Age-Related Changes in Neurons and Satellite Glial Cells in Mouse Dorsal Root Ganglia
Abstract
:1. Introduction
2. Results
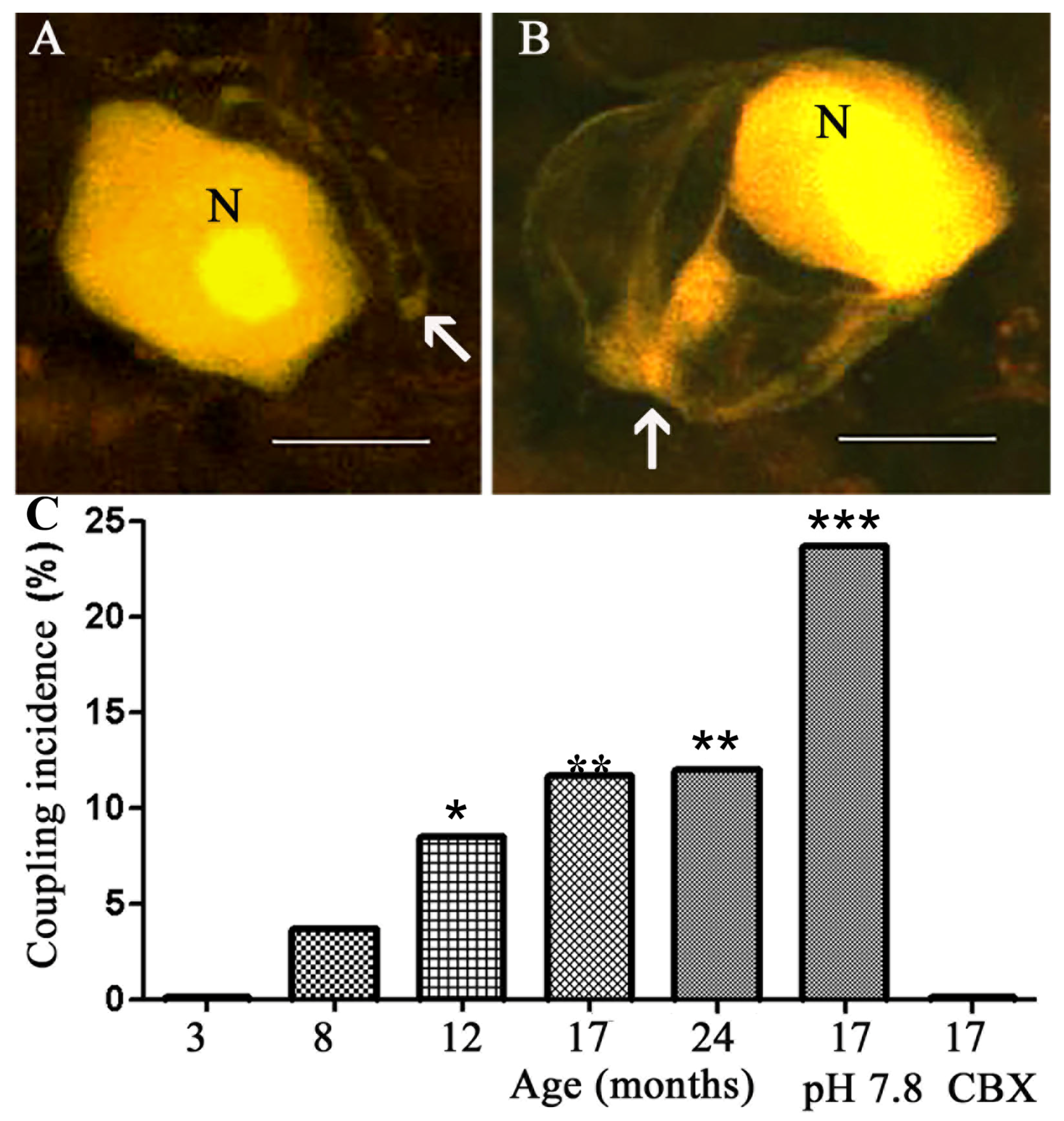
2.1. Coupling between Neurons and SGCs
2.2. Neuronal Excitability Increases with Age
2.3. Age-Related Change in Tactile Sensitivity
3. Discussion
4. Materials and Methods
4.1. Animals and Preparations
4.2. Intracellular Labeling and Recording
4.3. Measurement of Withdrawal Threshold
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Domenichiello, A.F.; Ramsden, C.E. The silent epidemic of chronic pain in older adults. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 93, 284–290. [Google Scholar] [CrossRef]
- Jones, M.R.; Ehrhardt, K.P.; Ripoll, J.G.; Sharma, B.; Padnos, I.W.; Kaye, R.J.; Kaye, A.D. Pain in the Elderly. Curr. Pain Headache Rep. 2016, 20, 23. [Google Scholar] [CrossRef]
- Patel, K.V.; Guralnik, J.M.; Dansie, E.J.; Turk, D.C. Prevalence and impact of pain among older adults in the United States: Findings from the 2011 National Health and aging trends study. Pain 2013, 154, 2649–2657. [Google Scholar] [CrossRef] [Green Version]
- Kohno, K.; Tsuda, M. Role of microglia and P2X4 receptors in chronic pain. Pain Rep. 2021, 6, e864. [Google Scholar] [CrossRef]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Milligan, E.D.; Watkins, L.R. Pathological and protective roles of glia in chronic pain. Nat. Rev. Neurosci. 2009, 10, 23–36. [Google Scholar] [CrossRef] [Green Version]
- Jasmin, L.; Vit, J.P.; Bhargava, A.; Ohara, P.T. Can satellite glial cells be therapeutic targets for pain control? Neuron Glia Biol. 2010, 6, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Shinoda, M.; Kubo, A.; Hayashi, Y.; Iwata, K. Peripheral and Central Mechanisms of Persistent Orofacial Pain. Front. Neurosci. 2019, 13, 1227. [Google Scholar] [CrossRef]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef]
- Spray, D.C.; Hanani, M. Gap junctions, pannexins and pain. Neurosci. Lett. 2019, 695, 46–52. [Google Scholar] [CrossRef]
- Huang, T.Y.; Hanani, M.; Ledda, M.; De Palo, S.; Pannese, E. Aging is associated with an increase in dye coupling and in gap junction number in satellite glial cells of murine dorsal root ganglia. Neuroscience 2006, 137, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Hanstein, R.; Zhao, J.B.; Basak, R.; Smith, D.N.; Zuckerman, Y.Y.; Hanani, M.; Spray, D.C.; Gulinello, M. Focal Inflammation Causes Carbenoxolone-Sensitive Tactile Hypersensitivity in Mice. Open Pain J. 2010, 3, 123–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.Y.; Belzer, V.; Hanani, M. Gap junctions in dorsal root ganglia: Possible contribution to visceral pain. Eur. J. Pain 2010, 14, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kaji, K.; Shinoda, M.; Honda, K.; Unno, S.; Shimizu, N.; Iwata, K. Connexin 43 contributes to ectopic orofacial pain following inferior alveolar nerve injury. Mol. Pain 2016, 12, 1744806916633704. [Google Scholar] [CrossRef] [Green Version]
