Association of High LAT1 Expression with Poor Prognosis and Recurrence in Colorectal Cancer Patients Treated with Oxaliplatin-Based Adjuvant Chemotherapy
Abstract
1. Introduction
2. Results
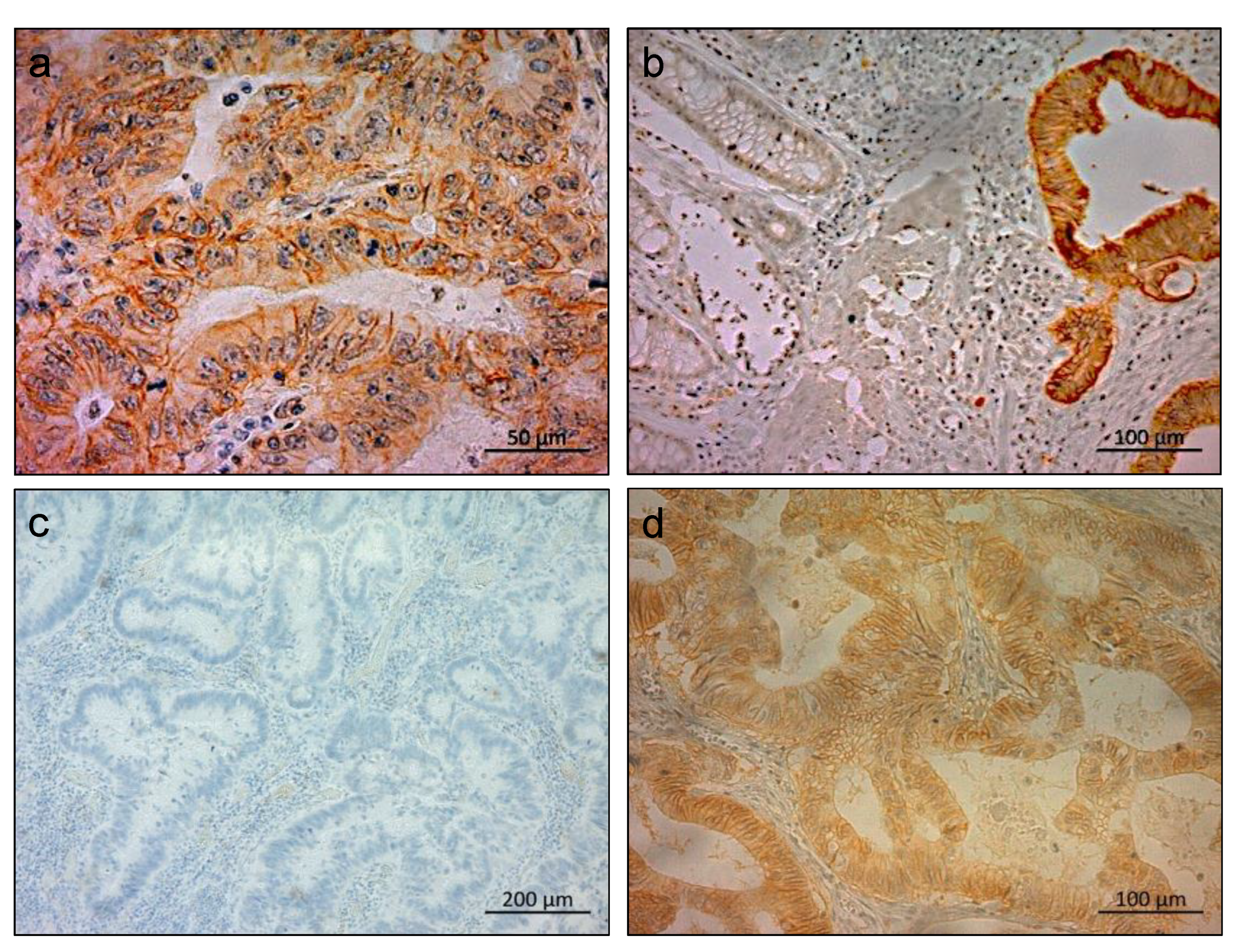
2.1. Immunohistochemical Staining of LAT1 in Clinical CRC Specimens
2.2. Clinicopathological Significance of LAT1 Expression in Patients with CRC
2.3. Prognostic Significance of LAT1 Expression in Patients with CRC
2.4. Functional Analysis of LAT1 in CRC Cell Lines
2.5. Association between mTOR and Oxaliplatin in LAT1-Suppressed CRC Cells
3. Discussion
4. Materials and Methods
4.1. Clinical Samples and Cell Lines
4.2. Immunohistochemical Analysis
4.3. siRNA Transfection
4.4. Protein Extraction and Western Blot Analysis
4.5. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4.6. Cell Proliferation Assay
4.7. Oxaliplatin Sensitivity Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baidoun, F.; Elshiwy, K.; Elkeraie, Y.; Merjaneh, Z.; Khoudari, G.; Sarmini, M.T.; Gad, M.; Al-Husseini, M.; Saad, A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets 2021, 22, 998–1009. [Google Scholar]
- Hori, M.; Matsuda, T.; Shibata, A.; Katanoda, K.; Sobue, T.; Nishimoto, H. Cancer incidence and incidence rates in Japan in 2009: A study of 32 population-based cancer registries for the Monitoring of Cancer Incidence in Japan (MCIJ) project. Jpn. J. Clin. Oncol. 2015, 45, 884–891. [Google Scholar] [CrossRef]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Goodman, P.J.; Ungerleider, J.S.; Emerson, W.A.; Tormey, D.C.; Glick, J.H.; et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 1990, 322, 352–358. [Google Scholar] [CrossRef]
- Andre, T.; Boni, C.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Bonetti, A.; Clingan, P.; Bridgewater, J.; Rivera, F.; et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J. Clin. Oncol. 2009, 27, 3109–3116. [Google Scholar] [CrossRef]
- Andre, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Laurie, J.A.; Moertel, C.G.; Fleming, T.R.; Wieand, H.S.; Leigh, J.E.; Rubin, J.; McCormack, G.W.; Gerstner, J.B.; Krook, J.E.; Malliard, J. Surgical adjuvant therapy of large-bowel carcinoma: An evaluation of levamisole and the combination of levamisole and fluorouracil. The North Central Cancer Treatment Group and the Mayo Clinic. J. Clin. Oncol. 1989, 7, 1447–1456. [Google Scholar] [CrossRef]
- Ueno, H.; Ishiguro, M.; Nakatani, E.; Ishikawa, T.; Uetake, H.; Matsuda, C.; Nakamoto, Y.; Kotake, M.; Kurachi, K.; Egawa, T.; et al. Prospective Multicenter Study on the Prognostic and Predictive Impact of Tumor Budding in Stage II Colon Cancer: Results From the SACURA Trial. J. Clin. Oncol. 2019, 37, 1886–1894. [Google Scholar] [CrossRef]
