Investigation of Glucose–Water Mixtures as a Function of Concentration and Temperature by Infrared Spectroscopy
Abstract
:1. Introduction
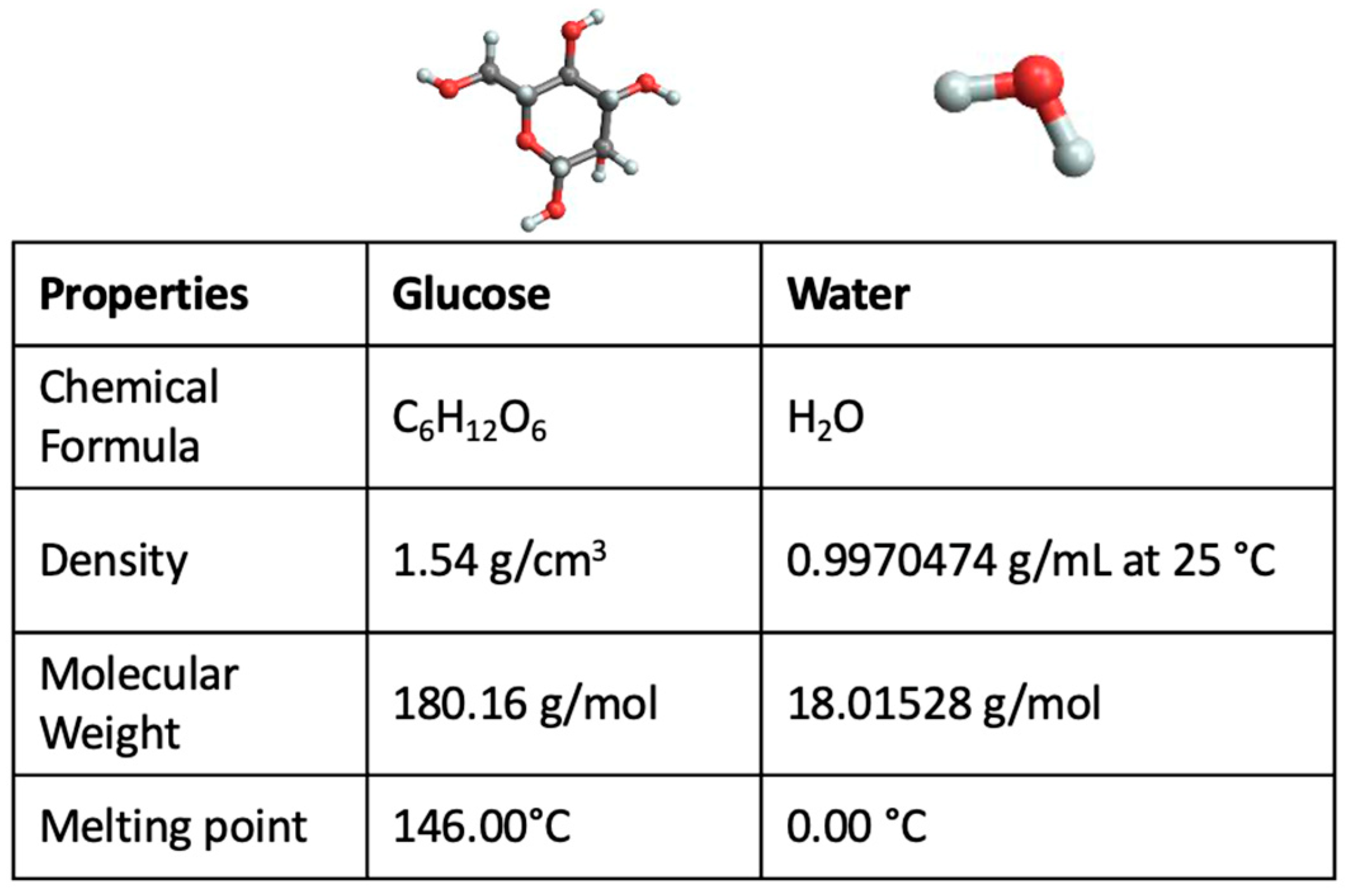
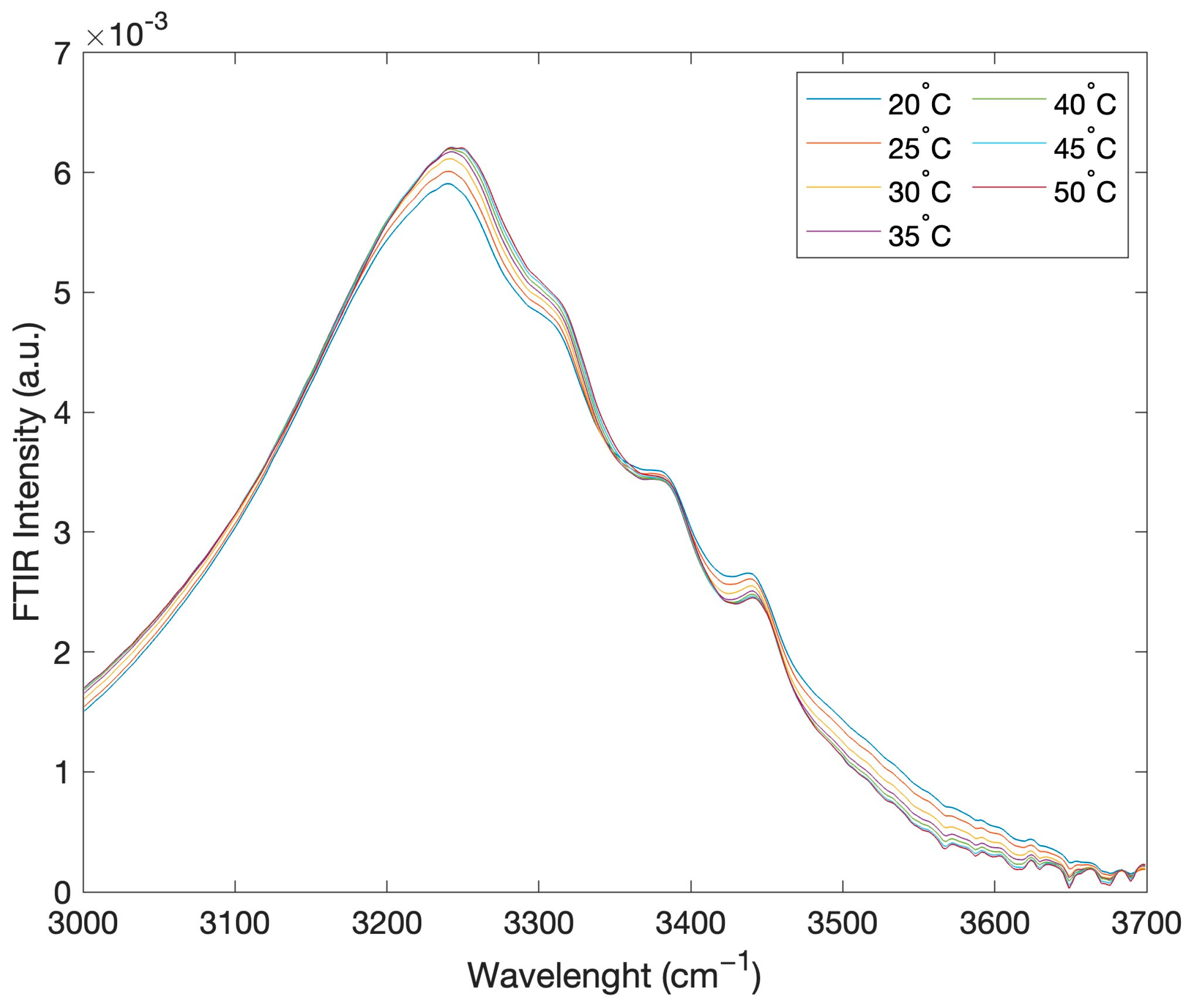
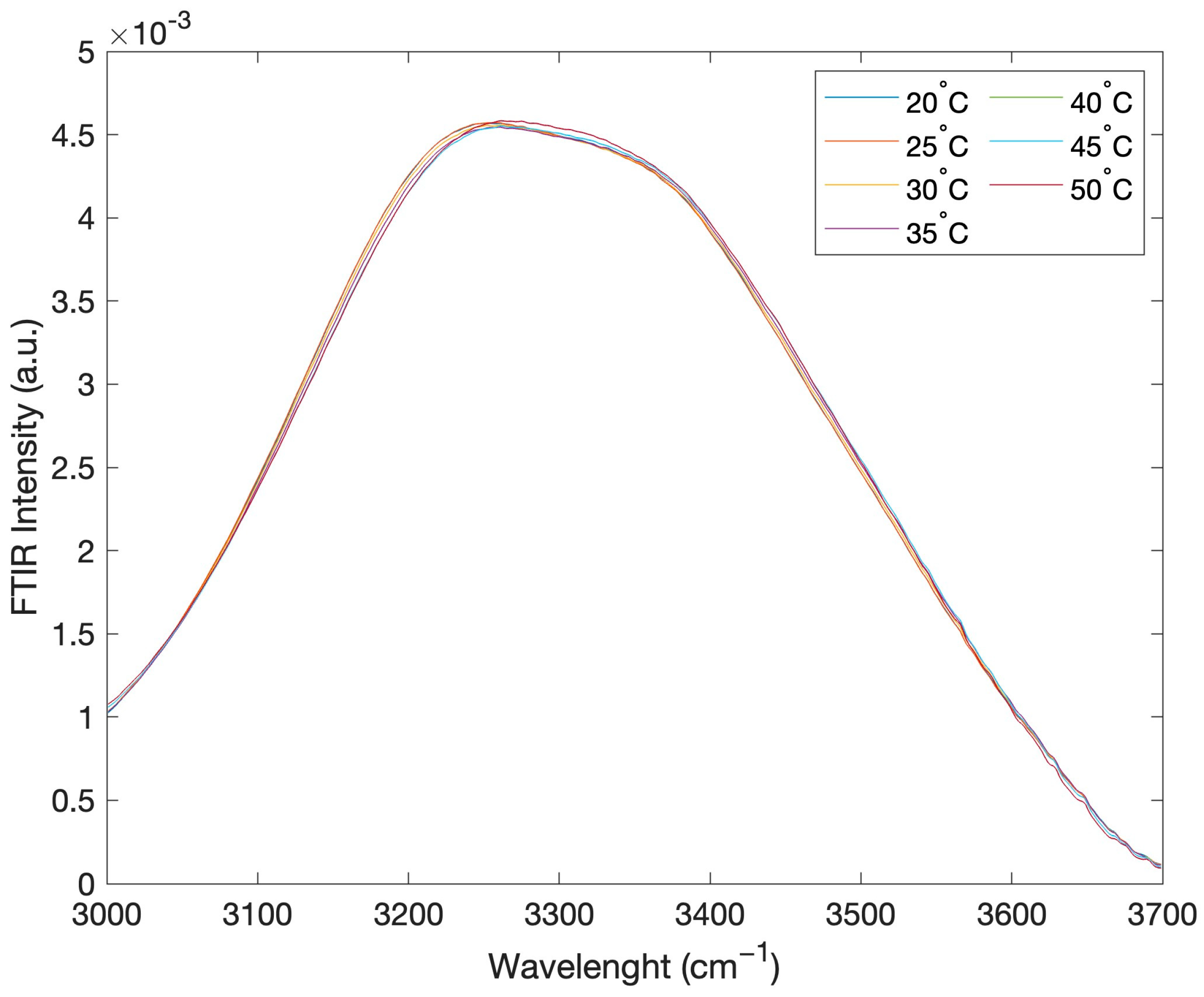
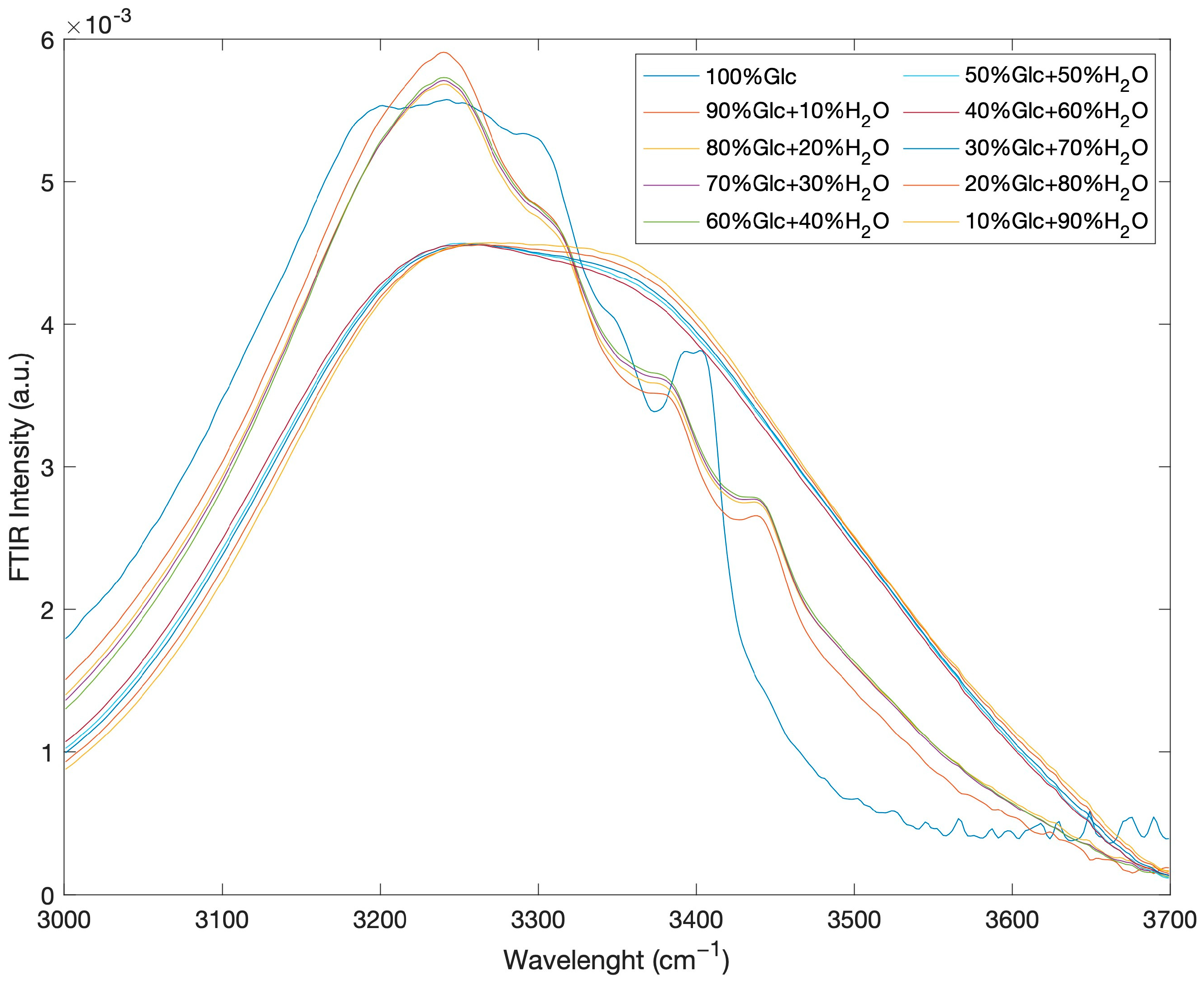
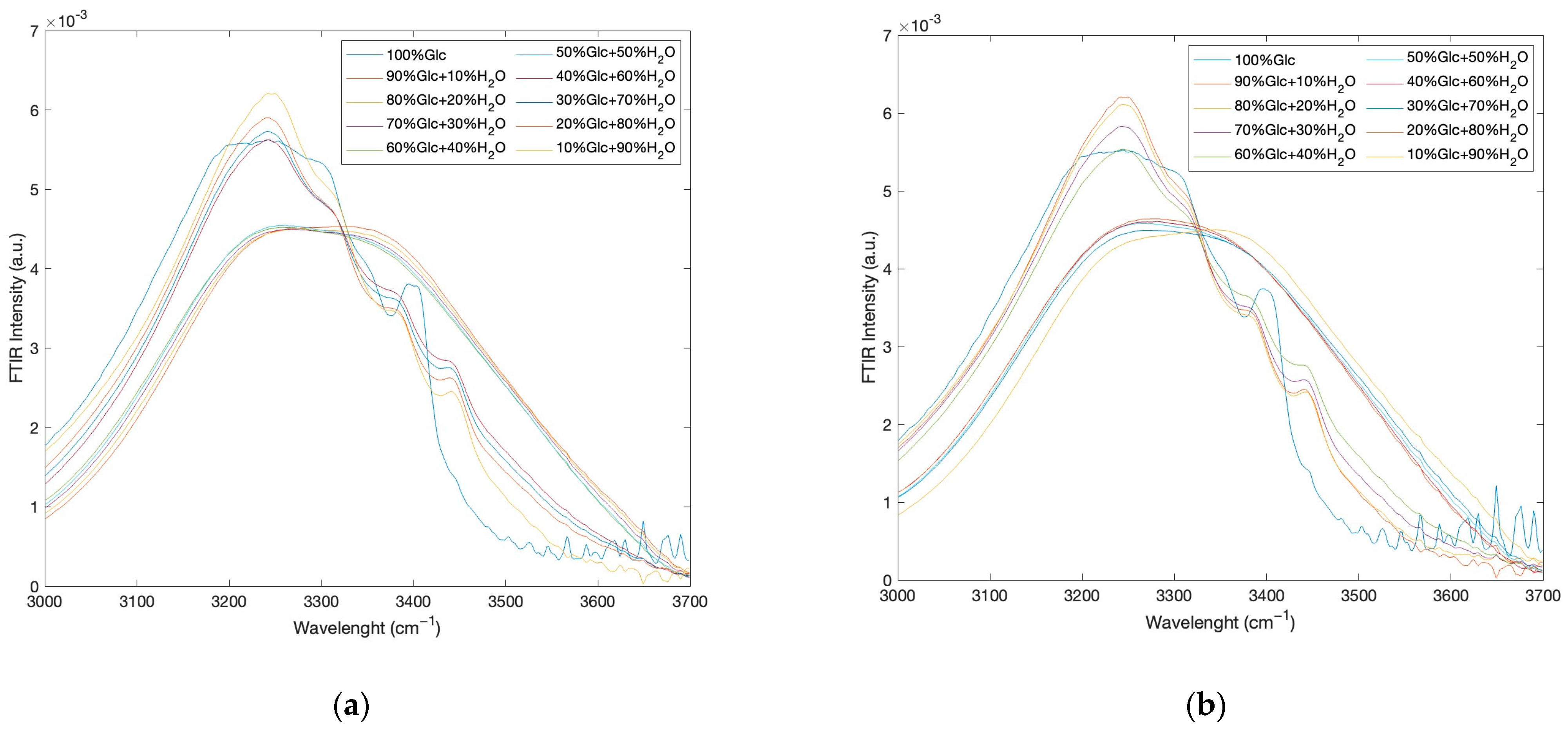
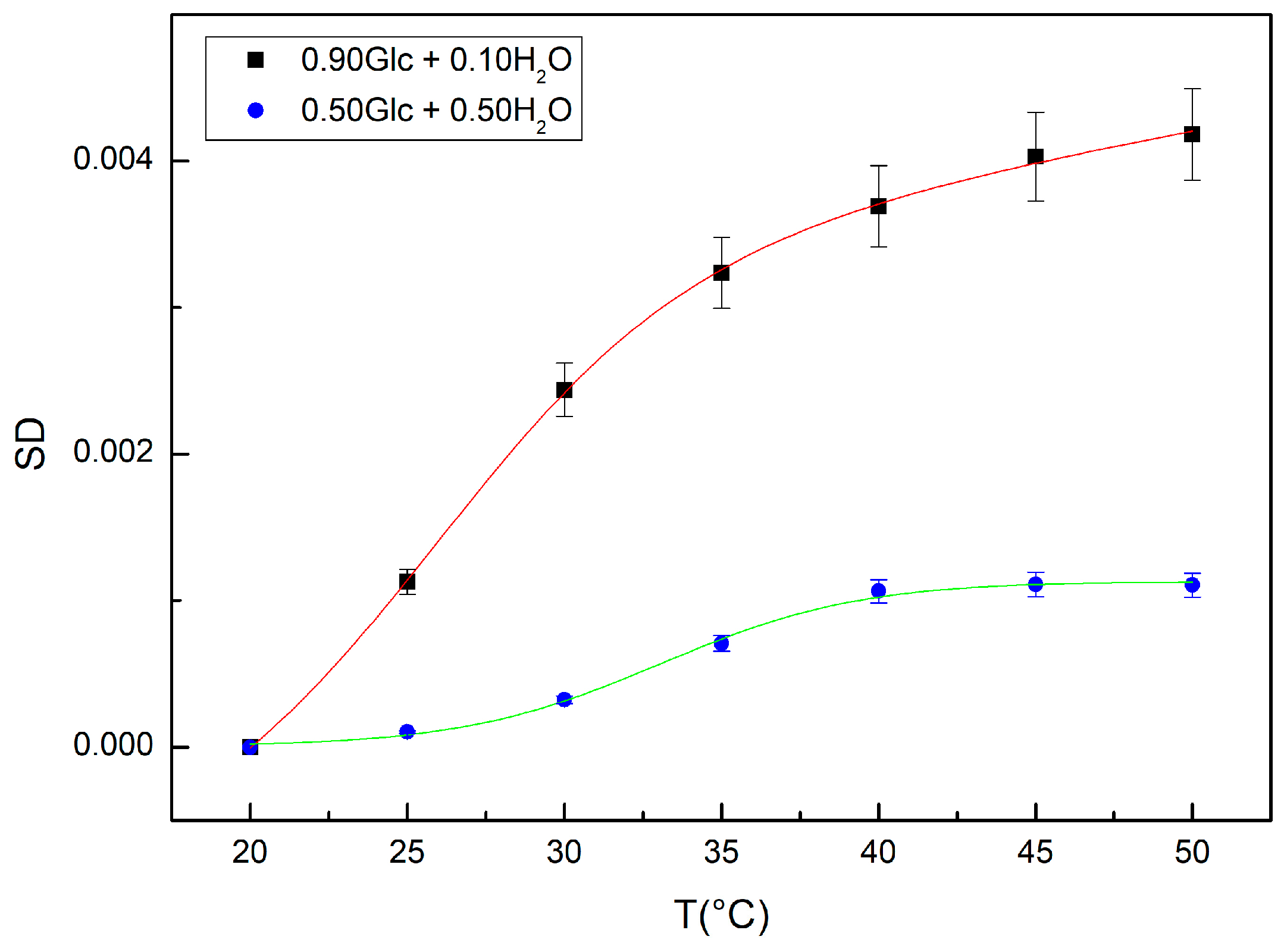
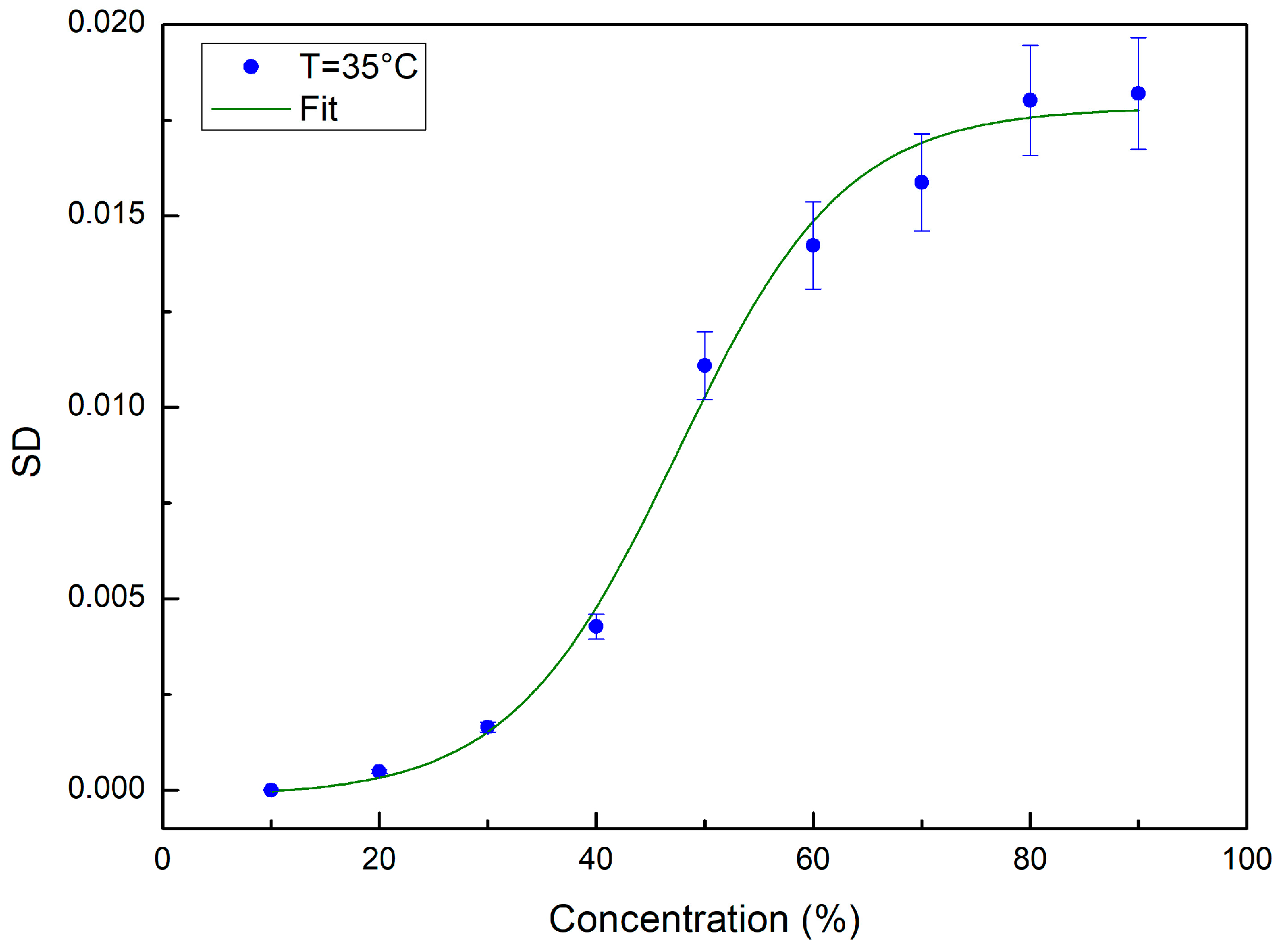
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kohlmeier, M. Glucose. In Food Science and Technology, Nutrient Metabolism; Kohlmeier, M., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 193–210. [Google Scholar]
- Tonova, K.; Lazarova, M.; Dencheva-Zarkova, M.; Paniovska, S.; Tsibranska, I.; Stanoev, V.; Dzhonova, D.; Genova, J. Separation of glucose, other reducing sugars and phenolics from natural extract by nanofiltration: Effect of pressure and cross-flow velocity. Chem. Eng. Res. Design 2020, 162, 107–116. [Google Scholar] [CrossRef]
- Mitchell, H.H.; Hamilton, T.S.; Steggerda, F.R.; Bean, H.W. The chemical composition of the adult human body and its bearing on the biochemistry of growth. J. Biol. Chem. 1945, 158, 625–637. [Google Scholar] [CrossRef]
