Characterizing the Specific Recognition of Xanthurenic Acid by GEP1 and GEP1-GCα Interactions in cGMP Signaling Pathway in Gametogenesis of Malaria Parasites
Abstract
:1. Introduction
2. Results
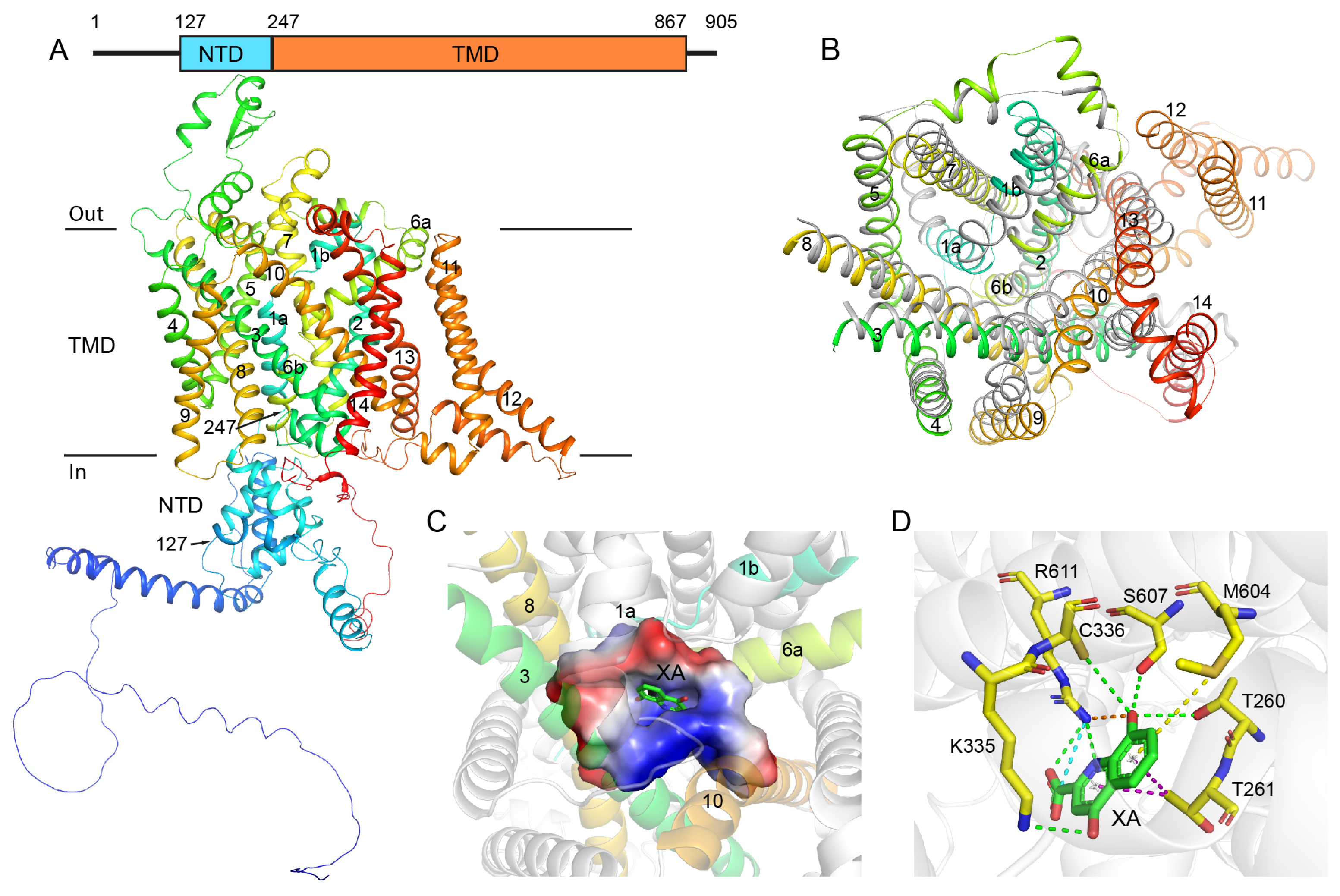
2.1. Structural Analysis of GEP1 by AlphaFold2 and the Recognition Model of XA
2.2. Expression of GEP1 in Insect Cells
2.3. N-terminal Truncation Facilitates Protein Expression and Solubility
2.4. Protein Purification and Trapping in Amphipol Enhances Homogeneity
2.5. CD Spectroscopy of GEP1 Protein at Different pH and Temperatures
2.6. GEP1 Interacts with GCα via Its C-terminal Domain
3. Discussion
4. Materials and Methods
4.1. Regents
4.2. De Novo Structure Prediction Using AlphaFold2 and Molecular Docking Using Discovery Studio
4.3. Construct Design
4.4. Protein Expression
4.5. Observation Using Inverted Fluorescence Microscopy
4.6. Detection of GFP Intensity and FSEC
4.7. Pull-Down Experiment
4.8. Protein Purification
4.9. Negative Stain Electron Microscopy
4.10. Trapping in Amphipol A8-35
4.11. CD Spectroscopy Analysis
4.12. Detection of Interaction between GEP1 and GCα
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cowman, A.F.; Healer, J.; Marapana, D.; Marsh, K. Malaria: Biology and Disease. Cell 2016, 167, 610–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tun, K.M.; Imwong, M.; Lwin, K.M.; Win, A.A.; Hlaing, T.M.; Hlaing, T.; Lin, K.; Kyaw, M.P.; Plewes, K.; Faiz, M.A.; et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: A cross-sectional survey of the K13 molecular marker. Lancet Infect. Dis. 2015, 15, 415–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Mao, S.; Sopha, C.; Sam, B.; Dek, D.; Try, V.; Amato, R.; et al. Dihydroartemisinin-piperaquine resistance in Plasmodium falciparum malaria in Cambodia: A multisite prospective cohort study. Lancet Infect. Dis. 2016, 16, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straimer, J.; Gnädig, N.F.; Witkowski, B.; Amaratunga, C.; Duru, V.; Ramadani, A.P.; Dacheux, M.; Khim, N.; Zhang, L.; Lam, S.; et al. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 2015, 347, 428–431. [Google Scholar] [CrossRef] [Green Version]
- Birnbaum, J.; Scharf, S.; Schmidt, S.; Jonscher, E.; Hoeijmakers, W.A.M.; Flemming, S.; Toenhake, C.G.; Schmitt, M.; Sabitzki, R.; Bergmann, B.; et al. A Kelch13-defined endocytosis pathway mediates artemisinin resistance in malaria parasites. Science 2020, 367, 51–59. [Google Scholar] [CrossRef]
- Jiang, X.; Yuan, Y.; Huang, J.; Zhang, S.; Luo, S.; Wang, N.; Pu, D.; Zhao, N.; Tang, Q.; Hirata, K.; et al. Structural Basis for Blocking Sugar Uptake into the Malaria Parasite Plasmodium falciparum. Cell 2020, 183, 258–268. [Google Scholar] [CrossRef]
- Jonscher, E.; Flemming, S.; Schmitt, M.; Sabitzki, R.; Reichard, N.; Birnbaum, J.; Bergmann, B.; Höhn, K.; Spielmann, T. PfVPS45 Is Required for Host Cell Cytosol Uptake by Malaria Blood Stage Parasites. Cell Host Microbe 2019, 25, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Bansal, A.; Molina-Cruz, A.; Brzostowski, J.; Liu, P.; Luo, Y.; Gunalan, K.; Li, Y.; Ribeiro, J.M.C.; Miller, L.H. PfCDPK1 is critical for malaria parasite gametogenesis and mosquito infection. Proc. Natl. Acad. Sci. USA 2018, 115, 774–779. [Google Scholar] [CrossRef] [Green Version]
