Characterization of Stress Granule Protein Turnover in Neuronal Progenitor Cells Using Correlative STED and NanoSIMS Imaging
Abstract
1. Introduction
2. Results and Discussion
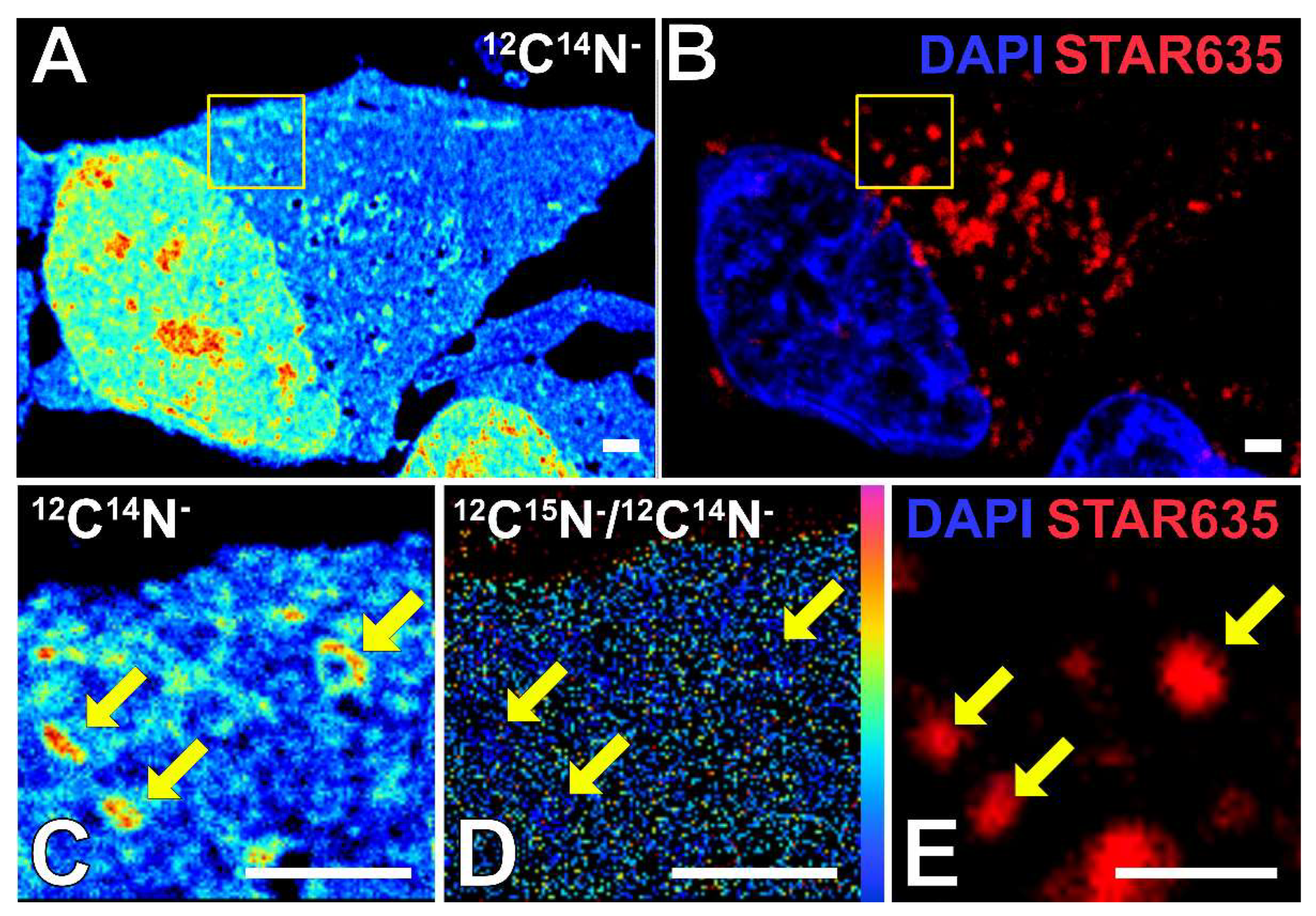
2.1. Correlative STED and NanoSIMS Imaging for Protein Turnover of SGs
2.2. Effect of Cellular Stress on Amino Acid Uptake and Cellular Protein Turnover
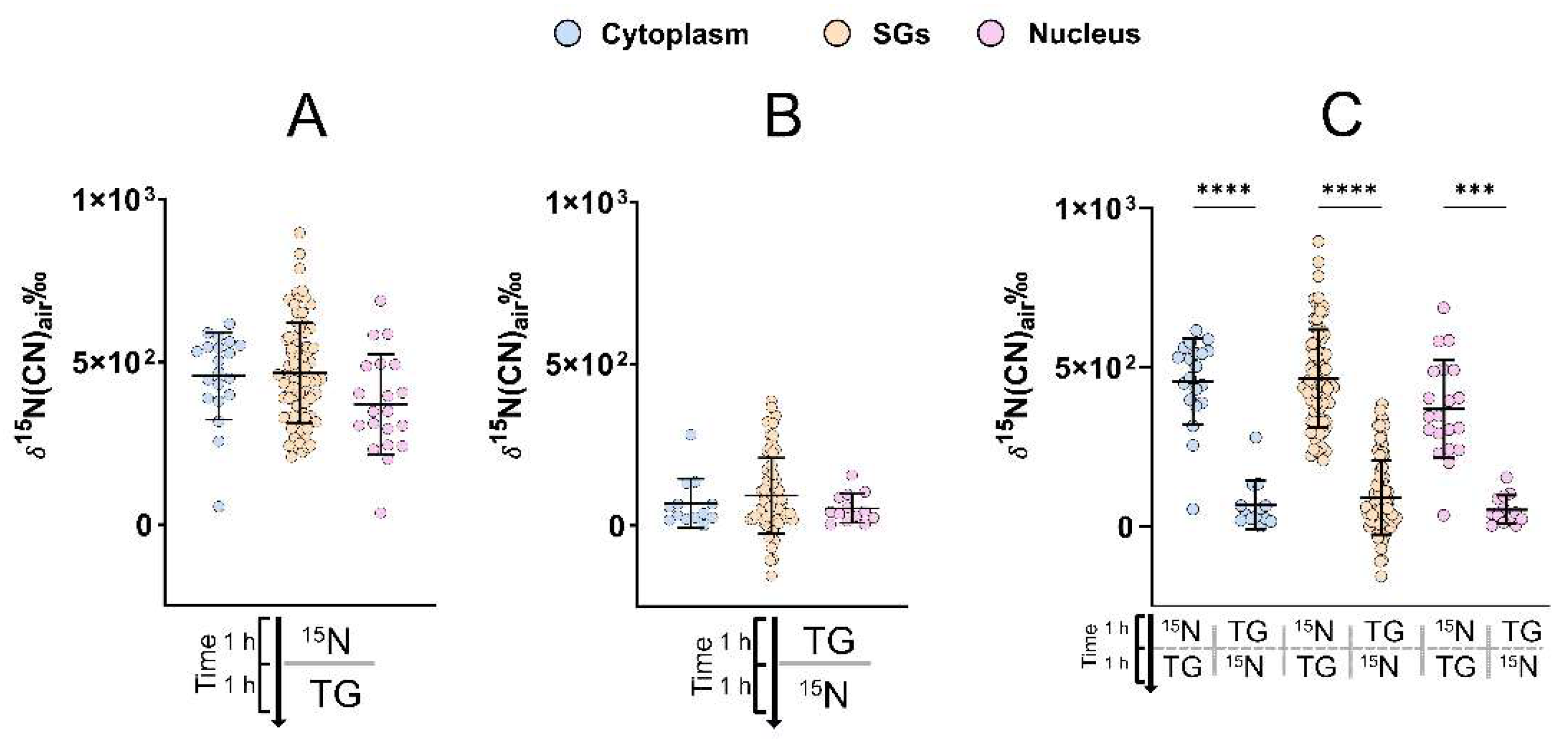
2.3. Protein Turnover during SG Assembly
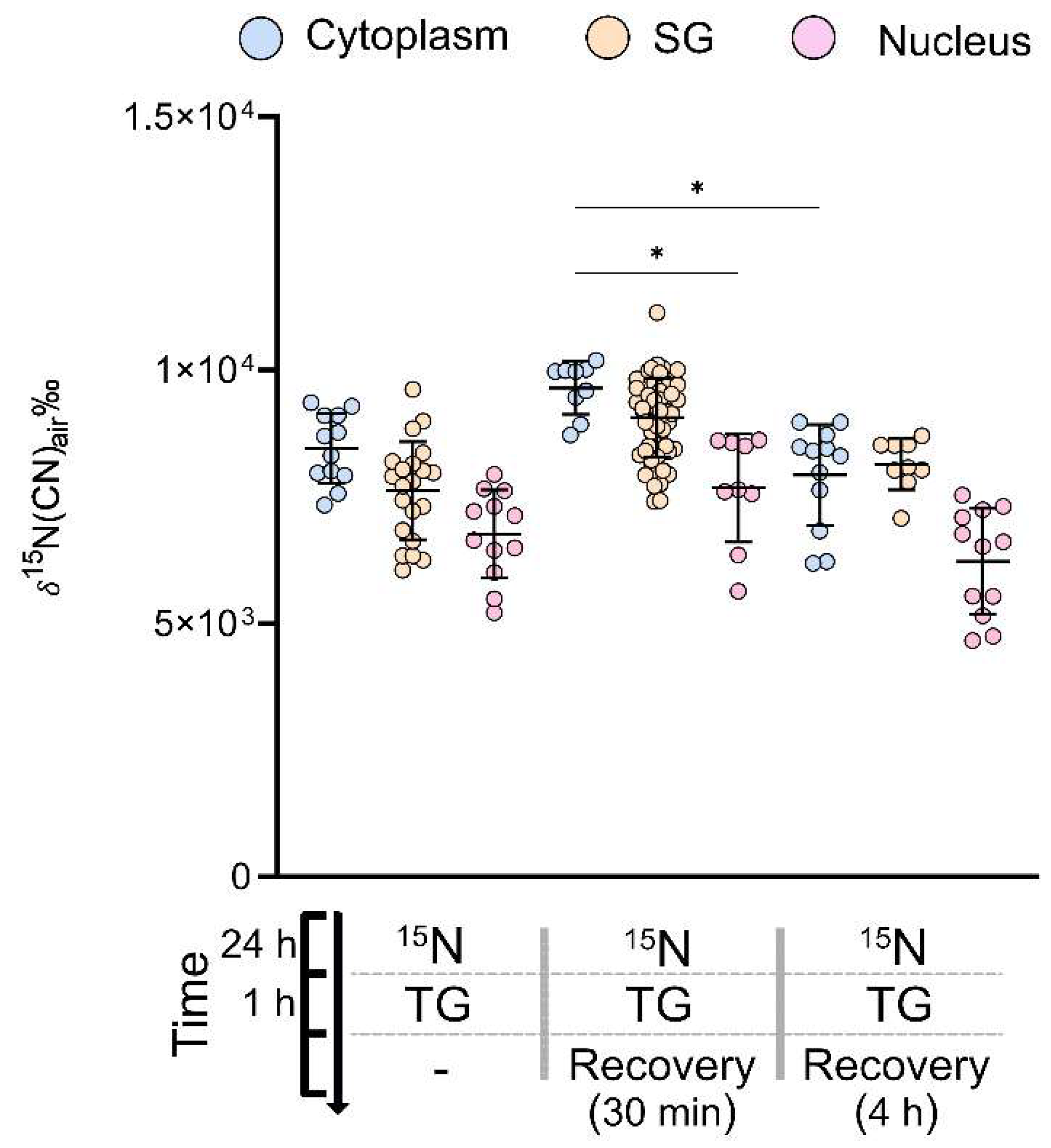
2.4. SG Protein Turnover during Stress Recovery
3. Materials and Methods
4. Limitations and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kültz, D. Defining biological stress and stress responses based on principles of physics. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D. Evolution of cellular stress response mechanisms. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Yamazaki, T.; Kroemer, G. Linking cellular stress responses to systemic homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 731–745. [Google Scholar] [CrossRef] [PubMed]
- Senft, D.; Ronai, Z.A. UPR, autophagy, and mitochondria crosstalk underlies the ER stress response. Trends Biochem. Sci. 2015, 40, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kurop, M.K.; Huyen, C.M.; Kelly, J.H.; Blagg, B.S. The heat shock response and small molecule regulators. Eur. J. Med. Chem. 2021, 226, 113846. [Google Scholar] [CrossRef]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Adams, C.J.; Kopp, M.C.; Larburu, N.; Nowak, P.R.; Ali, M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019, 6, 11. [Google Scholar] [CrossRef]
