1. Introduction
Autosomal dominant cerebellar ataxias, also known as spinocerebellar ataxias (SCAs) are a group of progressive hereditary neurodegenerative disorders with significant clinical and genetic diversity [
1,
2]. Currently more than 40 subtypes of SCA have been distinguished. Spinocerebellar ataxia type 40 (SCA40) was first described in a Chinese family suffering from adult-onset cerebellar ataxia [
3]. A missense allele of the coiled-coil domain-containing protein 88C (
CCDC88C) gene was identified as a putative causative gene. Located in the long arm of chromosome 14, this gene encodes the Dvl-associating protein, which exhibits a high frequency of leucine residues (DAPLE) [
3]. In addition to SCA40, mutations of this gene were proposed to be causative for nonsyndromic congenital hydrocephalus type 1 in an autosomal recessive manner [
4].
Recently, the phenotypic spectrum of SCA40 has been expanded with new cases. The second reported incidence was identified in a Polish family in which members exhibited the clinical symptoms of hand tremor at rest and in action, slight ataxia, parkinsonism and cognitive decline [
5]. Yahia et al. published a case study on a Sudanese patient with childhood-onset spastic paraparesis without cerebellar signs caused by another missense mutation of the
CCDC88C gene [
6]. An additional seven SCA40 patients of Kurdish, Chinese and Indian ancestry with dominantly cerebellar signs and variable movement disorders have been reported [
7,
8,
9,
10]. Segregation analysis clearly proved the putative disease-causing effect of the identified variants in four cases (p.R464H, p.D43N, p.E665K and p.R197Q). This analysis was not performed in two families (p.S1974R and p.F1024L), and analysis results were controversial in one case (p.R629Q) [
3,
5,
6,
7,
8,
9,
10].
The effect of three identified missense mutations (p.R464H, p.D43N and p.E665K) was confirmed with in vitro functional analyses demonstrating that mutant (MT) DAPLE triggers c-Jun N-terminal kinase 1 (JNK1) activation and caspase-3 apoptotic signaling in cells [
3,
5,
6]. However, the pathological effect of further four identified
CCDC88C gene mutations (p.S1974R, p.R197Q, p.R629Q and p.F1024L) were not verified with functional analyses.
In this work, we describe the clinical features of a Hungarian spinocerebellar ataxia patient carrying a heterozygous novel CCDC88C missense mutation and compare it with the phenotypes of the previously reported SCA40 subjects. Furthermore, we report results of in vitro functional tests aimed at confirming the causative role of this new CCDC88C variant.
3. Discussion
SCA40 is a hereditary neurodegenerative disorder with significant clinical and genetic diversity. The first report by Tsoi and colleagues described a
CCDC88C missense mutation in an autosomal-dominant form of SCA40 [
2]. Subsequently, similar results have been reported for additional missense mutations of the gene [
5,
6,
7,
8,
9,
10] (
Supplementary Table S3). Interestingly, the CCDC88C mutation has also been found in 2010 to cause a complex hydrocephalic brain malformation in a large family [
4]. Since this report, several new cases with these types of mutations have been identified [
13,
14], suggesting that these two conditions may be
CCDC88C-related allelic disorders. Segregation analysis of four variants (p.R464H, p.D43N, p.E665K and p.R197Q) also indicate the pathogenic role of the
CCDC88C mutations [
3,
5,
6].
It was suggested that the described
CCDC88C mutations may cause a loss of protein function through the truncation of binding motifs vital to the noncanonical Wnt pathway [
4,
15,
16] or by modulating the phosphorylation status of the JNK pathway, thereby inducing caspase-3 cleavage and triggering apoptosis [
3,
5,
6,
17]. According to the literature, cellular functions, such as protein trafficking and cilium formation, are also affected by these mutations.
We identified a novel missense CCDC88C mutation (c.607C > T) resulting in an p.R203W substitution in the hook domain of the DAPLE protein in a Hungarian female patient presenting late-onset ataxia.
