silveira, J.A.G. Ascorbic acid toxicity is related to oxidative stress and enhanced by high light and knockdown of chloroplast ascorbate peroxidases in rice plants. Theor. Exp. Plant Physiol. 2018, 30, 41–55. [Google Scholar] [CrossRef] [Green Version]
- Bassi, R.; Dall’Osto, L. Dissipation of light energy absorbed in excess: The molecular mechanisms. Ann. Rev. Plant Biol. 2021, 72, 47–76. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Shimakawa, G. Regulation of the generation of reactive oxygen species during photosynthetic electron transport. Biochem. Soc. Trans. 2022, 50, 1025–1034. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Pinnola, A.; Bassi, R. Molecular mechanisms involved in plant photoprotection. Biochem. Soc. Trans. 2018, 46, 467–482. [Google Scholar] [CrossRef]
- Kaiser, E.; Correa-Galvis, V.; Armbruster, U. Efficient photosynthesis in dynamic light environments: A chloroplast’s perspective. Biochem. J. 2019, 476, 2725–2741. [Google Scholar] [CrossRef] [Green Version]
- Correa-Galvis, V.; Poschmann, G.; Melzer, M.; Stühler, K.; Jahns, P. PsbS interactions involved in the activation of energy dissipation in Arabidopsis. Nat. Plants 2016, 2, 15225. [Google Scholar] [CrossRef]
- Sacharz, J.; Giovagnetti, V.; Ungerer, P.; Mastroianni, G.; Ruban, A.V. The xanthophyll cycle affects reversible interactions between PsbS and light-harvesting complex II to control non-photochemical quenching. Nat. Plants 2017, 3, 16225. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Caffarri, S.; Bassi, R. A mechanism of nonphotochemical energy dissipation, independent from PsbS, revealed by a conformational change in the antenna protein CP26. Plant Cell 2005, 17, 1217–1232. [Google Scholar] [CrossRef] [Green Version]
- Nilkens, M.; Kress, E.; Lambrev, P.; Miloslavina, Y.; Müller, M.; Holzwarth, A.R.; Jahns, P. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta 2010, 1797, 466–475. [Google Scholar] [CrossRef] [Green Version]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochim. Biophys. Acta 2009, 1787, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Bratt, C.; Arvidsson, P.; Carlsson, M.; Akerlund, H. Regulation of violaxanthin de-epoxidase activity by pH and ascorbate. Photosynth. Res. 1995, 45, 169–175. [Google Scholar] [CrossRef]
- Pfündel, E.E.; Renganathan, M.; Gilmore, A.M.; Yamamoto, H.Y.; Dilley, R.A. Intrathylakoid pH in isolated pea chloroplasts as probed by violaxanthin deepoxidation. Plant Physiol. 1994, 106, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Hieber, A.D.; Bugos, R.C.; Verhoeven, A.S.; Yamamoto, H.Y. Overexpression of violaxanthin de-epoxidase: Properties of C-terminal deletions on activity and pH-dependent lipid binding. Planta 2002, 214, 476–483. [Google Scholar] [CrossRef]
- Arnoux, P.; Morosinotto, T.; Saga, G.; Bassi, R.; Pignol, D. A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 2009, 21, 2036–2044. [Google Scholar] [CrossRef] [Green Version]
- Hallin, E.I.; Hasan, M.; Guo, K.; Åkerlund, H.-E. Molecular studies on structural changes and oligomerisation of violaxanthin de-epoxidase associated with the pH-dependent activation. Photosynth. Res. 2016, 129, 29–41. [Google Scholar] [CrossRef]
- Saga, G.; Giorgetti, A.; Fufezan, C.; Giacometti, G.M.; Bassi, R.; Morosinotto, T. Mutation analysis of violaxanthin de-epoxidase identifies substrate-binding sites and residues involved in catalysis. J. Biol. Chem. 2010, 285, 23763–23770. [Google Scholar] [CrossRef]
- Hartel, H.; Lokstein, H.; Grimm, B.; Rank, B. Kinetic studies on the xanthophyll cycle in barley leaves (Influence of antenna size and relations to nonphotochemical chlorophyll fluorescence quenching). Plant Physiol. 1996, 110, 471–482. [Google Scholar] [CrossRef] [Green Version]
- De Souza, A.P.; Burgess, S.J.; Doran, L.; Hansen, J.; Manukyan, L.; Maryn, N.; Gotarkar, D.; Leonelli, L.; Niyogi, K.K.; Long, S.P. Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 2022, 377, 851–854. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef] [Green Version]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Bethmann, S.; Melzer, M.; Schwarz, N.; Jahns, P. The zeaxanthin epoxidase is degraded along with the D1 protein during photoinhibition of photosystem II. Plant Direct 2019, 3, e00185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Peers, G.; Dent, R.M.; Bai, Y.; Yang, S.Y.; Apel, W.; Leonelli, L.; Niyogi, K.K. Evolution of an atypical de-epoxidase for photoprotection in the green lineage. Nat. Plants 2016, 2, 16140. [Google Scholar] [CrossRef] [Green Version]