- Jaskulska, A.; Janecka, A.E.; Gach-Janczak, K. Thapsigargin—From Traditional Medicine to Anticancer Drug. Int. J. Mol. Sci. 2020, 22, 4. [Google Scholar] [CrossRef]
- Varadarajan, S.; Tanaka, K.; Smalley, J.L.; Bampton, E.T.W.; Pellecchia, M.; Dinsdale, D.; Willars, G.B.; Cohen, G.M. Endoplasmic Reticulum Membrane Reorganization Is Regulated by Ionic Homeostasis. PLoS ONE 2013, 8, e56603. [Google Scholar] [CrossRef]
- Wolozin, B.; Ivanov, P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019, 20, 649–666. [Google Scholar] [CrossRef]
- Protter, D.S.; Parker, R. Principles and Properties of Stress Granules. Trends Cell Biol. 2016, 26, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Tolay, N.; Buchberger, A. Role of the Ubiquitin System in Stress Granule Metabolism. Int. J. Mol. Sci. 2022, 23, 3624. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Kedersha, N. Stress granules: The Tao of RNA triage. Trends Biochem. Sci. 2008, 33, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kedersha, N.; Cho, M.R.; Li, W.; Yacono, P.W.; Chen, S.; Gilks, N.; Golan, D.E.; Anderson, P. Dynamic Shuttling of Tia-1 Accompanies the Recruitment of mRNA to Mammalian Stress Granules. J. Cell Biol. 2000, 151, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Aulas, A.; Fay, M.M.; Lyons, S.M.; Achorn, C.A.; Kedersha, N.; Anderson, P.; Ivanov, P. Stress-specific differences in assembly and composition of stress granules and related foci. J. Cell Sci. 2017, 130, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, S.; Kedersha, N.; Anderson, P.; Ivanov, P. Molecular mechanisms of stress granule assembly and disassembly. Biochim. Et Biophys. Acta BBA-Mol. Cell Res. 2020, 1868, 118876. [Google Scholar] [CrossRef]