- Quasar Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet 2007, 370, 2020–2029. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Murugan, A.K. mTOR: Role in cancer, metastasis and drug resistance. Semin. Cancer Biol. 2019, 59, 92–111. [Google Scholar] [CrossRef]
- Mossmann, D.; Park, S.; Hall, M.N. mTOR signalling and cellular metabolism are mutual determinants in cancer. Nat. Rev. Cancer 2018, 18, 744–757. [Google Scholar] [CrossRef]
- Afzal, O.; Altamimi, A.S.; Mubeen, B.; Alzarea, S.I.; Almalki, W.H.; Al-Qahtani, S.D.; Atiya, E.M.; Al-Abbasi, F.A.; Ali, F.; Ullah, I.; et al. mTOR as a Potential Target for the Treatment of Microbial Infections, Inflammatory Bowel Diseases, and Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 12470. [Google Scholar] [CrossRef]
- Indini, A.; Fiorilla, I.; Ponzone, L.; Calautti, E.; Audrito, V. NAD/NAMPT and mTOR Pathways in Melanoma: Drivers of Drug Resistance and Prospective Therapeutic Targets. Int. J. Mol. Sci. 2022, 23, 9985. [Google Scholar] [CrossRef]
- Kanai, Y. Amino acid transporter LAT1 (SLC7A5) as a molecular target for cancer diagnosis and therapeutics. Pharmacol. Ther. 2022, 230, 107964. [Google Scholar] [CrossRef]
- Lu, M.; Zessin, A.S.; Glover, W.; Hsu, D.S. Activation of the mTOR Pathway by Oxaliplatin in the Treatment of Colorectal Cancer Liver Metastasis. PLoS ONE 2017, 12, e0169439. [Google Scholar] [CrossRef]
- Ohshima, Y.; Kaira, K.; Yamaguchi, A.; Oriuchi, N.; Tominaga, H.; Nagamori, S.; Kanai, Y.; Yokobori, T.; Miyazaki, T.; Asao, T.; et al. Efficacy of system l amino acid transporter 1 inhibition as a therapeutic target in esophageal squamous cell carcinoma. Cancer Sci. 2016, 107, 1499–1505. [Google Scholar] [CrossRef]
- Suzuki, S.; Kaira, K.; Ohshima, Y.; Ishioka, N.S.; Sohda, M.; Yokobori, T.; Miyazaki, T.; Oriuchi, N.; Tominaga, H.; Kanai, Y.; et al. Biological significance of fluorine-18-alpha-methyltyrosine (FAMT) uptake on PET in patients with oesophageal cancer. Br. J. Cancer 2014, 110, 1985–1991. [Google Scholar] [CrossRef]
- Yazawa, T.; Shimizu, K.; Kaira, K.; Nagashima, T.; Ohtaki, Y.; Atsumi, J.; Obayashi, K.; Nagamori, S.; Kanai, Y.; Oyama, T.; et al. Clinical significance of coexpression of L-type amino acid transporter 1 (LAT1) and ASC amino acid transporter 2 (ASCT2) in lung adenocarcinoma. Am. J. Transl. Res. 2015, 7, 1126–1139. [Google Scholar]
- Honjo, H.; Kaira, K.; Miyazaki, T.; Yokobori, T.; Kanai, Y.; Nagamori, S.; Oyama, T.; Asao, T.; Kuwano, H. Clinicopathological significance of LAT1 and ASCT2 in patients with surgically resected esophageal squamous cell carcinoma. J. Surg. Oncol. 2016, 113, 381–389. [Google Scholar] [CrossRef]
