Ultrastructural and Immunohistochemical Detection of Hydroxyapatite Nucleating Role by rRNA and Nuclear Chromatin Derivatives in Aortic Valve Calcification: In Vitro and In Vivo Pro-Calcific Animal Models and Actual Calcific Disease in Humans
Abstract
1. Introduction
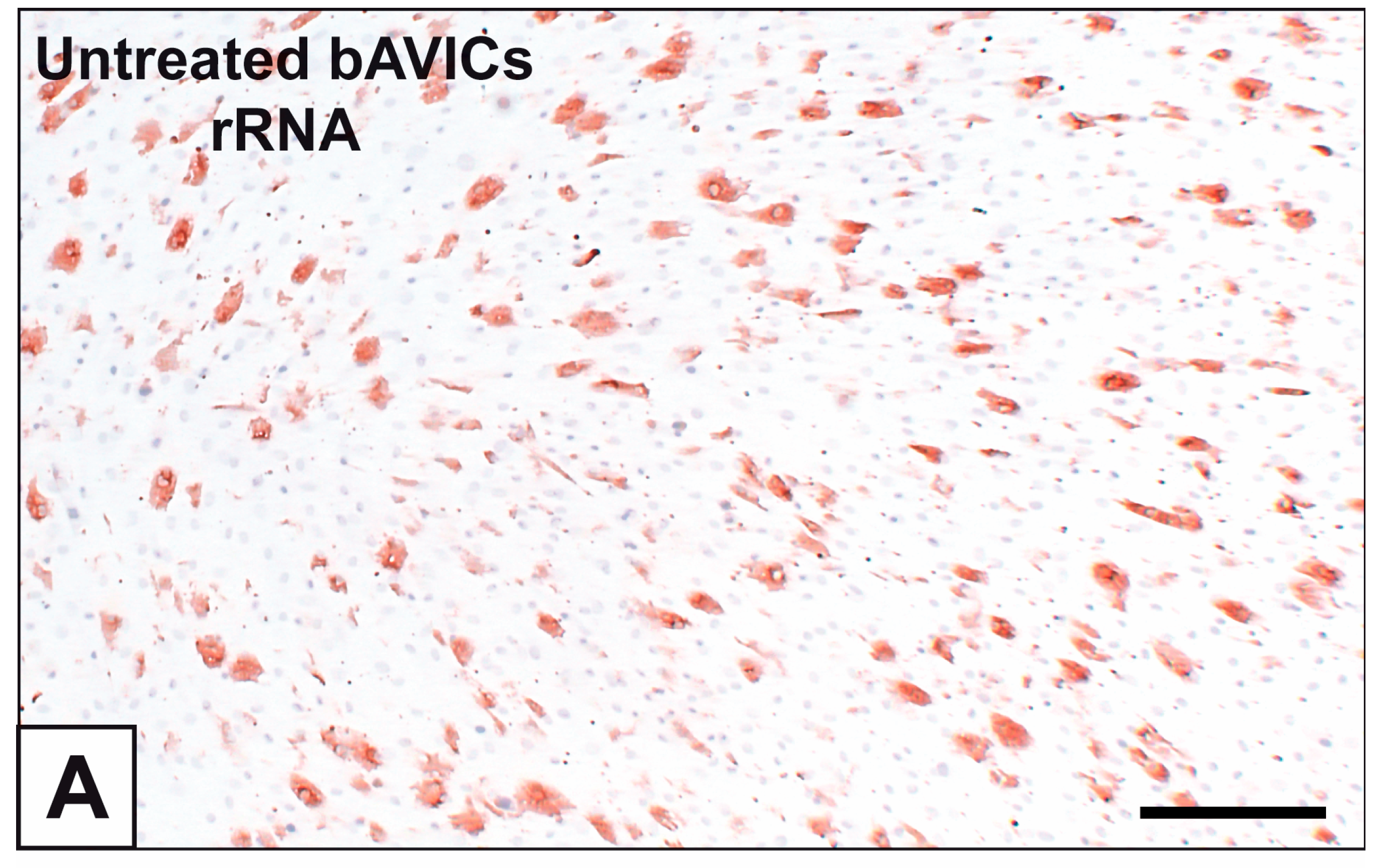
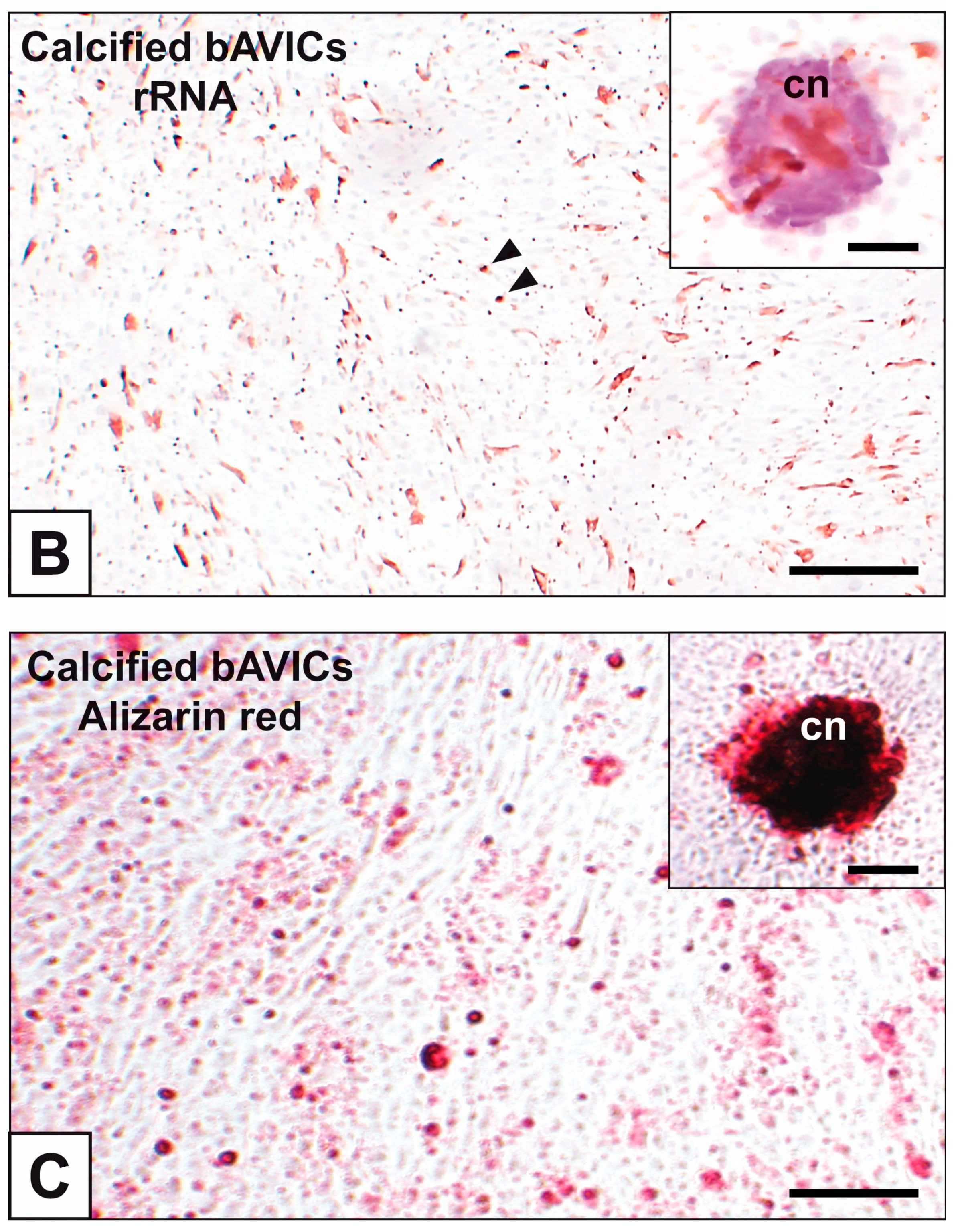
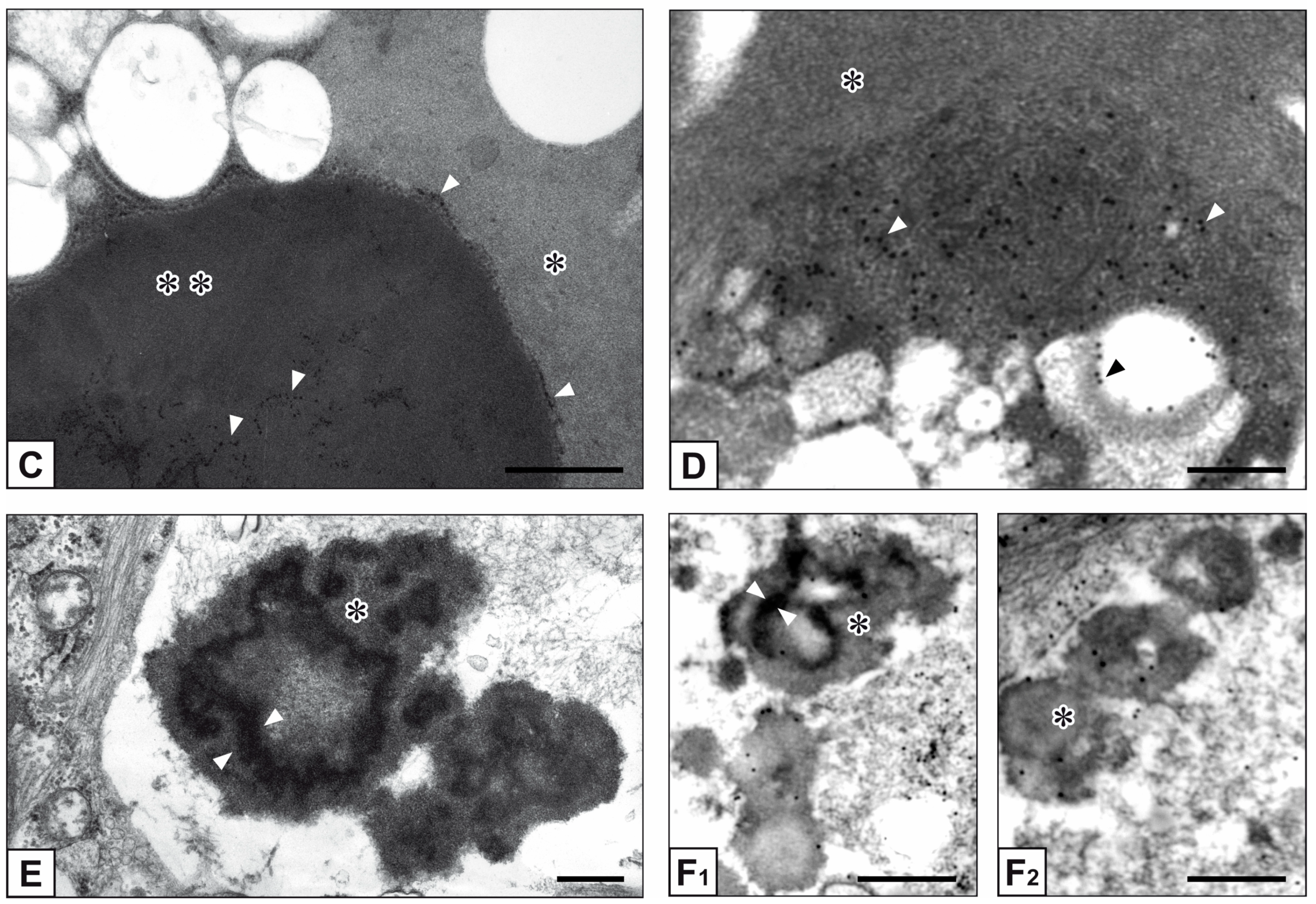
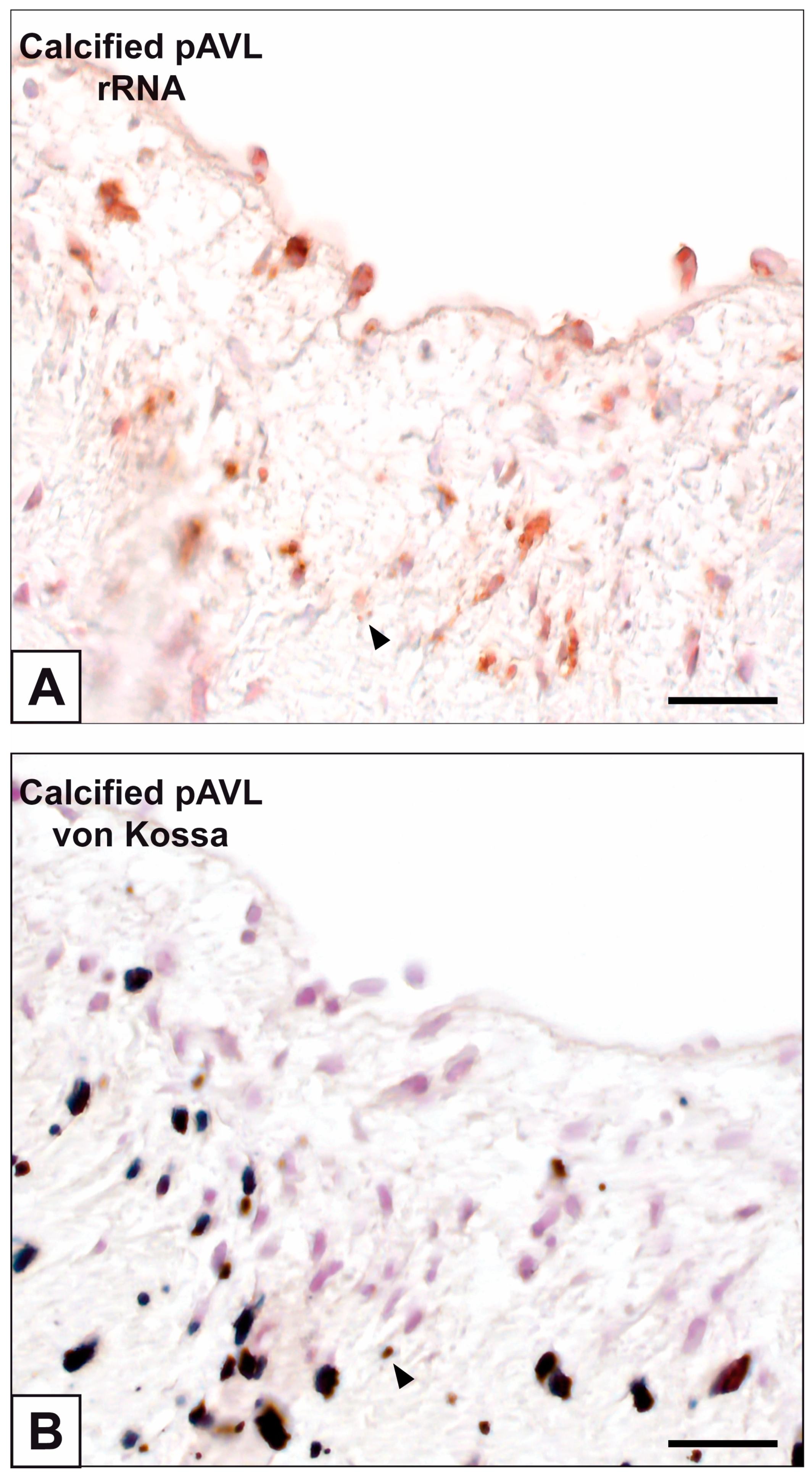
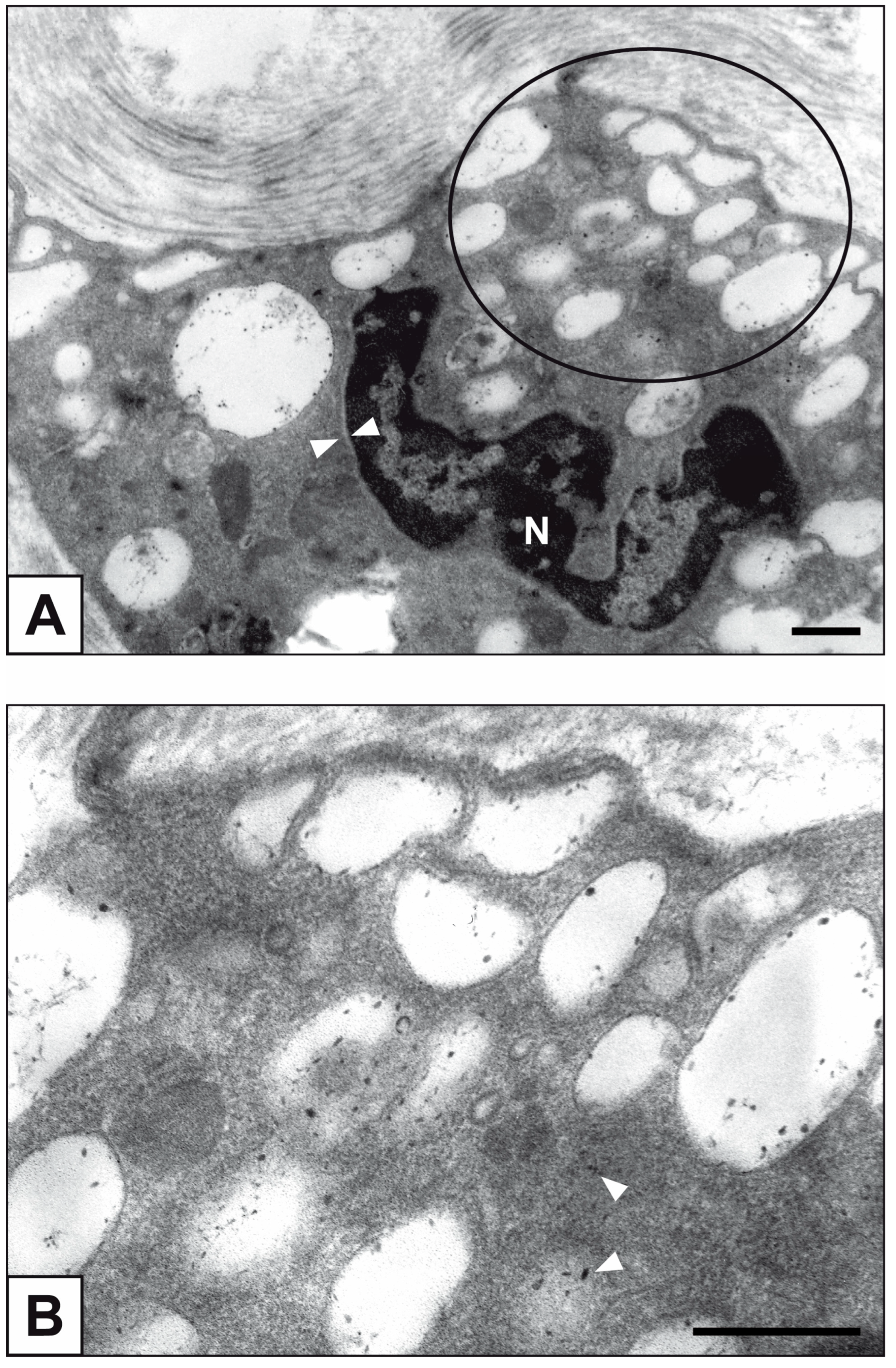
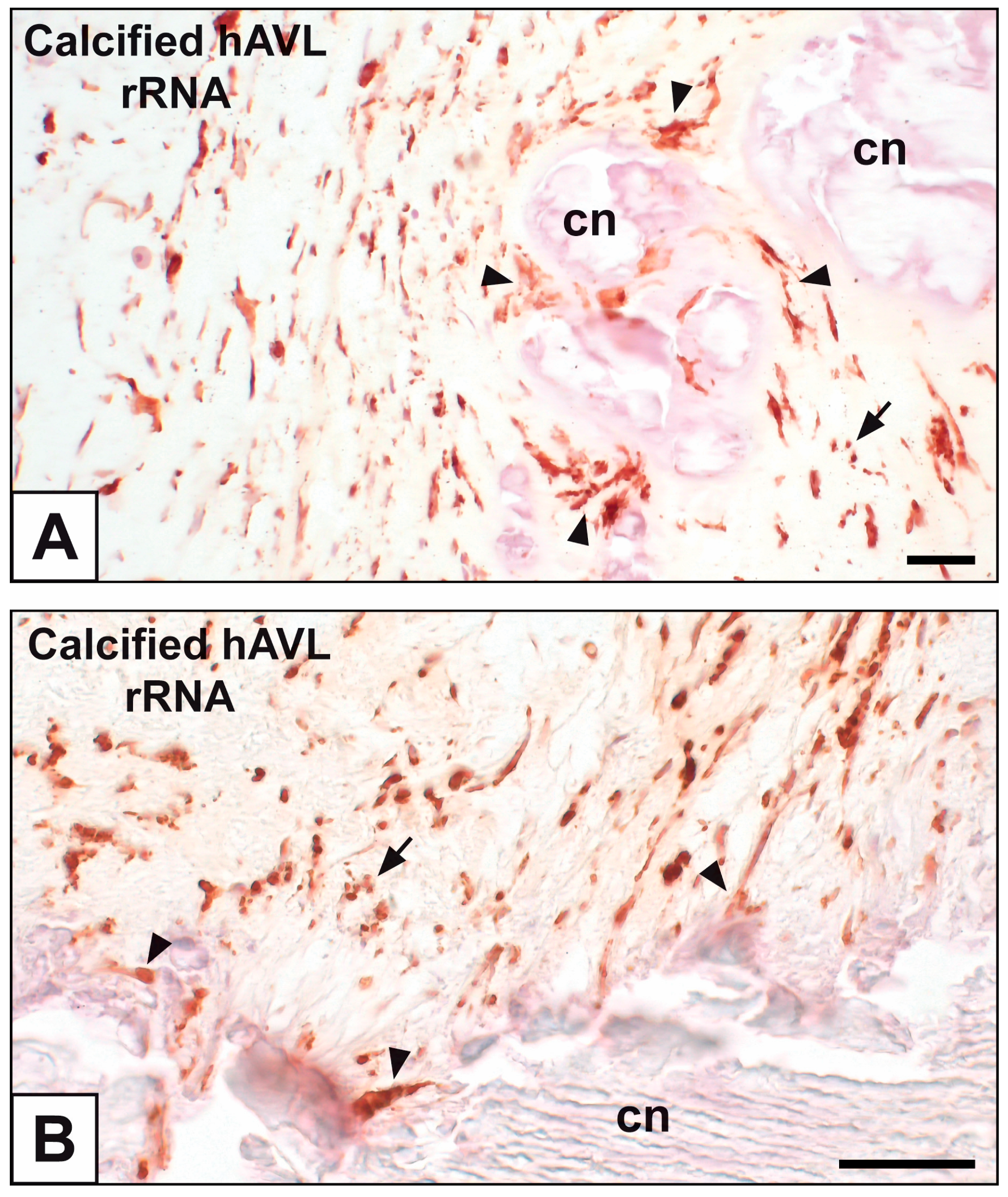
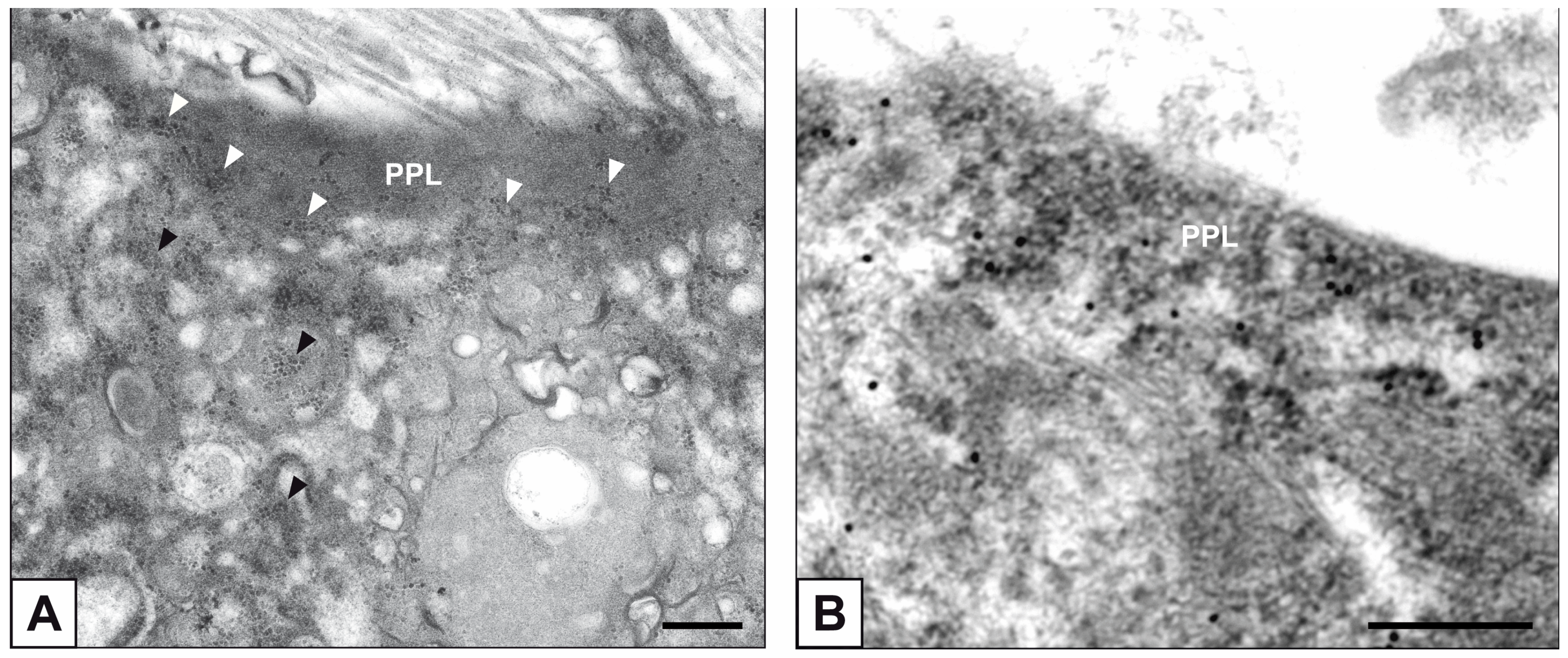
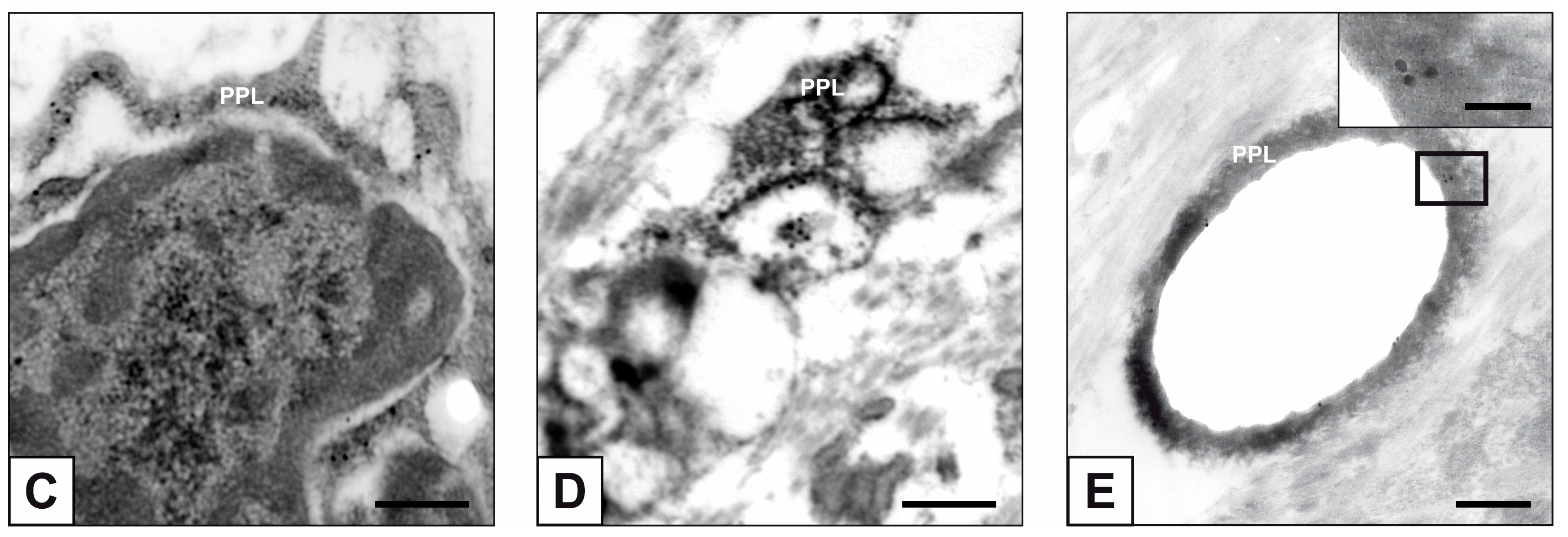
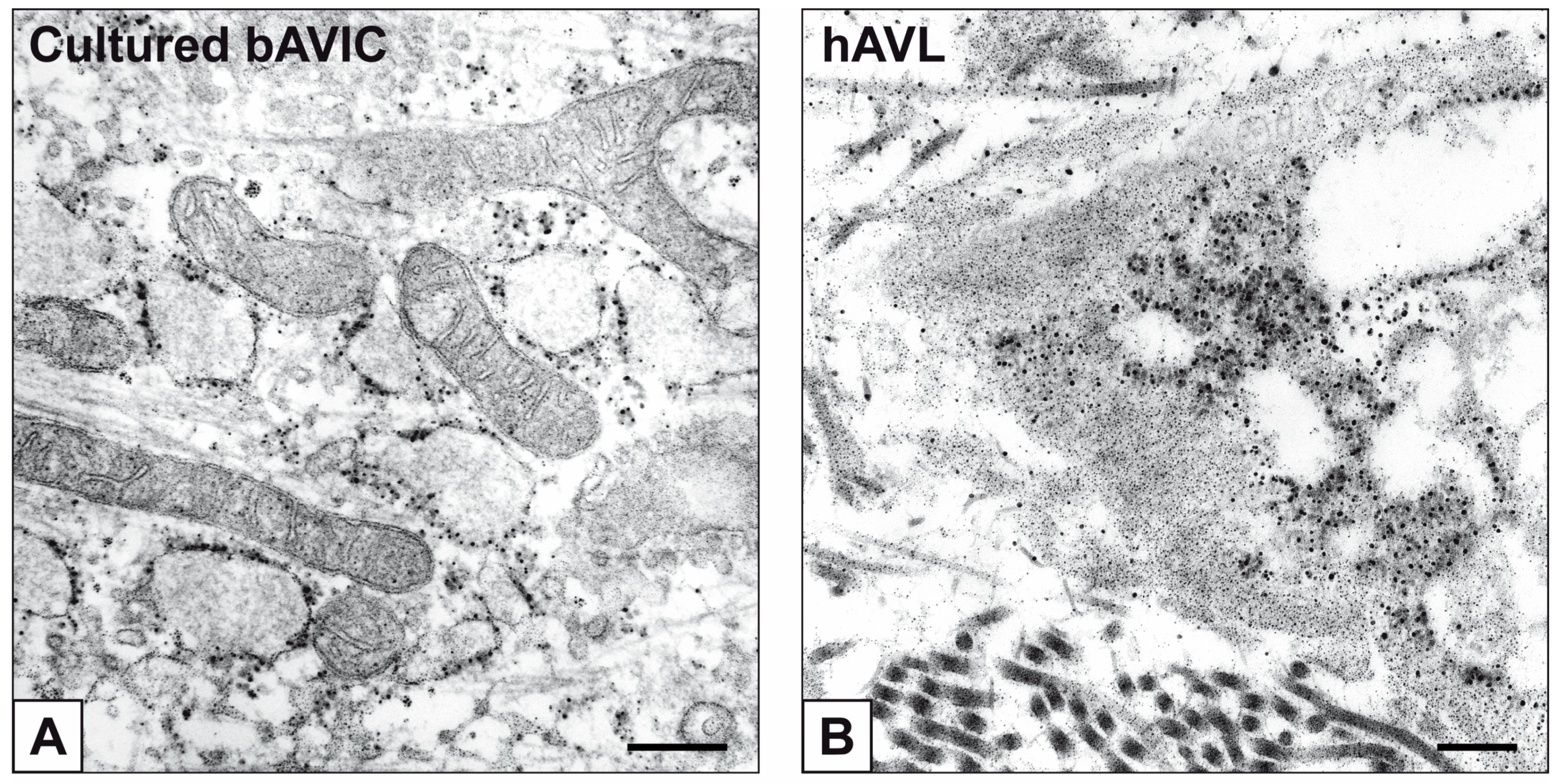
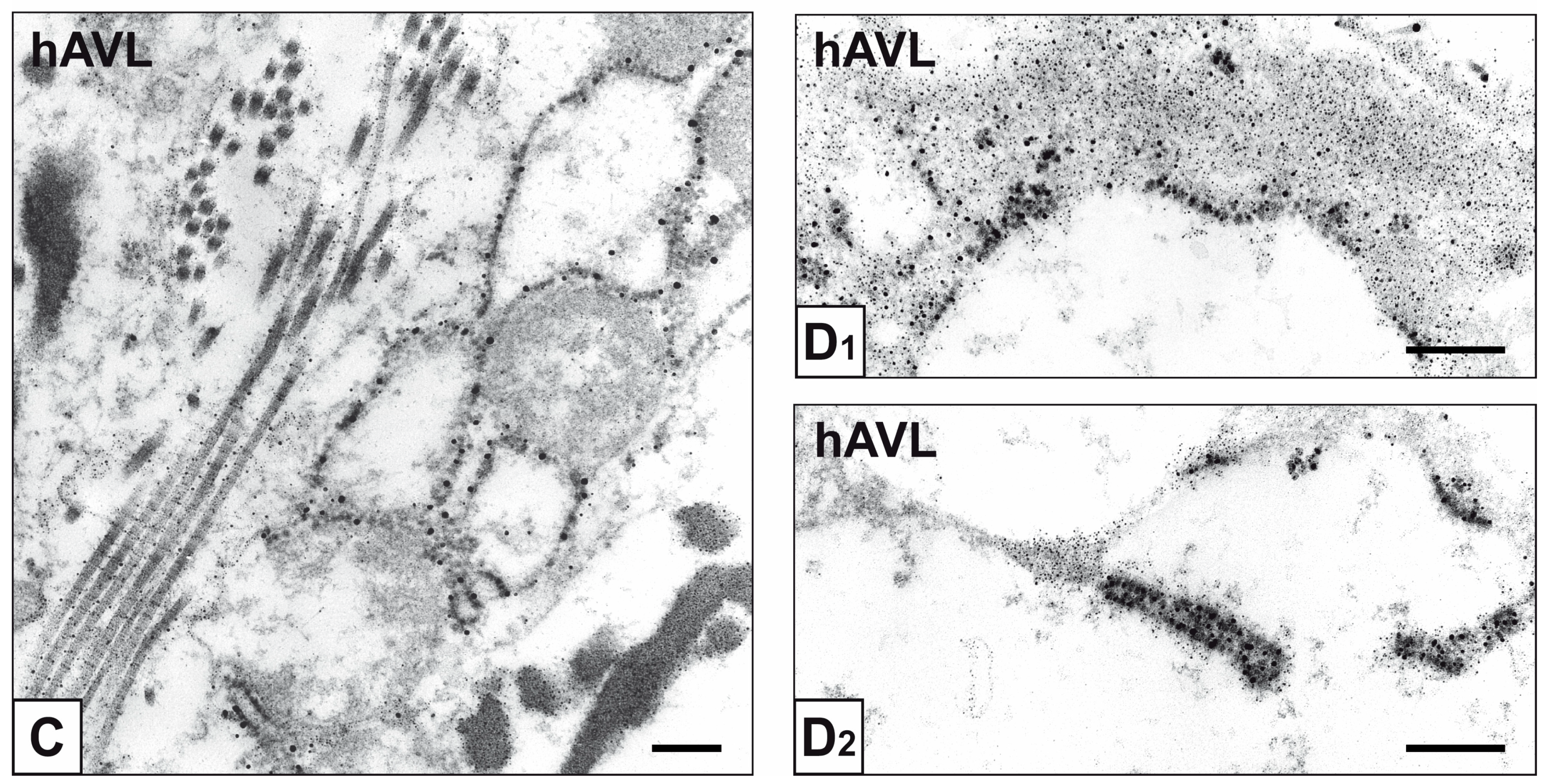
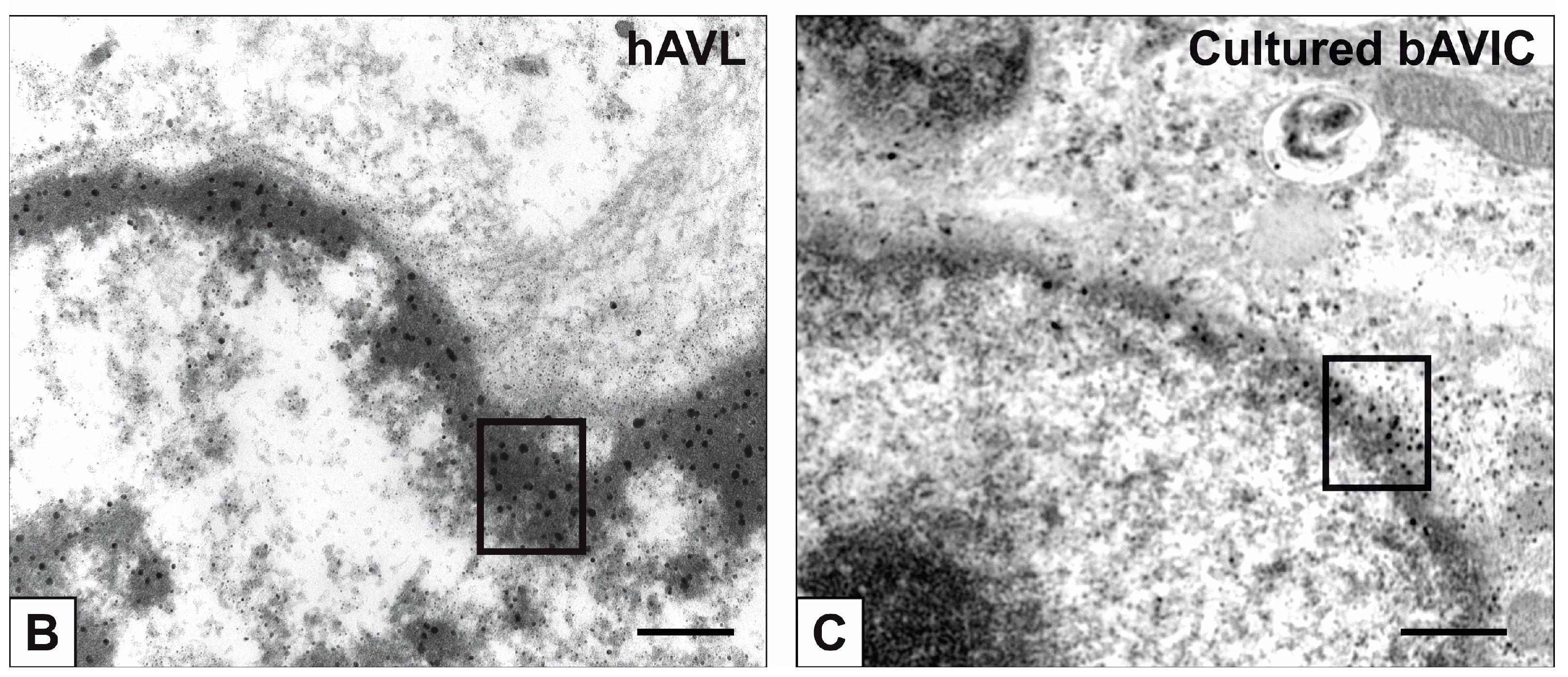
2. Results
3. Discussion
4. Materials and Methods
4.1. bAVIC Treatment
4.2. Subdermally Implanted pAVLs
4.3. Stenotic hAVLs
4.4. Immunocytochemical Assay of rRNA in bAVIC Cultures
4.5. Alizarin Red S Calcium Staining of bAVIC Cultures
4.6. Immunohistochemical Detection of rRNA in AVLs
4.7. Von Kossa Silver Staining for Calcium Binding Site Visualization in AVLs
4.8. Transmission Electron Microscopy
4.9. Immunogold Labelling of rRNA
4.10. Post-Embedding von Kossa Silver Staining for Ultrastructural Calcium Binding Site Visualization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fishbein, M.C.; Gissen, S.A.; Collins, J.J., Jr.; Barsamian, E.M.; Cohn, L.H. Pathologic findings after cardiac valve replacement with glutaraldehyde-fixed porcine valves. Am. J. Cardiol. 1977, 40, 331–337. [Google Scholar] [CrossRef]
- Ferrans, V.J.; Boyce, S.W.; Billingham, M.E.; Jones, M.; Ishihara, T.; Roberts, W.C. Calcific deposits in porcine bioprostheses: Structure and pathogenesis. Am. J. Cardiol. 1980, 46, 721–734. [Google Scholar] [CrossRef]
- Nimni, M.E. Ectopic calcification of glutaraldehyde crosslinked collagen bioprosthesis. Bone 1986, 7, 406–407. [Google Scholar] [CrossRef]