The clinical phenotype of the subject is characterized by slowly progressive episodic mild cerebellar symptoms, slight spasticity in the ankles and vibration hypesthesia in her feet. Similar neurological abnormalities were described in a Chinese family, although with more severe signs, which is supported by the difference in the SARA scores as well (
Supplementary Table S1) [
3]. Except mild spasticity in the ankles, no other upper motor neuron symptoms or movement disorders were noticed in the patient described in this case, although these neurological alterations were frequently observed in other cases [
5,
6,
7,
8,
9,
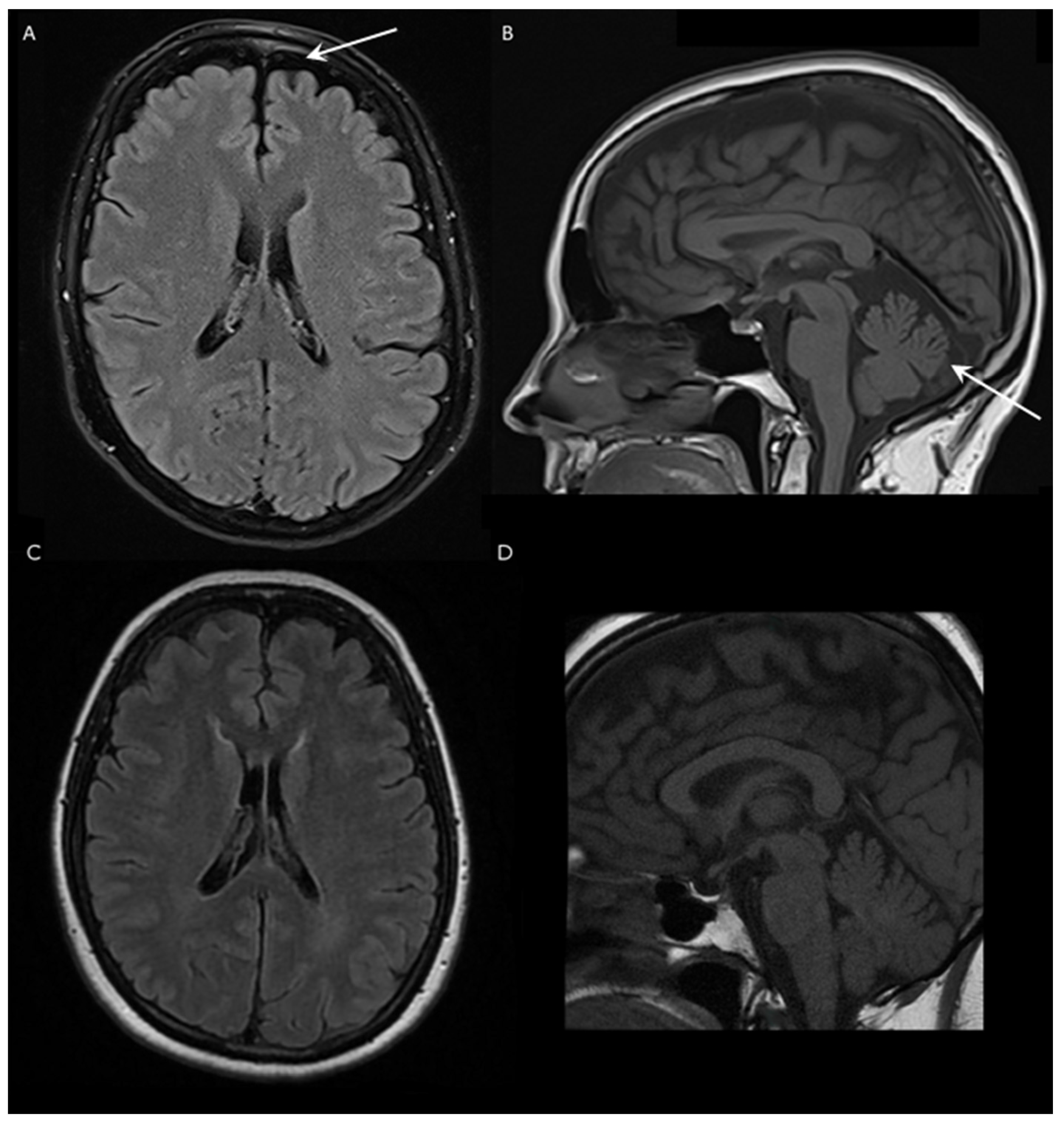
10]. Brain MRI examination showed mild cerebellar and frontal lobe atrophy, but no brainstem shrinkage, bilateral olivary degeneration or any other specific structural alterations were found.