- Kurozumi, S.; Kaira, K.; Matsumoto, H.; Kurosumi, M.; Yokobori, T.; Kanai, Y.; Sekine, C.; Honda, C.; Katayama, A.; Furuya, M.; et al. Association of L-type amino acid transporter 1 (LAT1) with the immune system and prognosis in invasive breast cancer. Sci. Rep. 2022, 12, 2742. [Google Scholar] [CrossRef]
- Shimizu, A.; Kaira, K.; Okubo, Y.; Utsumi, D.; Yasuda, M.; Tominaga, H.; Oriuchi, N.; Kanai, Y.; Takahashi, K.; Ishikawa, O. Prognostic impact of LAT1 and CD98 expression in cutaneous angiosarcoma. Neoplasma 2017, 64, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Altan, B.; Kaira, K.; Watanabe, A.; Kubo, N.; Bao, P.; Dolgormaa, G.; Bilguun, E.O.; Araki, K.; Kanai, Y.; Yokobori, T.; et al. Relationship between LAT1 expression and resistance to chemotherapy in pancreatic ductal adenocarcinoma. Cancer Chemother. Pharmacol. 2018, 81, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Kaira, K.; Motegi, Y.; Yokobori, T.; Takada, T.; Katoh, R.; Osone, K.; Takahashi, R.; Katayama, C.; Oyama, T.; et al. Role of Amino Acid Transporter Expression as a Prognostic Marker in Patients with Surgically Resected Colorectal Cancer. Anticancer Res. 2019, 39, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Yamaga, T.; Suehiro, J.; Wada, Y.; Sakurai, H. Induction of CTH expression in response to amino acid starvation confers resistance to anti-LAT1 therapy in MDA-MB-231 cells. Sci. Rep. 2022, 12, 1021. [Google Scholar] [CrossRef]
- Bergers, G.; Fendt, S.M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 2021, 21, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, N.; Sohda, M.; Ide, M.; Shimoda, Y.; Ubukata, Y.; Kuriyama, K.; Hara, K.; Sano, A.; Sakai, M.; Yokobori, T.; et al. High L-Type Amino Acid Transporter 1 Levels Are Associated with Chemotherapeutic Resistance in Gastric Cancer Patients. Oncology 2021, 99, 732–739. [Google Scholar] [CrossRef]
- Jewell, J.L.; Russell, R.C.; Guan, K.L. Amino acid signalling upstream of mTOR. Nat. Rev. Mol. Cell Biol. 2013, 14, 133–139. [Google Scholar] [CrossRef]
- Broer, S.; Broer, A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef]
- Lou, G.; Chen, L.; Xia, C.; Wang, W.; Qi, J.; Li, A.; Zhao, L.; Chen, Z.; Zheng, M.; Liu, Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J. Exp. Clin. Cancer Res. 2020, 39, 4. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Z.; Jiang, G.; Sun, H.; Yu, D. Nobiletin sensitizes colorectal cancer cells to oxaliplatin by PI3K/Akt/MTOR pathway. Front. Biosci. (Landmark Ed.) 2019, 24, 303–312. [Google Scholar]