- Vyavahare, N.; Ogle, M.; Schoen, F.J.; Zand, R.; Gloeckner, D.C.; Sacks, M.; Levy, R.J. Mechanisms of bioprosthetic heart valve failure: Fatigue causes collagen denaturation and glycosaminoglycan loss. J. Biomed. Mater. Res. 1999, 46, 44–50. [Google Scholar] [CrossRef]
- Simionescu, D.T.; Lovekamp, J.J.; Vyavahare, N.R. Glycosaminoglycan-degrading enzymes in porcine aortic heart valves: Implications for bioprosthetic heart valve degeneration. J. Heart Valve Dis. 2003, 12, 217–225. [Google Scholar] [PubMed]
- Aikawa, E.; Aikawa, M.; Libby, P.; Figueiredo, J.L.; Rusanescu, G.; Iwamoto, Y.; Fukuda, D.; Kohler, R.H.; Shi, G.P.; Jaffer, F.A.; et al. Arterial and aortic valve calcification abolished by elastolytic cathepsin S deficiency in chronic renal disease. Circulation 2009, 119, 1785–1794. [Google Scholar] [CrossRef]
- Perrotta, I.; Russo, E.; Camastra, C.; Filice, G.; Di Mizio, G.; Colosimo, F.; Ricci, P.; Tripepi, S.; Amorosi, A.; Triumbari, F.; et al. New evidence for a critical role of elastin in calcification of native heart valves: Immunohistochemical and ultrastructural study with literature review. Histopathology 2011, 59, 504–513. [Google Scholar] [CrossRef]
- Perrotta, I.; Davoli, M. Collagen mineralization in human aortic valve stenosis: A field emission scanning electron microscopy and energy dispersive spectroscopy analysis. Ultrastruct. Pathol. 2014, 38, 281–284. [Google Scholar] [CrossRef]
- Kim, K.M.; Huang, S. Ultrastructural study of calcification of human aortic valve. Lab. Investig. 1971, 25, 357–366. [Google Scholar]
- Kim, K.M.; Trump, B.F. Amorphous calcium precipitations in human aortic valve. Calcif. Tissue Res. 1975, 18, 155–160. [Google Scholar] [CrossRef]
- Kim, K.M. Calcification of matrix vesicles in human aortic valve and aortic media. Fed. Proc. 1976, 35, 156–162. [Google Scholar] [PubMed]
- Schoen, F.J.; Levy, R.J.; Nelson, A.C.; Berhhard, W.F.; Nashef, A.; Hawley, M. Onset and progression of experimental bioprosthetic heart valve calcification. Lab. Investig 1985, 52, 523–532. [Google Scholar] [PubMed]