To explore the molecular consequences of CCDC88C mutations, we expressed both the newly identified (p.R203W) and the previously described alleles (p.D43N, p.R464H and p.E665K) in HEK293 cells to examine JNK pathway activation.
WT and MT DAPLE proteins showed similar expression levels in the cells. Western blot analysis revealed no difference in JNK1 phosphorylation induced by WT or MT DAPLE proteins, and transfection of the cells by control plasmid constructs resulted in similar JNK1 phosphorylation. Furthermore, results from caspase-3 activation and detailed TUNEL assay did not support an increased proapoptotic effect of the novel and previously characterized
CCDC88C mutations as compared to the WT allele, despite the fact that these experiments were performed by following the experimental design and conditions detailed in previous publications [
3,
5,
6]. To further support these results, we included AP1–luciferase activity measurements. An increase in the luciferase reporter gene activation through an AP1 response element due to
CCDC88C mutations—most prominently by p.D43N and, to a lesser extent, by p.R203W and p.R464H—was observed, indicating the activation of the JNK pathway.
In conclusion, we have identified a novel mutation of the CCDC88C gene in a patient with spinocerebellar ataxia. We found that DAPLE protein might be a positive regulator of the JNK pathway, and its mutations slightly but not significantly increased JNK induced AP1–LUC activity. At the same time, we observed that neither the novel CCDC88C allele nor the earlier described alleles of CCDC88C led to an increased rate of apoptosis through JNK1 hyperphosphorylation. Thus, our findings partly disagree with previous reports suggesting the role of DAPLE in ataxia by activation of the JNK pathway via dominant gain of function mutations.
Based on data from the literature and our own findings, we can conclude that (i) the pathogenic role of
CCDC88C gene mutations in SCA40 is strongly supported by segregation analysis [
2,
4,
5], (ii) the broad range of clinical symptoms in patients with SCA40 also depends on the site of the genetic mutation causing impaired function in different domains of the DAPLE protein (
Supplementary Figure S3), (iii)
CCDC88C mutations may exert their effect not only by affecting the JNK pathway but also by different and possibly in much broader biological processes and (iv) the development of the disease might be caused by mutations in
CCDC88C and in other genes together, thus affecting not only JNK but different pathways as well. The interplay between a complex genetic background and the potential effects of harmful environmental factors could account for the diversity in both the age at disease onset and symptoms of the patients.
4. Materials and Methods
4.1. Clinical Examination
The patient was detected at the outpatient clinic of Department of Neurology University of Szeged by a movement disorder specialist having deep insight into ataxias and underwent a detailed diagnostic approach including neurological examination, laboratory and radiological investigations to exclude acquired causes of ataxia. After obtaining written informed consent, genomic DNA was extracted from peripheral blood leukocytes by standard protocol. First, the most common repeat expansion hereditary ataxias (SCA1, 2, 3, 6, 7, Friedreich’s ataxia and CANVAS) were tested. After the negative results of these tests, clinical exome sequencing was performed.