- Andary, J.; Maalouly, J.; Ouaini, R.; Chebib, H.; Rutledge, D.N.; Ouaini, N. Stability study of furans, glucose and xylose under overliming conditions: Effect of sugar degradation products. Biores. Technol. Rep. 2021, 15, 100722. [Google Scholar] [CrossRef]
- Feng, P. The energy spectrum of vibration of molecules in water and its properties. J. Mol. Liq. 2014, 195, 182–187. [Google Scholar] [CrossRef]
- De Ninno, A.; Castellano, A.C. Co-ordination of water molecules in isotopic mixtures. J. Mol. Struc. 2011, 1006, 434–440. [Google Scholar] [CrossRef]
- Rondonuwu, F.; Setiawan, A.; Muninggar, J.; Karwur, F. Glucose-water interactions at increasing concentrations and temperatures as revealed by Near-Infrared Spectroscopy. IOP Conf. Ser. Mat. Sci. Eng. 2020, 959, 012003. [Google Scholar] [CrossRef]
- Jung, Y.; Hwang, J. Near-infrared studies of glucose and sucrose in aqueous solutions: Water displacement effect and red shift in water absorption from water-solute interaction. Appl. Spectrosc. 2013, 67, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Wensheng, C.; Xueguang, S. Glucose induced variation of water structure by temperature dependent near infrared spectra. RSC Adv. 2016, 6, 105729–105736. [Google Scholar] [CrossRef]
- Elliott, G.D.; Wang, S.; Fuller, B.J. Cryoprotectants: A review of the actions and applications of cryoprotective solutes that modulate cell recovery from ultra-low temperatures. Cryobiology 2017, 76, 74–91. [Google Scholar] [CrossRef]
- Ball, P. Water as an Active Constituent in Cell Biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Magazù, S.; Migliardo, P.; Musolino, A.M.; Sciortino, M.T. α, α-trehalose-water solutions. 1. Hydration phenomena and anomalies in the acoustic properties. J. Phys. Chem. B 1997, 101, 2348–2351. [Google Scholar] [CrossRef]
- Gallina, M.E.; Sassi, P.; Paolantoni, M.; Morresi, A.; Cataliotti, R.S. Vibrational Analysis of Molecular Interactions in Aqueous Glucose Solutions. Temperature and Concentration Effects. J. Phys. Chem. B 2006, 110, 8856–8864. [Google Scholar] [CrossRef]
- Song, Y.C. Hydrogen Bonding Analysis of Hydroxyl Groups in Glucose Aqueous Solutions by a Molecular Dynamics Simulation Study. Bull. Kor. Chem. Soc. 2012, 33, 2238–2246. [Google Scholar]