- Hitz, E.; Balestra, A.C.; Brochet, M.; Voss, T.S. PfMAP-2 is essential for male gametogenesis in the malaria parasite Plasmodium falciparum. Sci. Rep. 2020, 10, 11930. [Google Scholar] [CrossRef]
- Bennink, S.; Kiesow, M.J.; Pradel, G. The development of malaria parasites in the mosquito midgut. Cell. Microbiol. 2016, 18, 905–918. [Google Scholar] [CrossRef]
- Billker, O.; Lindo, V.; Panico, M.; Etienne, A.E.; Paxton, T.; Dell, A.; Rogers, M.; Sinden, R.E.; Morris, H.R. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature 1998, 392, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Muhia, D.K.; Swales, C.A.; Deng, W.; Kelly, J.M.; Baker, D.A. The gametocyte-activating factor xanthurenic acid stimulates an increase in membrane-associated guanylyl cyclase activity in the human malaria parasite Plasmodium falciparum. Mol. Microbiol. 2001, 42, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Brochet, M.; Collins, M.O.; Smith, T.K.; Thompson, E.; Sebastian, S.; Volkmann, K.; Schwach, F.; Chappell, L.; Gomes, A.R.; Berriman, M.; et al. Phosphoinositide metabolism links cGMP-dependent protein kinase G to essential Ca2+ signals at key decision points in the life cycle of malaria parasites. PLoS Biol. 2014, 12, e1001806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Billker, O.; Dechamps, S.; Tewari, R.; Wenig, G.; Franke-Fayard, B.; Brinkmann, V. Calcium and a calcium-dependent protein kinase regulate gamete formation and mosquito transmission in a malaria parasite. Cell 2004, 117, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Wei, J.; Cui, H.; Liu, C.; Zhi, Y.; Jiang, Z.; Li, Z.; Li, S.; Yang, Z.; Wang, X.; et al. An intracellular membrane protein GEP1 regulates xanthurenic acid induced gametogenesis of malaria parasites. Nat. Commun. 2020, 11, 1764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verrey, F.; Closs, E.I.; Wagner, C.A.; Palacin, M.; Endou, H.; Kanai, Y. CATs and HATs: The SLC7 family of amino acid transporters. Pflug. Arch. 2004, 447, 532–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brochet, M.; Balestra, A.C.; Brusini, L. cGMP homeostasis in malaria parasites-The key to perceiving and integrating environmental changes during transmission to the mosquito. Mol. Microbiol. 2021, 115, 829–838. [Google Scholar] [CrossRef]
- Brown, K.M.; Sibley, L.D. Essential cGMP signaling in Toxoplasma is initiated by a hybrid P-type ATPase-guanylate cyclase. Cell Host Microbe 2018, 24, 804–816. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Sorrentino, S.; Medalia, O.; Korkhov, V.M. The structure of a membrane adenylyl cyclase bound to an activated stimulatory G protein. Science 2019, 364, 389–394. [Google Scholar] [CrossRef]