- Valente, M.; Bortolotti, U.; Thiene, G. Ultrastructural substrates of dystrophic calcification in porcine bioprosthetic valve failure. Am. J. Pathol. 1985, 119, 12–21. [Google Scholar] [PubMed]
- Boskey, A.L.; Bullough, P.G.; Vigorita, V.; Di Carlo, E. Calcium-acidic phospholipid-phosphate complexes in human hydroxyapatite-containing pathologic deposits. Am. J. Pathol. 1988, 133, 22–29. [Google Scholar]
- Kim, K.M. Apoptosis and calcification. Scanning Microsc. 1995, 9, 1137–1178. [Google Scholar]
- Kim, K.M. Cells, rather than extracellular matrix, nucleate apatite in glutaraldehyde-treated vascular tissue. J. Biomed. Mater. Res. 2002, 59, 639–645. [Google Scholar] [CrossRef]
- Leopold, J.A. Cellular mechanisms of aortic valve calcification. Circ. Cardiovasc. Interv. 2012, 5, 605–614. [Google Scholar] [CrossRef]
- Olsson, M.; Thyberg, J.; Nilsson, J. Presence of oxidized low density lipoprotein in nonrheumatic stenotic aortic valves. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1218–1222. [Google Scholar] [CrossRef]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bossé, Y.; Dumesnil, J.G.; Tam, J.W.; Teo, K.K.; Mahmut, A.; Yang, X.; et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J. Am. Coll. Cardiol. 2015, 66, 1236–1246. [Google Scholar] [CrossRef]
- Hirsch, D.; Drader, J.; Thomas, T.J.; Schoen, F.J.; Levy, J.T.; Levy, R.J. Inhibition of calcification of glutaraldehyde pretreated porcine aortic valve cusps with sodium dodecyl sulfate: Preincubation and controlled release studies. J. Biomed. Mater. Res. 1993, 27, 1477–1484. [Google Scholar] [CrossRef]
- Jorge-Herrero, E.; Fernández, P.; de la Torre, N.; Escudero, C.; García-Páez, J.M.; Buján, J.; Castillo-Olivares, J.L. Inhibition of the calcification of porcine valve tissue by selective lipid removal. Biomaterials 1994, 15, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Vyavahare, N.; Hirsch, D.; Lerner, E.; Baskin, J.Z.; Schoen, F.J.; Bianco, R.; Kruth, H.S.; Zand, R.; Levy, R.J. Prevention of bioprosthetic heart valve calcification by ethanol