Metabolomic Analyses to Identify Candidate Biomarkers of Cystinosis
Abstract
1. Introduction
2. Results
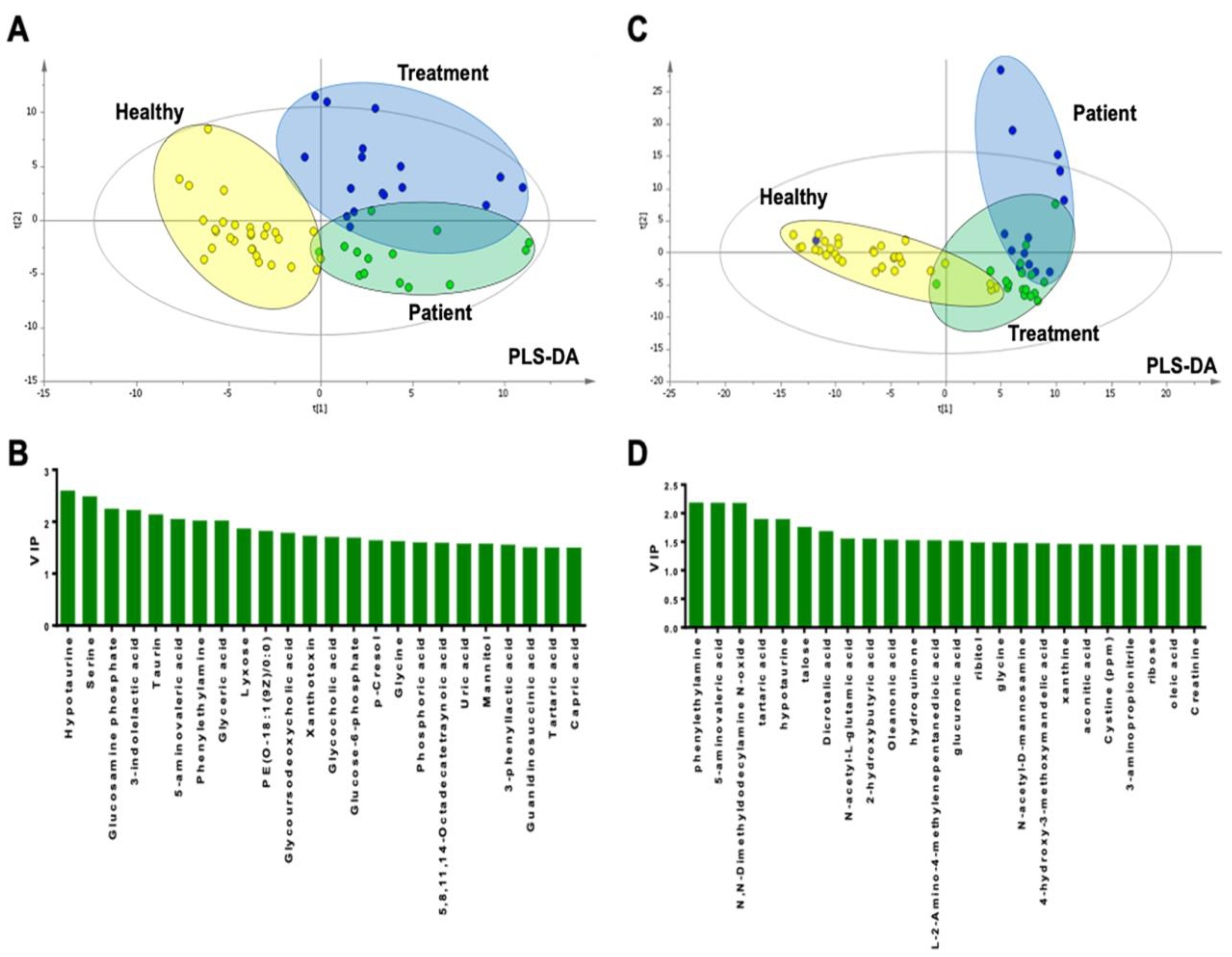
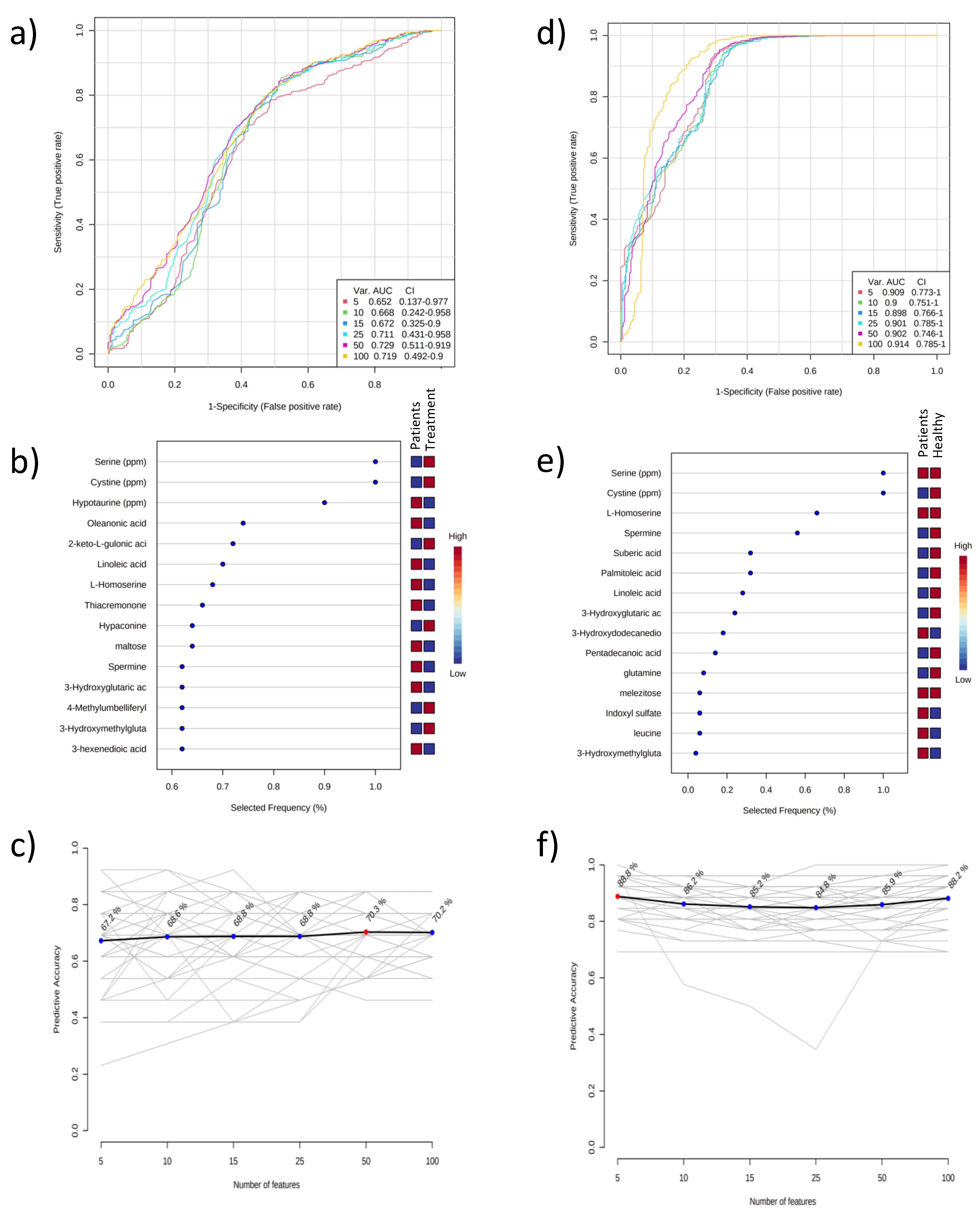
2.1. Metabolomic Results of Plasma Samples
2.2. Metabolomic Results of Urine Samples
2.3. Proteomic Results of Serum Samples
2.4. Pathway Analysis
3. Discussion
4. Materials and Methods
4.1. Definitions
4.2. Patients and Controls
4.3. Genomic Studies
4.4. Omics Analysis
4.5. Metabolomic Analysis
4.5.1. Sample Preparation
4.5.2. Targeted Pathway Analysis for Sulfur Metabolism
4.5.3. Proteomic Analysis
4.6. Data Processing
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emma, F.; Nesterova, G.; Langman, C.; Labbé, A.; Cherqui, S.; Goodyer, P.; Janssen, M.C.; Greco, M.; Topaloglu, R.; Elenberg, E.; et al. Nephropathic cystinosis: An international consensus document. Nephrol. Dial. Transpl. 2014, 29 (Suppl. 4), iv87–iv94. [Google Scholar] [CrossRef] [PubMed]
- Gahl, W.A.; Thoene, J.G.; Schneider, J.A. Cystinosis. N. Engl. J. Med. 2002, 347, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Town, M.; Jean, G.; Cherqui, S.; Attard, M.; Forestier, L.; Whitmore, S.A.; Callen, D.F.; Gribouval, O.; Broyer, M.; Bates, G.P.; et al. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat. Genet. 1998, 18, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gahl, W.A.; Kuehl, E.M.; Iwata, F.; Lindblad, A.; Kaiser-Kupfer, M.I. Corneal crystals in nephropathic cystinosis: Natural history and treatment with cysteamine eyedrops. Mol. Genet. Metab. 2000, 71, 100–120. [Google Scholar] [CrossRef]
- Nesterova, G.; Gahl, W. Nephropathic cystinosis: Late complications of a multisystemic disease. Pediatr. Nephrol. 2008, 23, 863–878. [Google Scholar] [CrossRef]
- Cherqui, S.; Courtoy, P.J. The renal Fanconi syndrome in cystinosis: Pathogenic insights and therapeutic perspectives. Nat. Rev. Nephrol. 2017, 13, 115–131. [Google Scholar] [CrossRef]
- Ivanova, E.A.; van den Heuvel, L.P.; Elmonem, M.A.; De Smedt, H.; Missiaen, L.; Pastore, A.; Mekahli, D.; Bultynck, G.; Levtchenko, E.N. Altered mTOR signalling in nephropathic cystinosis. J. Inherit. Metab. Dis. 2016, 39, 457–464. [Google Scholar] [CrossRef]
- Napolitano, G.; Johnson, J.L.; He, J.; Rocca, C.J.; Monfregola, J.; Pestonjamasp, K.; Cherqui, S.; Catz, S.D. Impairment of chaperone-mediated autophagy leads to selective lysosomal degradation defects in the lysosomal storage disease cystinosis. EMBO Mol. Med. 2015, 7, 158–174. [Google Scholar] [CrossRef]