- Pandey, A.; Shin, K.; Patterson, R.E.; Liu, X.-Q.; Rainey, J.K. Current strategies for protein production and purification enabling membrane protein structural biology. Biochem. Cell Biol. 2016, 94, 507–527. [Google Scholar] [CrossRef]
- Bernaudat, F.; Frelet-Barrand, A.; Pochon, N.; Dementin, S.; Hivin, P.; Boutigny, S.; Rioux, J.-B.; Salvi, D.; Seigneurin-Berny, D.; Richaud, P.; et al. Heterologous expression of membrane proteins: Choosing the appropriate host. PLoS ONE 2011, 6, e29191. [Google Scholar] [CrossRef] [PubMed]
- Kermani, A.A. A guide to membrane protein X-ray crystallography. FEBS J. 2021, 288, 5788–5804. [Google Scholar] [CrossRef]
- Drew, D.; Lerch, M.; Kunji, E.; Slotboom, D.-J.; de Gier, J.-W. Optimization of membrane protein overexpression and purification using GFP fusions. Nat. Methods 2006, 3, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Hibbs, R.E.; Gouaux, E. A fluorescence-detection size-exclusion chromatography-based thermostability assay for membrane protein precrystallization screening. Structure 2012, 20, 1293–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lü, W.; Du, J.; Goehring, A.; Gouaux, E. Cryo-EM structures of the triheteromeric NMDA receptor and its allosteric modulation. Science 2017, 355, eaal3729. [Google Scholar] [CrossRef] [Green Version]
- Virolainen, M.S.; Soltoft, C.L.; Pedersen, P.A.; Ellgaard, L. Production of an Active, Human Membrane Protein in Saccharomyces cerevisiae: Full-Length FICD. Int. J. Mol. Sci. 2022, 23, 2458. [Google Scholar] [CrossRef]
- Stetsenko, A.; Guskov, A. An Overview of the Top Ten Detergents Used for Membrane Protein Crystallization. Crystals 2017, 7, 197. [Google Scholar] [CrossRef] [Green Version]
- Popot, J.-L. Amphipols, nanodiscs, and fluorinated surfactants: Three nonconventional approaches to studying membrane proteins in aqueous solutions. Annu. Rev. Biochem. 2010, 79, 737–775. [Google Scholar] [CrossRef] [Green Version]
- Mazhab-Jafari, M.T.; Rohou, A.; Schmidt, C.; Bueler, S.A.; Benlekbir, S.; Robinson, C.V.; Rubinstein, J.L. Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase. Nature 2016, 539, 118–122. [Google Scholar] [CrossRef]
- Etzkorn, M.; Zoonens, M.; Catoire, L.J.; Popot, J.-L.; Hiller, S. How amphipols embed membrane proteins: Global solvent accessibility and interaction with a flexible protein terminus. J. Membr. Biol. 2014, 247, 965–970. [Google Scholar] [CrossRef]
- Yang, D.; Gouaux, E. Illumination of serotonin transporter mechanism and role of the allosteric site. Sci. Adv. 2021, 7, eabl3857. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Sun, S.; Wang, P.; Sun, Y.; Hu, Q.; Wang, X. The Mechanism of Secretion and Metabolism of Gut-Derived 5-Hydroxytryptamine. Int. J. Mol. Sci. 2021, 22, 7931. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.S.; Rohane, S.H. Review on Discovery Studio: An important Tool for Molecular Docking. Asian J. Res. Chem. 2021, 14, 86–88. [Google Scholar] [CrossRef]
- Singh, S.K.; Yamashita, A.; Gouaux, E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature 2007, 448, 952–956. [Google Scholar] [CrossRef]