- Yoshida, G.J. The Harmonious Interplay of Amino Acid and Monocarboxylate Transporters Induces the Robustness of Cancer Cells. Metabolites 2021, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Kahya, U.; Koseer, A.S.; Dubrovska, A. Amino Acid Transporters on the Guard of Cell Genome and Epigenome. Cancers 2021, 13, 125. [Google Scholar] [PubMed]
- Hayashi, K.; Jutabha, P.; Endou, H.; Sagara, H.; Anzai, N. LAT1 is a critical transporter of essential amino acids for immune reactions in activated human T cells. J. Immunol. 2013, 191, 4080–4085. [Google Scholar] [CrossRef] [PubMed]
- Okanishi, H.; Ohgaki, R.; Okuda, S.; Endou, H.; Kanai, Y. Proteomics and phosphoproteomics reveal key regulators associated with cytostatic effect of amino acid transporter LAT1 inhibitor. Cancer Sci. 2021, 112, 871–883. [Google Scholar] [CrossRef]
- Liu, Y.H.; Li, Y.L.; Shen, H.T.; Chien, P.J.; Sheu, G.T.; Wang, B.Y.; Chang, W.W. L-Type Amino Acid Transporter 1 Regulates Cancer Stemness and the Expression of Programmed Cell Death 1 Ligand 1 in Lung Cancer Cells. Int. J. Mol. Sci. 2021, 22, 10955. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Kato, K.; Cho, B.C.; Takahashi, M.; Okada, M.; Lin, C.Y.; Chin, K.; Kadowaki, S.; Ahn, M.J.; Hamamoto, Y.; Doki, Y.; et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 1506–1517. [Google Scholar] [CrossRef]



| Variable | Membrane LAT1 Expression | p-Value | |
|---|---|---|---|
| Negative (n = 45) | Positive (n = 53) | ||
| Age | |||
| 70≧ | 35 | 36 | 0.274 |
| 70< | 10 | 17 | |
| Sex | |||
| Male | 26 | 33 | 0.651 |
| Female | 19 | 20 | |
| Smoking | |||
| Yes | 23 | 28 | 0.865 |
| No | 22 | 25 | |
| Tumor factor | |||
| T1–T3 | 36 | 40 | 0.591 |
| T4 | 9 | 13 | |
| N factor | |||
| Absent | 17 | 9 | 0.020 * |
| Present | 28 | 44 | |
| Tumor location | |||
| Right | 12 | 18 | 0.434 |
| Left | 33 | 35 | |
| Pathological stage | |||
| Ⅱ | 17 | 11 | 0.063 |
| Ⅲ | 28 | 42 | |
| Histology Type | |||
| Well | 26 | 35 | 0.233 |
| Not-well | 19 | 18 | |
| Lymphatic invasion | |||
| Absent | 11 | 5 | 0.044 * |
| Present | 34 | 48 | |
| Vascular invasion | |||
| Absent | 10 | 17 | 0.274 |
| Present | 35 | 36 | |
| Perineural invasion | |||
| Absent | 26 | 30 | 0.091 |
| Present | 19 | 23 | |
| Recurrence | |||
| Absent | 41 | 37 | 0.007 * |
| Present | 4 | 16 | |
| Adjuvant chemotherapy | |||
| CAPOX | 21 | 22 | 0.627 |
| UFT + LV | 14 | 21 | |
| Capecitabine | 6 | 8 | |
| Others | 4 | 2 | |
| Factors | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | ||
| Age | 70≥ | Reference | |||||
| 70< | 1.241 | 0.477–3.232 | 0.658 | ||||
| Sex | Male | Reference | |||||
| Female | 0.477 | 0.173–1.314 | 0.152 | ||||
| Smoking | Yes | Reference | |||||
| No | 0.695 | 0.284–1.702 | 0.426 | ||||
| T factor | T1–3 | Reference | Reference | ||||