- Prencipe, G.; Caiello, I.; Cherqui, S.; Whisenant, T.; Petrini, S.; Emma, F.; De Benedetti, F. Inflammasome activation by cystine crystals: Implications for the pathogenesis of cystinosis. J. Am. Soc. Nephrol. 2014, 25, 1163–1169. [Google Scholar] [CrossRef]
- Raggi, C.; Luciani, A.; Nevo, N.; Antignac, C.; Terryn, S.; Devuyst, O. Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis. Hum. Mol. Genet. 2014, 23, 2266–2278. [Google Scholar] [CrossRef]
- Rega, L.R.; Polishchuk, E.; Montefusco, S.; Napolitano, G.; Tozzi, G.; Zhang, J.; Bellomo, F.; Taranta, A.; Pastore, A.; Polishchuk, R.; et al. Activation of the transcription factor EB rescues lysosomal abnormalities in cystinotic kidney cells. Kidney Int. 2016, 89, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Sansanwal, P.; Yen, B.; Gahl, W.A.; Ma, Y.; Ying, L.; Wong, L.J.; Sarwal, M.M. Mitochondrial autophagy promotes cellular injury in nephropathic cystinosis. J. Am. Soc. Nephrol. 2010, 21, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Chabli, A.; Aupetit, J.; Raehm, M.; Ricquier, D.; Chadefaux-Vekemans, B. Measurement of cystine in granulocytes using liquid chromatography-tandem mass spectrometry. Clin. Biochem. 2007, 40, 692–698. [Google Scholar] [CrossRef]
- Elmonem, M.A.; Makar, S.H.; van den Heuvel, L.; Abdelaziz, H.; Abdelrahman, S.M.; Bossuyt, X.; Janssen, M.C.; Cornelissen, E.A.; Lefeber, D.J.; Joosten, L.A.; et al. Clinical utility of chitotriosidase enzyme activity in nephropathic cystinosis. Orphanet J. Rare Dis. 2014, 9, 155. [Google Scholar] [CrossRef]
- Nemutlu, E.; Zhang, S.; Gupta, A.; Juranic, N.O.; Macura, S.I.; Terzic, A.; Jahangir, A.; Dzeja, P. Dynamic phosphometabolomic profiling of human tissues and transgenic models by 18O-assisted 31P NMR and mass spectrometry. Physiol. Genom. 2012, 44, 386–402. [Google Scholar] [CrossRef]
- Bellomo, F.; De Leo, E.; Taranta, A.; Giaquinto, L.; Di Giovamberardino, G.; Montefusco, S.; Rega, L.R.; Pastore, A.; Medina, D.L.; Di Bernardo, D.; et al. Drug Repurposing in Rare Diseases: An Integrative Study of Drug Screening and Transcriptomic Analysis in Nephropathic Cystinosis. Int. J. Mol. Sci. 2021, 22, 12829. [Google Scholar] [CrossRef] [PubMed]