- Wang, M.; Cui, X.; Cai, W.; Shao, X. Temperature-dependent near-infrared spectroscopy for studying the interactions in protein aqueous solutions. NIR News 2019, 30, 15–17. [Google Scholar] [CrossRef]
- Song, C.; Fan, W.H.; Ding, L.; Chen, X.; Chen, Z.Y.; Wang, K. Terahertz and infrared characteristic absorption spectra of aqueous glucose and fructose solutions. Sci. Rep. 2018, 8, 8964. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Mizaikoff, B. Advances in Mid-Infrared Spectroscopy for Chemical Analysis. Ann. Rev. Anal. Chem. 2016, 12, 45–68. [Google Scholar] [CrossRef]
- Medhat, I.; Mousa, A.; Hanan, E.; Aned, L. Analysis of the Structure and Vibrational Spectra of Glucose and Fructose. Eclet. Quim. 2006, 31, 15–21. [Google Scholar]
- Bureau, S.; Ruiz, D.; Reich, M.; Gouble, B.; Bertrand, D.; Audergon, J.M.; Renard, C. Application of ATR-FTIR for a rapid and simultaneous determination of sugars and organic acids in apricot fruit. Food Chem. 2009, 115, 1133–1140. [Google Scholar] [CrossRef]
- King, J.M.; Eigenmann, C.A.; Colagiuri, S. Effect of ambient temperature and humidity on performance of blood glucose meters. Diabet Med. 1995, 12, 337–340. [Google Scholar] [CrossRef]
- Jensen, P.S.; Bak, J.; Andersson-Engels, S. Influence of Temperature on Water and Aqueous Glucose Absorption Spectra in the Near- and Mid-Infrared Regions at Physiologically Relevant Temperatures. Appl. Spectr. 2003, 57, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Deborah, H.; Richard, N.; Hardeberg, J.Y. A Comprehensive Evaluation of Spectral Distance Functions and Metrics for Hyperspectral Image Processing. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2015, 8, 3224–3234. [Google Scholar] [CrossRef]
- Lavine, B.; Almirall, J.; Muehlethaler, C.; Neumann, C.; Workman, J. Criteria for comparing infrared spectra—A review of the forensic and analytical chemistry literature. Foren. Chem. 2020, 18, 100224. [Google Scholar] [CrossRef]
- Shah, N.K.; Gemperline, P.J. Combination of the Mahalanobis distance and residual variance pattern recognition techniques for classification of near-infrared reflectance spectra. Analyt. Chem. 1990, 62, 465–470. [Google Scholar] [CrossRef]