| T4 | 3.808 | 1.583–9.161 | 0.003 * | 3.282 | 1.309–8.234 | 0.011 * | |
| N factor | Absent | Reference | |||||
| Present | 3.493 | 0.810–15.06 | 0.093 | ||||
| Tumor location | Right | Reference | |||||
| Left | 0.803 | 0.320–2.014 | 0.640 | ||||
| Pathological stage | Ⅱ | Reference | |||||
| Ⅲ | 2.356 | 0.690–8.040 | 0.171 | ||||
| Histology | Well | Reference | |||||
| Not-well | 0.87 | 0.347–2.182 | 0.767 | ||||
| Lymphatic invasion | Absent | Reference | |||||
| Present | 1.93 | 0.448–8.333 | 0.377 | ||||
| Vascular invasion | Absent | Reference | |||||
| Present | 4.11 | 0.953–17.75 | 0.058 | ||||
| Perineural invasion | Absent | Reference | Reference | ||||
| Present | 2.661 | 1.061–6.674 | 0.037 * | 1.91 | 0.729–4.994 | 0.189 | |
| Adjuvant chemotherapy | UFT + LV | Reference | |||||
| CAPOX | 2.42 | 0.770–7.601 | 0.13 | ||||
| Capecitabine | 1.301 | 0.238–7.107 | 0.761 | ||||
| LAT1 | Negative | Reference | Reference | ||||
| Positive | 4.003 | 1.337–11.99 | 0.013 * | 3.99 | 1.327–11.99 | 0.014 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shibasaki, Y.; Yokobori, T.; Sohda, M.; Shioi, I.; Ozawa, N.; Komine, C.; Suga, K.; Nakazawa, N.; Osone, K.; Shiraishi, T.; et al. Association of High LAT1 Expression with Poor Prognosis and Recurrence in Colorectal Cancer Patients Treated with Oxaliplatin-Based Adjuvant Chemotherapy. Int. J. Mol. Sci. 2023, 24, 2604. https://doi.org/10.3390/ijms24032604
Shibasaki Y, Yokobori T, Sohda M, Shioi I, Ozawa N, Komine C, Suga K, Nakazawa N, Osone K, Shiraishi T, et al. Association of High LAT1 Expression with Poor Prognosis and Recurrence in Colorectal Cancer Patients Treated with Oxaliplatin-Based Adjuvant Chemotherapy. International Journal of Molecular Sciences. 2023; 24(3):2604. https://doi.org/10.3390/ijms24032604
Chicago/Turabian StyleShibasaki, Yuta, Takehiko Yokobori, Makoto Sohda, Ikuma Shioi, Naoya Ozawa, Chika Komine, Kunihiko Suga, Nobuhiro Nakazawa, Katsuya Osone, Takuya Shiraishi, and et al. 2023. "Association of High LAT1 Expression with Poor Prognosis and Recurrence in Colorectal Cancer Patients Treated with Oxaliplatin-Based Adjuvant Chemotherapy" International Journal of Molecular Sciences 24, no. 3: 2604. https://doi.org/10.3390/ijms24032604
APA StyleShibasaki, Y., Yokobori, T., Sohda, M., Shioi, I., Ozawa, N., Komine, C., Suga, K., Nakazawa, N., Osone, K., Shiraishi, T., Okada, T., Sano, A., Sakai, M., Ogawa, H., Kaira, K., Shirabe, K., & Saeki, H. (2023). Association of High LAT1 Expression with Poor Prognosis and Recurrence in Colorectal Cancer Patients Treated with Oxaliplatin-Based Adjuvant Chemotherapy. International Journal of Molecular Sciences, 24(3), 2604. https://doi.org/10.3390/ijms24032604