- Aldahmesh, M.A.; Humeidan, A.; Almojalli, H.A.; Khan, A.O.; Rajab, M.; AA, A.L.-A.; Meyer, B.F.; Alkuraya, F.S. Characterization of CTNS mutations in Arab patients with cystinosis. Ophthalmic Genet. 2009, 30, 185–189. [Google Scholar] [CrossRef]
- Topaloglu, R.; Gulhan, B.; İnözü, M.; Canpolat, N.; Yilmaz, A.; Noyan, A.; Dursun, İ.; Gökçe, İ.; Gürgöze, M.K.; Akinci, N.; et al. The Clinical and Mutational Spectrum of Turkish Patients with Cystinosis. Clin. J. Am. Soc. Nephrol. 2017, 12, 1634–1641. [Google Scholar] [CrossRef]
- Topaloglu, R.; Vilboux, T.; Coskun, T.; Ozaltin, F.; Tinloy, B.; Gunay-Aygun, M.; Bakkaloglu, A.; Besbas, N.; van den Heuvel, L.; Kleta, R.; et al. Genetic basis of cystinosis in Turkish patients: A single-center experience. Pediatr. Nephrol. 2012, 27, 115–121. [Google Scholar] [CrossRef]
- Topaloglu, R.; Baskın, E.; Bahat, E.; Kavukcu, S.; Cakar, N.; Donmez, O.; Guven, A.G.; Calıskan, S.; Erdogan, O.; Yalcınkaya, F. Hereditary renal tubular disorders in Turkey: Demographic, clinical, and laboratory features. Clin. Exp. Nephrol. 2011, 15, 108–113. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Zeki, Ö.C.; Eylem, C.C.; Reçber, T.; Kır, S.; Nemutlu, E. Integration of GC-MS and LC-MS for untargeted metabolomics profiling. J. Pharm. Biomed. Anal. 2020, 190, 113509. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, M. Amino acids and nitrogen compounds. In Nutrient Metabolism; Academic Press: Cambridge, MA, USA, 2015; pp. 265–477. [Google Scholar]
- De Koning, T.J.; Snell, K.; Duran, M.; Berger, R.; Poll-The, B.T.; Surtees, R. L-serine in disease and development. Biochem. J. 2003, 371 Pt 3, 653–661. [Google Scholar] [CrossRef]
- Sumayao, R., Jr.; Newsholme, P.; McMorrow, T. The Role of Cystinosin in the Intermediary Thiol Metabolism and Redox Homeostasis in Kidney Proximal Tubular Cells. Antioxid 2018, 7, 179. [Google Scholar] [CrossRef] [PubMed]
- Kalatzis, V.; Cherqui, S.; Antignac, C.; Gasnier, B. Cystinosin, the protein defective in cystinosis, is a H+-driven lysosomal cystine transporter. Embo J. 2001, 20, 5940–5949. [Google Scholar] [CrossRef]
- Gavazza, M.B.; Català, A. Protective effect of N-acetyl-serotonin on the nonenzymatic lipid peroxidation in rat testicular microsomes and mitochondria. J. Pineal Res. 2004, 37, 153–160. [Google Scholar] [CrossRef]