preincubation. Efficacy and mechanisms. Circulation 1997, 95, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Pathak, C.P.; Adams, A.K.; Simpson, T.; Phillips, R.E., Jr.; Moore, M.A. Treatment of bioprosthetic heart valve tissue with long chain alcohol solution to lower calcification potential. J. Biomed. Mater. Res. A 2004, 69, 140–144. [Google Scholar] [CrossRef]
- Spina, M.; Ortolani, F.; El Messlemani, A.; Gandaglia, A.; Bujan, J.; Garcia-Honduvilla, N.; Vesely, I.; Gerosa, G.; Casarotto, D.; Petrelli, L.; et al. Isolation of intact aortic valve scaffolds for heart-valve bioprostheses: Extracellular matrix structure, prevention from calcification, and cell repopulation features. J. Biomed. Mater. Res. A 2003, 67, 1338–1350. [Google Scholar] [CrossRef]
- Hopkins, R.A.; Linthurst Jones, A.; Wolfinbarger, L.; Moore, M.A.; Bert, A.A.; Lofland, G.K. Decellularization reduces calcification while improving both durability and 1-year functional results of pulmonary homograft valves in juvenile sheep. J. Thorac. Cardiovasc. Surg. 2009, 137, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.G.; Kim, S.H.; Choi, S.Y.; Kim, Y.J. Anticalcification effects of decellularization, solvent, and detoxification treatment for genipin and glutaraldehyde fixation of bovine pericardium. Eur. J. Cardiothorac. Surg. 2012, 41, 383–390. [Google Scholar] [CrossRef]
- Collatusso, C.; Roderjan, J.G.; de Noronha, L.; Klosowski, A.; Suss, P.H.; Guarita-Souza, L.C.; Diniz, F.; da Costa, A. Decellularization as a method to reduce calcification in bovine pericardium bioprosthetic valves. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 302–311. [Google Scholar] [CrossRef]
- Luo, Y.; Huang, S.; Ma, L. A novel detergent-based decellularization combined with carbodiimide crosslinking for improving anti-calcification of bioprosthetic heart valve. Biomed. Mater. 2021, 16, 045022. [Google Scholar] [CrossRef]