- Penmatsa, A.; Wang, K.H.; Gouaux, E. X-ray structure of dopamine transporter elucidates antidepressant mechanism. Nature 2013, 503, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Cao, A.-M.; Quast, R.B.; Fatemi, F.; Rondard, P.; Pin, J.-P.; Margeat, E. Allosteric modulators enhance agonist efficacy by increasing the residence time of a GPCR in the active state. Nat. Commun. 2021, 12, 5426. [Google Scholar] [CrossRef]
- Youn, T.; Yoon, S.; Byrne, B.; Chae, P.S. Foldable Detergents for Membrane Protein Stability. Chembiochem 2022, 23, e202200276. [Google Scholar] [CrossRef]
- Zhao, L.-H.; Ma, S.; Sutkeviciute, I.; Shen, D.-D.; Zhou, X.E.; de Waal, P.W.; Li, C.-Y.; Kang, Y.; Clark, L.J.; Jean-Alphonse, F.G.; et al. Structure and dynamics of the active human parathyroid hormone receptor-1. Science 2019, 364, 148–153. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Bernson, D.; Mecinovic, A.; Abed, M.T.; Limé, F.; Jageland, P.; Palmlöf, M.; Esbjörner, E.K. Amyloid formation of bovine insulin is retarded in moderately acidic pH and by addition of short-chain alcohols. Eur. Biophys. J. 2020, 49, 145–153. [Google Scholar] [CrossRef]
- McNamara, C.; Zinkernagel, A.S.; Macheboeuf, P.; Cunningham, M.W.; Nizet, V.; Ghosh, P. Coiled-coil irregularities and instabilities in group A Streptococcus M1 are required for virulence. Science 2008, 319, 1405–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Günay-Esiyok, Ö.; Scheib, U.; Noll, M.; Gupta, N. An unusual and vital protein with guanylate cyclase and P4-ATPase domains in a pathogenic protist. Life Sci. Alliance 2019, 2, e201900402. [Google Scholar] [CrossRef] [PubMed]
- del Alamo, D.; DeSousa, L.; Nair, R.M.; Rahman, S.; Meiler, J.; McHaourab, H.S. Integrated AlphaFold2 and DEER investigation of the conformational dynamics of a pH-dependent APC antiporter. Proc. Natl. Acad. Sci. USA 2022, 119, e2206129119. [Google Scholar] [CrossRef]
- Coleman, J.A.; Green, E.M.; Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 2016, 532, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.H.; Penmatsa, A.; Gouaux, E. Neurotransmitter and psychostimulant recognition by the dopamine transporter. Nature 2015, 521, 322–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnamurthy, H.; Gouaux, E. X-ray structures of LeuT in substrate-free outward-open and apo inward-open states. Nature 2012, 481, 469–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carucci, D.J.; Witney, A.A.; Muhia, D.K.; Warhurst, D.C.; Schaap, P.; Meima, M.; Li, J.-L.; Taylor, M.C.; Kelly, J.M.; Baker, D.A. Guanylyl cyclase activity associated with putative bifunctional integral membrane proteins in Plasmodium falciparum. J. Biol. Chem. 2000, 275, 22147–22156. [Google Scholar] [CrossRef] [Green Version]
- Baker, D.A.; Drought, L.G.; Flueck, C.; Nofal, S.D.; Patel, A.; Penzo, M.; Walker, E.M. Cyclic nucleotide signalling in malaria parasites. Open Biol. 2017, 7, 170213. [Google Scholar] [CrossRef] [Green Version]