- Oxenkrug, G. Antioxidant effects of N-acetylserotonin: Possible mechanisms and clinical implications. Ann. N. Y. Acad. Sci. 2005, 1053, 334–347. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, Y.; Xi, L.; Li, G.; Zhao, F.; Qi, Y.; Liu, J.; Zhao, D. A Nested Case-Control Study of Association between Metabolome and Hypertension Risk. Biomed Res. Int. 2016, 2016, 7646979. [Google Scholar] [CrossRef]
- Marques, F.Z.; Mackay, C.R.; Kaye, D.M. Beyond gut feelings: How the gut microbiota regulates blood pressure. Nat. Rev. Cardiol. 2018, 15, 20–32. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.N.; Liu, G.; Bai, M.M.; Peng, C.; Li, T.J.; Yin, Y.L. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell. Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef]
- Brown, D.G.; Rao, S.; Weir, T.L.; O’Malia, J.; Bazan, M.; Brown, R.J.; Ryan, E.P. Metabolomics and metabolic pathway networks from human colorectal cancers, adjacent mucosa, and stool. Cancer Metab. 2016, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Hamer, H.M.; De Preter, V.; Windey, K.; Verbeke, K. Functional analysis of colonic bacterial metabolism: Relevant to health? Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1–G9. [Google Scholar] [CrossRef] [PubMed]
- Baumner, S.; Weber, L.T. Nephropathic Cystinosis: Symptoms, Treatment, and Perspectives of a Systemic Disease. Front. Pediatr. 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Ferrannini, E.; Natali, A.; Camastra, S.; Nannipieri, M.; Mari, A.; Adam, K.P.; Milburn, M.V.; Kastenmüller, G.; Adamski, J.; Tuomi, T.; et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes 2013, 62, 1730–1737. [Google Scholar] [CrossRef] [PubMed]
- Gall, W.E.; Beebe, K.; Lawton, K.A.; Adam, K.P.; Mitchell, M.W.; Nakhle, P.J.; Ryals, J.A.; Milburn, M.V.; Nannipieri, M.; Camastra, S.; et al. alpha-hydroxybutyrate is an early biomarker of insulin resistance and glucose intolerance in a nondiabetic population. PLoS ONE 2010, 5, e10883. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Robey, R.W.; Massey, P.R.; Amiri-Kordestani, L.; Bates, S.E. ABC transporters: Unvalidated therapeutic targets in cancer and the CNS. Anticancer Agents Med. Chem. 2010, 10, 625–633. [Google Scholar] [CrossRef]
- Blachier, F.; Boutry, C.; Bos, C.; Tomé, D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 2009, 90, 814s–821s. [Google Scholar] [CrossRef]