- Ortolani, F.; Petrelli, L.; Tubaro, F.; Spina, M.; Marchini, M. Novel ultrastructural features as revealed by phthalocyanine reactions indicate cell priming for calcification in subdermally implanted aortic valves. Connect. Tissue Res. 2002, 43, 44–55. [Google Scholar] [CrossRef]
- Ortolani, F.; Tubaro, F.; Petrelli, L.; Gandaglia, A.; Spina, M.; Marchini, M. Copper retention, calcium release and ultrastructural evidence indicate specific Cuprolinic Blue uptake and peculiar modifications in mineralizing aortic valves. Histochem. J. 2002, 34, 41–50. [Google Scholar] [CrossRef]
- Ortolani, F.; Petrelli, L.; Nori, S.L.; Gerosa, G.; Spina, M.; Marchini, M. Malachite green and phthalocyanine-silver reactions reveal acidic phospholipid involvement in calcification of porcine aortic valves in rat subdermal model. Histol. Histopathol. 2003, 18, 1131–1140. [Google Scholar] [PubMed]
- Ortolani, F.; Bonetti, A.; Tubaro, F.; Petrelli, L.; Contin, M.; Nori, S.L.; Spina, M.; Marchini, M. Ultrastructural characterization of calcification onset and progression in subdermally implanted aortic valves. Histochemical and spectrometric data. Histol. Histopathol. 2007, 22, 261–272. [Google Scholar] [PubMed]
- Ortolani, F.; Rigonat, L.; Bonetti, A.; Contin, M.; Tubaro, F.; Rattazzi, M.; Marchini, M. Pro-calcific responses by aortic valve interstitial cells in a novel in vitro model simulating dystrophic calcification. Ital. J. Anat. Embryol. 2010, 115, 135–139. [Google Scholar] [PubMed]
- Bonetti, A.; Marchini, M.; Ortolani, F. Ectopic mineralization in heart valves: New insights from in vivo and in vitro procalcific models and promising perspectives on noncalcifiable bioengineered valves. J. Thorac. Dis. 2019, 11, 2126–2143. [Google Scholar] [CrossRef]
- Bonetti, A.; Contin, M.; Tonon, F.; Marchini, M.; Ortolani, F. Calcium-dependent cytosolic phospholipase A2α as key factor in calcification of subdermally implanted aortic valve leaflets. Int. J. Mol. Sci. 2022, 23, 1988. [Google Scholar] [CrossRef]