- Magazù, S.; Migliardo, F.; Benedetto, A.; Calabrò, E.; La Torre, R.; Caccamo, M.T. Bioprotective effects of sucrose and trehalose on proteins. In Sucrose: Properties, Biosynthesis and Health Implications; Magazù, S., Ed.; Nova Science Publisher: New York, NY, USA, 2013; pp. 35–46. [Google Scholar]
- Caccamo, M.T.; Gugliandolo, C.; Zammuto, V.; Magazù, S. Thermal properties of an exopolysaccharide produced by a marine thermotolerant Bacillus licheniformis by ATR-FTIR spectroscopy. Int. J. Biol. Macromol. 2020, 145, 77–83. [Google Scholar] [CrossRef]
- Faraone, A.; Magazù, S.; Maisano, G.; Ponterio, R.C.; Villari, V. Experimental Evidence of Slow Dynamics in Semidilute Polymer Solutions. Macromolecules 1999, 32, 1128–1133. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Magazù, S. Thermal restraint on PEG-EG mixtures by FTIR investigations and wavelet cross-correlation analysis. Polym. Test. 2017, 62, 311–318. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Cannuli, A.; Calabrò, E.; Magazù, S. Acoustic Levitator Power Device: Study of Ethylene-Glycol Water Mixtures. IOP Conf. Ser. Mater. Sci. Eng. ACPEE 2017, 199, 012119. [Google Scholar] [CrossRef] [Green Version]