- Walker, V. Ammonia metabolism and hyperammonemic disorders. Adv. Clin. Chem. 2014, 67, 73–150. [Google Scholar]
- Huls, M.; Kramers, C.; Levtchenko, E.N.; Wilmer, M.J.; Dijkman, H.B.; Kluijtmans, L.A.; van der Hoorn, J.W.; Russel, F.G.; Masereeuw, R. P-glycoprotein-deficient mice have proximal tubule dysfunction but are protected against ischemic renal injury. Kidney Int. 2007, 72, 1233–1241. [Google Scholar] [CrossRef]
- Nemutlu, E.; Orgul, G.; Recber, T.; Aydin, E.; Ozkan, E.; Turgal, M.; Alikasifoglu, M.; Kir, S.; Beksac, M.S. Metabolic Infrastructure of Pregnant Women With Trisomy 21 Fetuses; Metabolomic Analysis. Z. Geburtshilfe Neonatol. 2019, 223, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Eylem, C.C.; Yilmaz, M.; Derkus, B.; Nemutlu, E.; Camci, C.B.; Yilmaz, E.; Turkoglu, M.A.; Aytac, B.; Ozyurt, N.; Emregul, E. Untargeted multi-omic analysis of colorectal cancer-specific exosomes reveals joint pathways of colorectal cancer in both clinical samples and cell culture. Cancer Lett. 2020, 469, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Nemutlu, E.; Zhang, S.; Xu, Y.Z.; Terzic, A.; Zhong, L.; Dzeja, P.D.; Cha, Y.M. Cardiac resynchronization therapy induces adaptive metabolic transitions in the metabolomic profile of heart failure. J. Card. Fail. 2015, 21, 460–469. [Google Scholar] [CrossRef] [PubMed]


| (a) Patient Group | |||
| No | Gender | Mutation | Age at Sampling (Years) |
| 1 | Male | c.681G>A (p.Glu227Glu) (Hom) | 1.16 |
| 2 | Male | c.681G>A (p.Glu227Glu) (Hom) | 6.97 |
| 3 | Male | c.681G>A (p.Glu227Glu) (Hom) | 1.54 |
| 4 | Male | c.18_21 del (p.Thr7Phefs*7) (Hom) | 0.91 |
| 5 | Male | c.1015G>A (p.Gly339Arg) (Hom) | 1.68 |
| 6 | Male | c.18_21 del (p.Thr7Phefs*7) (Hom) | 0.76 |
| 7 | Female | c.681G>A (p.Glu227Glu) (Hom) | 10 |
| 8 | Female | c. 691C>T (Gln231*) (Hom) | 0.74 |
| 9 | Female | c.291_294del (p. Thr98Phefs*19) (Hom) | 0.6 |
| 10 | Female | c.681G>A (p.Glu227Glu) (Het)/c.1054 C>T(p.Gln352*) (Het) | 2.11 |
| 11 | Female | c.323delA (p.Gln108Argfs*10) (Hom) | 2.23 |
| 12 | Male | c.18_21 del (p.Thr7Phefs*7) (Hom) | 0.6 |
| 13 | Female | c.141–22 A>G (Hom) | 0.8 |
| 14 | Male | c.681G>A (p.Glu227Glu) (Hom) | 0.8 |
| (b) Treatment Group | |||
| No. | Gender | Mutation | Age at Sampling (Years) |
| 1 | Male | c.681G>A (p.Glu227Glu) (Hom) | 3.16 |
| 2 | Male | c.681G>A (p.Glu227Glu) (Hom) | 8.46 |