- Bonetti, A.; Della Mora, A.; Contin, M.; Tubaro, F.; Marchini, M.; Ortolani, F. Ultrastructural and spectrophotometric study on the effects of putative triggers on aortic valve interstitial cells in in vitro models simulating metastatic calcification. Anat. Rec. 2012, 295, 1117–1127. [Google Scholar] [CrossRef]
- Bonetti, A.; Della Mora, A.; Contin, M.; Gregoraci, G.; Tubaro, F.; Marchini, M.; Ortolani, F. Survival-related autophagic activity versus procalcific death in cultured aortic valve interstitial cells treated with critical normophosphatemic-like phosphate concentrations. J. Histochem. Cytochem. 2017, 65, 125–138. [Google Scholar] [CrossRef]
- Bonetti, A.; Allegri, L.; Baldan, F.; Contin, M.; Battistella, C.; Damante, G.; Marchini, M.; Ortolani, F. Critical involvement of calcium-dependent cytosolic phospholipase A2α in aortic valve interstitial cell calcification. Int. J. Mol. Sci. 2020, 21, 6398. [Google Scholar] [CrossRef]
- Bonetti, A.; Bonifacio, A.; Della Mora, A.; Livi, U.; Marchini, M.; Ortolani, F. Carotenoids co-localize with hydroxyapatite, cholesterol, and other lipids in calcified stenotic aortic valves. Ex vivo Raman maps compared to histological patterns. Eur. J. Histochem. 2015, 59, 2505. [Google Scholar] [CrossRef]
- Girardot, M.N.; Torrianni, M.; Dillehay, D.; Girardot, J.M. Role of glutaraldehyde in calcification of porcine heart valves: Comparing cusp and wall. J. Biomed. Mater. Res. 1995, 29, 793–801. [Google Scholar] [CrossRef]
- Cinatl, J.; Sprincl, L.; Kourilek, K. Calcification of cell nuclei in experimental necrosis in vivo. Nature 1967, 216, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Tsolaki, E.; Csincsik, L.; Xue, J.; Lengyel, I.; Bertazzo, S. Nuclear and cellular micro and nano calcification in Alzheimer’s disease patients and correlation to phosphorylated Tau. Acta Biomater. 2022, 143, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C. Vesicles associated with calcification in the matrix of epiphyseal cartilage. J. Cell Biol. 1969, 41, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Korolev, N.; Allahverdi, A.; Lyubartsev, A.P.; Nordenskiöld, L. The polyelectrolyte properties of chromatin. Soft Matter 2012, 8, 9322–9333. [Google Scholar] [CrossRef]