4.2. Cloning of the Constructs
The pcDNA3.1+/C−(K)DYK vector carrying the full length CCDC88C cDNA was purchased from GenScript Biotech Corp (Rijswijk, The Netherlands).
CCDC88C (NM_001080414.3, NP_001073883.2) (c.C607T), (c.G127A), (c.G1391A) and (c.G1993A) mutations were generated using the Quick Change Site-Directed Mutagenesis Kit (Agilent, Santa Clara, CA, USA), according to the instructions of the manufacturer. Oligoes used for the mutagenesis are listed in
Supplementary Table S1. The mutations were introduced into the CCDC88C pcDNA3.1+/C−(K)DYK vector by replacing wild type (WT) sequences with mutated ones.
The miniprom−LUC and AP1–LUC cis-reporter plasmids were created by inserting the synthesized minipromoter and three copies of the AP1 response element (
Supplementary Table S2) into the pGL4,201/luc2/Puro plasmid vector (Promega, Madison, WI, USA).
The final constructs carrying the MT CCDC88C cDNAs or the minipromoter and AP1 response element were verified by sequencing. Plasmid DNAs for transfection were purified with the QIAGEN Plasmid Maxi Kit (QIAGEN, Hilden, Germany).
4.3. Transfection of HEK293 Cell Line
The HEK293 cell line (Merck, Darmstadt, Germany) was maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS, Lonza, Basel, Switzerland), 1% L-glutamine (Lonza) and 1% antimycotic–antibiotic solution (Lonza) at 37 °C in a humidified atmosphere with 5% CO2.
For transfection, cells were seeded into 12-well plates at a density of 300,000 cells/mL in full medium. After 48 h, medium was changed to FBS-free medium, and cells were co-transfected with the AP1–LUC cis-reporter plasmid, the pGL4.75 (hRluc/CMV) plasmid (Promega), which was used as internal control, and 1µg pcDNA3.1+/C-(K)DYK vector (GenScript) carrying the WT or mutant CCDC88C cDNA sequences. Transfection was carried out with the Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions. Mock-transfection by transfection reagent and cotransfection of luciferase plasmids with the empty pcDNA3.1(+) plasmid served as control. Twenty-four hours after transfection, cell samples were collected for extraction of proteins, which were subjected to luciferase activity measurement or to TUNEL assay.
4.4. Cell Viability Test
The viability of transfected HEK293 cells was monitored by regular microscopic control and MTT assays.
To assess whether cell viability was affected by transfection, cells were seeded into 96-well plates at a density of 150,000 cells/mL. After 48 h, medium was changed to FBS-free medium, and cells were cotransfected with the pcDNA3.1+/C−(K)DYK vector carrying WT or MT CCDC88C cDNA sequences or with the pAP1–LUC cis-reporter plasmid using the Lipofectamine 3000 transfection reagent (Thermo Fisher Scientific), according to the manufacturer’s instructions. Twenty-four hours after transfection, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Merck) was added to a final concentration of 0.5 mg/mL, and cells were incubated at 37 °C in a humidified atmosphere with 5% CO
2 for 4 h. Subsequently, the medium was discarded, and the formazan crystals that had formed were solubilized in acidified isopropanol (20 mL 1N HCl and 500 mL isopropanol) supplemented with 2% SDS. Optical density (OD) was measured on a SPECTROstar Nano spectrophotometer (BMG Labtech, Ortenberg, Germany) at 540 nm. OD values were compared to the control wells transfected by the empty pcDNA3.1(+) vector and presented as a percentage (%) of living cells (
Supplementary Figure S1).
4.5. AP-1 Luciferase Reporter Assay
To determine luciferase activity, cells were rinsed in phosphate-buffered saline (PBS) and lysed in passive lysis buffer (Promega).