- Reineke, L.C.; Neilson, J.R. Differences between acute and chronic stress granules, and how these differences may impact function in human disease. Biochem. Pharmacol. 2018, 162, 123–131. [Google Scholar] [CrossRef]
- Hu, K.; Relton, E.; Locker, N.; Phan, N.T.N.; Ewing, A.G. Electrochemical Measurements Reveal Reactive Oxygen Species in Stress Granules. Angew. Chem. Int. Ed. 2021, 60, 15302–15306. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, J.; Sun, Q. Aberrant Stress Granule Dynamics and Aggrephagy in ALS Pathogenesis. Cells 2021, 10, 2247. [Google Scholar] [CrossRef]
- Buchan, J.R.; Kolaitis, R.M.; Taylor, J.P.; Parker, R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. Cell 2013, 153, 1461. [Google Scholar] [CrossRef]
- Wood, A.; Gurfinkel, Y.; Polain, N.; Lamont, W.; Rea, S.L. Molecular Mechanisms Underlying TDP-43 Pathology in Cellular and Animal Models of ALS and FTLD. Int. J. Mol. Sci. 2021, 22, 4705. [Google Scholar] [CrossRef]
- Wheeler, J.R.; Matheny, T.; Jain, S.; Abrisch, R.; Parker, R. Distinct stages in stress granule assembly and disassembly. eLife 2016, 5, e18413. [Google Scholar] [CrossRef]
- Hoppe, P.; Cohen, S.; Meibom, A. NanoSIMS: Technical Aspects and Applications in Cosmochemistry and Biological Geo-chemistry. Geostand Geoanal. Res. 2013, 37, 111–154. [Google Scholar] [CrossRef]
- Jähne, S.; Mikulasch, F.; Heuer, H.G.; Truckenbrodt, S.; Agüi-Gonzalez, P.; Grewe, K.; Vogts, A.; Rizzoli, S.O.; Priesemann, V. Presynaptic activity and protein turnover are correlated at the single-synapse level. Cell Rep. 2021, 34, 108841. [Google Scholar] [CrossRef] [PubMed]