- Patel, G.L. Isolation of the nuclear acidic proteins, their fractionation, and some general characteristics. In Acidic Proteins of the Nucleus; Cameron, I.L., Jeter, J.R., Jr., Eds.; Academic Press: New York, NY, USA; San Francisco, CA, USA; London, UK, 1974; pp. 29–57. [Google Scholar]
- Iop, L.; Renier, V.; Naso, F.; Piccoli, M.; Bonetti, A.; Gandaglia, A.; Pozzobon, M.; Paolin, A.; Ortolani, F.; Marchini, M.; et al. The influence of heart valve leaflet matrix characteristics on the interaction between human mesenchymal stem cells and decellularized scaffolds. Biomaterials 2009, 30, 4104–4116. [Google Scholar] [CrossRef]
- Iop, L.; Bonetti, A.; Naso, F.; Rizzo, S.; Cagnin, S.; Bianco, R.; Dal Lin, C.; Martini, P.; Poser, H.; Franci, P.; et al. Decellularized allogeneic heart valves demonstrate self-regeneration potential after a long-term preclinical evaluation. PLoS ONE 2014, 9, e99593. [Google Scholar] [CrossRef]
- Bertipaglia, B.; Ortolani, F.; Petrelli, L.; Gerosa, G.; Spina, M.; Pauletto, P.; Casarotto, D.; Marchini, M.; Sartore, S. Cell characterization of porcine aortic valve and decellularized leaflets repopulated with aortic valve interstitial cells: The VESALIO Project (Vitalitate Exornatum Succedaneum Aorticum Labore Ingenioso Obtenibitur). Ann. Thorac. Surg. 2003, 75, 1274–1282. [Google Scholar] [CrossRef]
- Rieder, E.; Kasimir, M.T.; Silberhumer, G.; Seebacher, G.; Wolner, E.; Simon, P.; Weigel, G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J. Thorac. Cardiovasc. Surg. 2004, 127, 399–405. [Google Scholar] [CrossRef]
- Roosens, A.; Somers, P.; De Somer, F.; Carriel, V.; Van Nooten, G.; Cornelissen, R. Impact of detergent-based decellularization methods on porcine tissues for heart valve engineering. Ann. Biomed. Eng. 2016, 44, 2827–2839. [Google Scholar] [CrossRef]