- Bisio, H.; Lunghi, M.; Brochet, M.; Soldati-Favre, D. Phosphatidic acid governs natural egress in Toxoplasma gondii via a guanylate cyclase receptor platform. Nat. Microbiol. 2019, 4, 420–428. [Google Scholar] [CrossRef] [Green Version]
- Jumper, J.; Hassabis, D. Protein structure predictions to atomic accuracy with AlphaFold. Nat. Methods 2022, 19, 11–12. [Google Scholar] [CrossRef]
- Mirdita, M.; Schütze, K.; Moriwaki, Y.; Heo, L.; Ovchinnikov, S.; Steinegger, M. ColabFold: Making protein folding accessible to all. Nat. Methods 2022, 19, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Chen, X.; Zhu, C.; Liang, X.; Gao, Z.; Luo, S. Identification of a novel substrate motif of yeast separase and deciphering the recognition specificity using AlphaFold2 and molecular dynamics simulation. Biochem. Biophys. Res. Commun. 2022, 620, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, Y.; Xu, G.; Wu, L.; Han, R.; Schwaneberg, U.; Rao, Y.; Zhao, Y.L.; Zhou, J.; Ni, Y. Structural Insight into Enantioselective Inversion of an Alcohol Dehydrogenase Reveals a “Polar Gate” in Stereorecognition of Diaryl Ketones. J. Am. Chem. Soc. 2018, 140, 12645–12654. [Google Scholar] [CrossRef]
- Fitzgerald, D.J.; Berger, P.; Schaffitzel, C.; Yamada, K.; Richmond, T.J.; Berger, I. Protein complex expression by using multigene baculoviral vectors. Nat. Methods 2006, 3, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Erijman, A.; Dantes, A.; Bernheim, R.; Shifman, J.M.; Peleg, Y. Transfer-PCR (TPCR): A highway for DNA cloning and protein engineering. J. Struct. Biol. 2011, 175, 171–177. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, C.; Liang, X.; Chen, X.; Liang, M.; Zheng, J.; Wan, B.; Luo, S. Characterizing the Specific Recognition of Xanthurenic Acid by GEP1 and GEP1-GCα Interactions in cGMP Signaling Pathway in Gametogenesis of Malaria Parasites. Int. J. Mol. Sci. 2023, 24, 2561. https://doi.org/10.3390/ijms24032561
Zhu C, Liang X, Chen X, Liang M, Zheng J, Wan B, Luo S. Characterizing the Specific Recognition of Xanthurenic Acid by GEP1 and GEP1-GCα Interactions in cGMP Signaling Pathway in Gametogenesis of Malaria Parasites. International Journal of Molecular Sciences. 2023; 24(3):2561. https://doi.org/10.3390/ijms24032561
Chicago/Turabian StyleZhu, Cheng, Xiaoge Liang, Xu Chen, Miaomiao Liang, Jianting Zheng, Bingbing Wan, and Shukun Luo. 2023. "Characterizing the Specific Recognition of Xanthurenic Acid by GEP1 and GEP1-GCα Interactions in cGMP Signaling Pathway in Gametogenesis of Malaria Parasites" International Journal of Molecular Sciences 24, no. 3: 2561. https://doi.org/10.3390/ijms24032561
APA StyleZhu, C., Liang, X., Chen, X., Liang, M., Zheng, J., Wan, B., & Luo, S. (2023). Characterizing the Specific Recognition of Xanthurenic Acid by GEP1 and GEP1-GCα Interactions in cGMP Signaling Pathway in Gametogenesis of Malaria Parasites. International Journal of Molecular Sciences, 24(3), 2561. https://doi.org/10.3390/ijms24032561