- Saka, S.K.; Vogts, A.; Kröhnert, K.; Hillion, F.; Rizzoli, S.O.; Wessels, J.T. Correlated optical and isotopic nanoscopy. Nat. Commun. 2014, 5, 3664. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Takahash, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Lunn, J.S.; Sakowski, S.A.; Hur, J.; Feldman, E.L. Stem cell technology for neurodegenerative diseases. Ann. Neurol. 2011, 70, 353–361. [Google Scholar] [CrossRef]
- Ng, S.-A.; Sullivan, K.M. Application of stem cell transplantation in autoimmune diseases. Curr. Opin. Hematol. 2019, 26, 392–398. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Agüi-Gonzalez, P.; Jähne, S.; Phan, N.T.N. SIMS imaging in neurobiology and cell biology. J. Anal. At. Spectrom. 2019, 34, 1355–1368. [Google Scholar] [CrossRef]
- Burrill, J.S.; Long, E.K.; Reilly, B.; Deng, Y.; Armitage, I.M.; Scherer, P.E.; Bernlohr, D.A. Inflammation and ER Stress Regulate Branched-Chain Amino Acid Uptake and Metabolism in Adipocytes. Mol. Endocrinol. 2015, 29, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Matheny, T.; Rao, B.S.; Parker, R. Transcriptome-Wide Comparison of Stress Granules and P-Bodies Reveals that Translation Plays a Major Role in RNA Partitioning. Mol. Cell. Biol. 2019, 39, e00313-19. [Google Scholar] [CrossRef] [PubMed]
- Namkoong, S.; Ho, A.; Woo, Y.M.; Kwak, H.; Lee, J.H. Systematic Characterization of Stress-Induced RNA Granulation. Mol. Cell 2018, 70, 175–187.e8. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Liu, X.; Liu, H. Mechanisms of Epidermal Growth Factor Effect on Animal Intestinal Phosphate Absorption: A Review. Front. Veter-Sci. 2021, 8, 670140. [Google Scholar] [CrossRef] [PubMed]