- Ramm, R.; Goecke, T.; Theodoridis, K.; Hoeffler, K.; Sarikouch, S.; Findeisen, K.; Ciubotaru, A.; Cebotari, S.; Tudorache, I.; Haverich, A.; et al. Decellularization combined with enzymatic removal of N-linked glycans and residual DNA reduces inflammatory response and improves performance of porcine xenogeneic pulmonary heart valves in an ovine in vivo model. Xenotransplantation 2020, 27, e12571. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonetti, A.; Contin, M.; Marchini, M.; Ortolani, F. Ultrastructural and Immunohistochemical Detection of Hydroxyapatite Nucleating Role by rRNA and Nuclear Chromatin Derivatives in Aortic Valve Calcification: In Vitro and In Vivo Pro-Calcific Animal Models and Actual Calcific Disease in Humans. Int. J. Mol. Sci. 2023, 24, 2667. https://doi.org/10.3390/ijms24032667
Bonetti A, Contin M, Marchini M, Ortolani F. Ultrastructural and Immunohistochemical Detection of Hydroxyapatite Nucleating Role by rRNA and Nuclear Chromatin Derivatives in Aortic Valve Calcification: In Vitro and In Vivo Pro-Calcific Animal Models and Actual Calcific Disease in Humans. International Journal of Molecular Sciences. 2023; 24(3):2667. https://doi.org/10.3390/ijms24032667
Chicago/Turabian StyleBonetti, Antonella, Magali Contin, Maurizio Marchini, and Fulvia Ortolani. 2023. "Ultrastructural and Immunohistochemical Detection of Hydroxyapatite Nucleating Role by rRNA and Nuclear Chromatin Derivatives in Aortic Valve Calcification: In Vitro and In Vivo Pro-Calcific Animal Models and Actual Calcific Disease in Humans" International Journal of Molecular Sciences 24, no. 3: 2667. https://doi.org/10.3390/ijms24032667
APA StyleBonetti, A., Contin, M., Marchini, M., & Ortolani, F. (2023). Ultrastructural and Immunohistochemical Detection of Hydroxyapatite Nucleating Role by rRNA and Nuclear Chromatin Derivatives in Aortic Valve Calcification: In Vitro and In Vivo Pro-Calcific Animal Models and Actual Calcific Disease in Humans. International Journal of Molecular Sciences, 24(3), 2667. https://doi.org/10.3390/ijms24032667