- Coesel, S.; Oborník, M.; Varela, J.; Falciatore, A.; Bowler, C. Evolutionary origins and functions of the carotenoid biosynthetic pathway in marine diatoms. PLoS ONE 2008, 3, e2896. [Google Scholar] [CrossRef] [Green Version]
- Girolomoni, L.; Bellamoli, F.; de la Cruz Valbuena, G.; Perozeni, F.; D’Andrea, C.; Cerullo, G.; Cazzaniga, S.; Ballottari, M. Evolutionary divergence of photoprotection in the green algal lineage: A plant-like violaxanthin de-epoxidase enzyme activates the xanthophyll cycle in the green alga Chlorella vulgaris modulating photoprotection. New Phytol. 2020, 228, 136–150. [Google Scholar] [CrossRef]
- Goss, R.; Jakob, T. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth. Res. 2010, 106, 103–122. [Google Scholar] [CrossRef]
- Dambek, M.; Eilers, U.; Breitenbach, J.; Steiger, S.; Büchel, C.; Sandmann, G. Biosynthesis of fucoxanthin and diadinoxanthin and function of initial pathway genes in Phaeodactylum tricornutum. J. Exp. Bot. 2012, 63, 5607–5612. [Google Scholar] [CrossRef]
- Grouneva, I.; Jakob, T.; Wilhelm, C.; Goss, R. Influence of ascorbate and pH on the activity of the diatom xanthophyll cycle-enzyme diadinoxanthin de-epoxidase. Physiol. Plant. 2006, 126, 205–211. [Google Scholar] [CrossRef]
- Dautermann, O.; Lyska, D.; Andersen-Ranberg, J.; Becker, M.; Fröhlich-Nowoisky, J.; Gartmann, H.; Kramer, L.C.; Mayr, K.; Pieper, D.; Rij, L.M.; et al. An algal enzyme required for biosynthesis of the most abundant marine carotenoids. Sci. Adv. 2020, 6, eaaw9183. [Google Scholar] [CrossRef] [Green Version]
- Pinnola, A.; Dall’Osto, L.; Gerotto, C.; Morosinotto, T.; Bassi, R.; Alboresi, A. Zeaxanthin binds to Light-Harvesting Complex Stress-Related Protein to enhance nonphotochemical quenching in Physcomitrella patens. Plant Cell 2013, 25, 3519–3534. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, B.; Borisova-Mubarakshina, M.; Vilyanen, D.; Vetoshkina, D.; Kozuleva, M. Cooperative pathway of O2 reduction to H2O2 in chloroplast thylakoid membrane: New insight into the Mehler reaction. Biophys. Rev. 2022, 14, 857–869. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Pastori, G.M.; Kiddle, G.; Antoniw, J.; Bernard, S.; Veljovic-Jovanovic, S.; Verrier, P.J.; Noctor, G.; Foyer, C.H. Leaf vitamin C contents modulate plant defense transcripts and regulate genes that control development through hormone signaling. Plant Cell 2003, 15, 939–951. [Google Scholar] [CrossRef] [Green Version]
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-related redox regulation and signaling in plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Luschin-Ebengreuth, N.; Zechmann, B. Compartment-specific investigations of antioxidants and hydrogen peroxide in leaves of Arabidopsis thaliana during dark-induced senescence. Acta Physiol. Plant. 2016, 38, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrow, S.C.; Facchini, P.J. Functional diversity of 2-oxoglutarate/Fe(II)-dependent dioxygenases in plant metabolism. Front. Plant Sci. 2004, 5, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyaji, T.; Kuromori, T.; Takeuchi, Y.; Yamaji, N.; Yokosho, K.; Shimazawa, A.; Sugimoto, E.; Omote, H.; Ma, J.F.; Shinozaki, K.; et al. AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat. Commun. 2015, 6, 5928. [Google Scholar] [CrossRef] [PubMed]
- Hoang, M.T.T.; Almeida, D.; Chay, S.; Alcon, C.; Corratge-Faillie, C.; Curie, C.; Mari, S. AtDTX25, a member of the multidrug and toxic compound extrusion family, is a vacuolar ascorbate transporter that controls intracellular iron cycling in Arabidopsis. New Phytol. 2021, 231, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tóth, S.Z. The Functions of Chloroplastic Ascorbate in Vascular Plants and Algae. Int. J. Mol. Sci. 2023, 24, 2537. https://doi.org/10.3390/ijms24032537
Tóth SZ. The Functions of Chloroplastic Ascorbate in Vascular Plants and Algae. International Journal of Molecular Sciences. 2023; 24(3):2537. https://doi.org/10.3390/ijms24032537
Chicago/Turabian StyleTóth, Szilvia Z. 2023. "The Functions of Chloroplastic Ascorbate in Vascular Plants and Algae" International Journal of Molecular Sciences 24, no. 3: 2537. https://doi.org/10.3390/ijms24032537
APA StyleTóth, S. Z. (2023). The Functions of Chloroplastic Ascorbate in Vascular Plants and Algae. International Journal of Molecular Sciences, 24(3), 2537. https://doi.org/10.3390/ijms24032537