- Waisman, A.; Norris, A.M.; Elias Costa, M.; Kopinke, D. Automatic and unbiased segmentation and quantification of myofibers in skeletal muscle. Sci. Rep. 2021, 11, 11793. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabasco, S.; Lork, A.A.; Berlin, E.; Nguyen, T.D.K.; Ernst, C.; Locker, N.; Ewing, A.G.; Phan, N.T.N. Characterization of Stress Granule Protein Turnover in Neuronal Progenitor Cells Using Correlative STED and NanoSIMS Imaging. Int. J. Mol. Sci. 2023, 24, 2546. https://doi.org/10.3390/ijms24032546
Rabasco S, Lork AA, Berlin E, Nguyen TDK, Ernst C, Locker N, Ewing AG, Phan NTN. Characterization of Stress Granule Protein Turnover in Neuronal Progenitor Cells Using Correlative STED and NanoSIMS Imaging. International Journal of Molecular Sciences. 2023; 24(3):2546. https://doi.org/10.3390/ijms24032546
Chicago/Turabian StyleRabasco, Stefania, Alicia A. Lork, Emmanuel Berlin, Tho D. K. Nguyen, Carl Ernst, Nicolas Locker, Andrew G. Ewing, and Nhu T. N. Phan. 2023. "Characterization of Stress Granule Protein Turnover in Neuronal Progenitor Cells Using Correlative STED and NanoSIMS Imaging" International Journal of Molecular Sciences 24, no. 3: 2546. https://doi.org/10.3390/ijms24032546
APA StyleRabasco, S., Lork, A. A., Berlin, E., Nguyen, T. D. K., Ernst, C., Locker, N., Ewing, A. G., & Phan, N. T. N. (2023). Characterization of Stress Granule Protein Turnover in Neuronal Progenitor Cells Using Correlative STED and NanoSIMS Imaging. International Journal of Molecular Sciences, 24(3), 2546. https://doi.org/10.3390/ijms24032546