Luciferase activity of the lysates was measured using the Firefly & Renilla Dual Luciferase Assay Kit (Promega) and a Synergy HTX multimode reader (Agilent, Santa Clara, CA), according to the manufacturer’s instructions. Luciferase activity derived from AP1–LUC plasmid was normalized to the activity of Renilla luciferase activity from the pGL4.75 (hRluc/CMV) plasmid.
4.6. Western Blot Analysis
Cells were washed twice with PBS, and protein was extracted by lysing the cells in lysis buffer containing 20 mM HEPES, 150 mM KCl, 1 mM MgCl2, 1 mM DTT, 0.5% Triton-X-100, 10% glycine, 0.1% NP-40 and 0.5% sodium dodecyl sulfate (SDS) (all chemicals were obtained from Merck) supplemented with 1% HALT™ Protease-Phosphatase Inhibitor 100X (Thermo Fisher Scientific). Samples were incubated for 30 min on ice with occasional vortexing, and cell debris was removed by centrifugation at 16,000 g for 10 min at 4 °C. The protein concentration of each sample was measured with the BCA-Kit (Thermo Fisher Scientific), and equal amounts of total protein were separated onto a 10% SDS polyacrylamide gel (SDS-PAGE) and blotted onto polyvinylidene difluoride membranes (Thermo Fisher Scientific).
After blocking with 5% milk in Tris-buffered saline with 0.05% Tween® 20 detergent (TBST), membranes were probed with monoclonal mouse anti-FLAG antibody (1:1.000, Merck) for visualization of the (K)DYK-tag. Total and phospho-JNK proteins were detected using anti-JNK 3708 (1:1.000, Cell Signaling Technology, Danvers, MA, USA) and anti-p-JNK 5136 (1:500; Cell Signaling Technology) antibodies. Endogenous caspase-3 and cleaved caspase-3 were detected by anticaspase-3 and antiactivated caspase-3 antibody Asp175 (1:1.000 and 1:500; Cell Signaling Technology), respectively.
As a loading control, actin was visualized with the monoclonal antihuman actin antibody (1:1.000, Merck). Horseradish peroxidase-conjugated antimouse goat antibody and antirabbit goat antibody (Southern Biotech, Birmingham, AL, USA) were used as secondary antibodies. Chemiluminescent signals were detected and visualized on the Omega Lum G Chemidoc Imaging System (Aplegen Inc., Pleasanton, CA, USA). Before the membranes were used for probing with each antibody, previous antibodies were stripped by incubating the membranes in 0.1 M glycine at pH 1.9. Representative results of three individual experiments are shown.
4.7. TUNEL Assay
TUNEL method was applied to detect apoptosis of the HEK-293 cells. Cells were collected onto a microscopic slide using a cytocentrifuge (6 min, 600 RPM, 35,000 cells/slide) to create cytospin preparations. Cytospin samples were fixed in 4% paraformaldehyde for 20 min and then permeabilized on ice for 5 min in 0.1% Triton X-100 and 0.1% sodium citrate containing PBS. The In Situ Cell Death Detection Kit TMR red (Roche, Basel, Switzerland) was used to detect the apoptotic cells according to the manufacturer’s instructions. For the TUNEL reaction, one part enzyme and nine parts label solution were used for each sample, all of which were incubated for 60 min at 37 °C in a humidified chamber. One negative control (without the enzyme solution) and one positive control (digested with QIAGEN DNase I together with the TUNEL reaction) were applied for each experimental series. Nuclei were visualized with 4’’,6-diamidino-2-phenylindole (DAPI, Merck) staining. Five pictures were taken from randomly selected fields of each sample using a Zeiss AxioVert A1 microscope (20× original magnification, Carl Zeiss AG, Oberkochen, Germany). The rate (%) of TUNEL positive apoptotic cells was determined using the ImageJ software.
4.8. Statistical Analysis
Experiments were carried out in duplicate with at least three biological repeats. For statistical analysis one-tailed paired Student’s t-test was used with correction for multiple comparisons. The significance level was set at p ≤ 0.05.