- Fenimore, P.; Frauenfelder, H.; Magazù, S.; McMahon, B.; Mezei, F.; Migliardo, F.; Young, R.; Stroe, I. Concepts and problems in protein dynamics. Chem. Phys. 2013, 424, 2–6. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Cannuli, A. PEG acoustic levitation treatment for historic wood preservation investigated by means of FTIR spectroscopy and wavelets. Curr. Chem Biol. 2019, 13, 60–72. [Google Scholar] [CrossRef]
- Magazù, S.; Calabrò, E.; Caccamo, M.T. Experimental study of thermal restraint in bio-protectant disaccharides by FTIR spectroscopy. Open Biotech. 2018, 12, 123–133. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Magazù, S. Multiscaling wavelet analysis of infrared and Raman data on polyethylene glycol 1000 aqueous solutions. Spectr. Lett. 2017, 50, 130–136. [Google Scholar] [CrossRef]
- Caccamo, M.T.; Mavilia, G.; Mavilia, L.; Lombardo, D.; Magazù, S. Self-assembly Processes in Hydrated Montmorillonite by FTIR Investigations. Materials 2020, 13, 1100. [Google Scholar] [CrossRef] [PubMed]
- Kovács, F.; Yan, H.; Li, H.; Kunsági-Máté, S. Temperature-Induced Change of Water Structure in Aqueous Solutions of Some Kosmotropic and Chaotropic Salts. Int. J. Mol. Sci. 2021, 22, 12896. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caccamo, M.T.; Magazù, S. Investigation of Glucose–Water Mixtures as a Function of Concentration and Temperature by Infrared Spectroscopy. Int. J. Mol. Sci. 2023, 24, 2564. https://doi.org/10.3390/ijms24032564
Caccamo MT, Magazù S. Investigation of Glucose–Water Mixtures as a Function of Concentration and Temperature by Infrared Spectroscopy. International Journal of Molecular Sciences. 2023; 24(3):2564. https://doi.org/10.3390/ijms24032564
Chicago/Turabian StyleCaccamo, Maria Teresa, and Salvatore Magazù. 2023. "Investigation of Glucose–Water Mixtures as a Function of Concentration and Temperature by Infrared Spectroscopy" International Journal of Molecular Sciences 24, no. 3: 2564. https://doi.org/10.3390/ijms24032564
APA StyleCaccamo, M. T., & Magazù, S. (2023). Investigation of Glucose–Water Mixtures as a Function of Concentration and Temperature by Infrared Spectroscopy. International Journal of Molecular Sciences, 24(3), 2564. https://doi.org/10.3390/ijms24032564