| 3 | Male | c.681G>A (p.Glu227Glu) (Hom) | 2.95 |
| 4 | Male | c.18_21 del (p.Thr7Phefs*7) (Hom) | 2.16 |
| 5 | Male | c.1015G>A (p.Gly339Arg) (Hom) | 2.93 |
| 6 | Male | c.18_21 del (p.Thr7Phefs*7) (Hom) | 1.70 |
| 7 | Female | c.681G>A (p.Glu227Glu) (Hom) | 10.63 |
| 8 | Female | c.681G>A (p.Glu227Glu) (Het)/c.1054 C>T(p.Gln352*) (Het) | 2.27 |
| 9 | Male | c.18_21 del (p.Thr7Phefs*7) (Hom) | 0.58 |
| 10 | Female | c.291_294del (p. Thr98Phefs*19) (Hom) | 1.08 |
| 11 | Female | c.681G>A (p.Glu227Glu) (Hom) | 10.26 |
| 12 | Female | c.681G>A (p.Glu227Glu) (Hom) | 1.84 |
| 13 | Male | c.681G>A (p.Glu227Glu) (Hom) | 17.3 |
| 14 | Male | c.681G>A (p.Glu227Glu) (Hom) | 8.80 |
| 15 | Male | c.1015G>A (p.Gly339Arg) (Hom) | 13.94 |
| 16 | Male | c.1015G>A (p.Gly339Arg) (Hom) | 6.48 |
| 17 | Male | c.18_21 del (p.Thr7Phefs*7) (Hom) | 3.50 |
| 18 | Male | c.323delA (p.Gln108Argfs*10) (Hom) | 7.97 |
| 19 | Male | c.141–22A>G (het)/c.681G>A (p.Glu227Glu) (Het) | 10.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemutlu, E.; Ozaltin, F.; Yabanoglu-Ciftci, S.; Gulhan, B.; Eylem, C.C.; Baysal, İ.; Gök-Topak, E.D.; Ulubayram, K.; Sezerman, O.U.; Ucar, G.; et al. Metabolomic Analyses to Identify Candidate Biomarkers of Cystinosis. Int. J. Mol. Sci. 2023, 24, 2603. https://doi.org/10.3390/ijms24032603
Nemutlu E, Ozaltin F, Yabanoglu-Ciftci S, Gulhan B, Eylem CC, Baysal İ, Gök-Topak ED, Ulubayram K, Sezerman OU, Ucar G, et al. Metabolomic Analyses to Identify Candidate Biomarkers of Cystinosis. International Journal of Molecular Sciences. 2023; 24(3):2603. https://doi.org/10.3390/ijms24032603
Chicago/Turabian StyleNemutlu, Emirhan, Fatih Ozaltin, Samiye Yabanoglu-Ciftci, Bora Gulhan, Cemil Can Eylem, İpek Baysal, Elif Damla Gök-Topak, Kezban Ulubayram, Osman Ugur Sezerman, Gulberk Ucar, and et al. 2023. "Metabolomic Analyses to Identify Candidate Biomarkers of Cystinosis" International Journal of Molecular Sciences 24, no. 3: 2603. https://doi.org/10.3390/ijms24032603
APA StyleNemutlu, E., Ozaltin, F., Yabanoglu-Ciftci, S., Gulhan, B., Eylem, C. C., Baysal, İ., Gök-Topak, E. D., Ulubayram, K., Sezerman, O. U., Ucar, G., Kır, S., & Topaloglu, R. (2023). Metabolomic Analyses to Identify Candidate Biomarkers of Cystinosis. International Journal of Molecular Sciences, 24(3), 2603. https://doi.org/10.3390/ijms24032603