- Ohara, P.T.; Vit, J.P.; Bhargava, A.; Jasmin, L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J. Neurophysiol. 2008, 100, 3064–3073. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.Z.; Zhang, P.; Hao, T.; Wang, L.M.; Guo, M.D.; Gan, Y.H. Connexin 43 contributes to temporomandibular joint inflammation induced-hypernociception via sodium channel 1.7 in trigeminal ganglion. Neurosci. Lett. 2019, 707, 134301. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.I.; Dudek, F.E.; Rash, J.E. Update on connexins and gap junctions in neurons and glia in the mammalian nervous system. Brain Res. Brain Res. Rev. 2004, 47, 191–215. [Google Scholar] [CrossRef] [PubMed]
- Theis, M.; Söhl, G.; Eiberger, J.; Willecke, K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005, 28, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 1994, 263, 1768–1771. [Google Scholar] [CrossRef]
- Froes, M.M.; Correia, A.H.; Garcia-Abreu, J.; Spray, D.C.; Campos de Carvalho, A.C.; Neto, A.V. Gap-junctional coupling between neurons and astrocytes in primary central nervous system cultures. Proc. Natl. Acad. Sci. USA 1999, 96, 7541–7546. [Google Scholar] [CrossRef]
- Rozental, R.; Andrade-Rozental, A.F.; Zheng, X.; Urban, M.; Spray, D.C.; Chiu, F.C. Gap junction-mediated bidirectional signaling between human fetal hippocampal neurons and astrocytes. Dev. Neurosci. 2001, 23, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Zündorf, G.; Kahlert, S.; Reiser, G. Gap-junction blocker carbenoxolone differentially enhances NMDA-induced cell death in hippocampal neurons and astrocytes in co-culture. J. Neurochem. 2007, 102, 508–521. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Maubecin, V.; García-Hernández, F.; Williams, J.T.; Van Bockstaele, E.J. Functional coupling between neurons and glia. J. Neurosci. 2000, 20, 4091–4098. [Google Scholar] [CrossRef] [Green Version]
- Pakhotin, P.; Verkhratsky, A. Electrical. Synapses between Bergmann glial cells and Purkinje neurones in rat cerebellar slices. Mol. Cell Neurosci. 2005, 28, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Anderson, M.; Park, K.; Zheng, Q.; Agarwal, A.; Gong, C.; Saijilafu; Young, L.; He, S.; LaVinka, P.C.; et al. Coupled Activation of Primary Sensory Neurons Contributes to Chronic Pain. Neuron 2016, 91, 1085–1896. [Google Scholar] [CrossRef] [Green Version]
- Ciglieri, E.; Vacca, M.; Ferrini, F.; Atteya, M.A.; Aimar, P.; Ficarra, E.; Di Cataldo, S.; Merighi, A.; Salio, C. Cytoarchitectural analysis of the neuron-to-glia association in the dorsal root ganglia of normal and diabetic mice. J. Anat. 2020, 237, 988–997. [Google Scholar] [CrossRef]
- Spray, D.C.; Iglesias, R.; Shraer, N.; Suadicani, S.O.; Belzer, V.; Hanstein, R.; Hanani, M. Gap junction mediated signaling between satellite glia and neurons in trigeminal ganglia. Glia 2019, 67, 791–801. [Google Scholar] [CrossRef]
- Hanani, M.; Huang, T.Y.; Cherkas, P.S.; Ledda, M.; Pannese, E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience 2002, 114, 279–283. [Google Scholar] [CrossRef]
- Zuriel, E.; Devor, M. Dye coupling does not explain functional crosstalk within dorsal root ganglia. J. Peripher. Nerv. Syst. 2001, 6, 227–231. [Google Scholar] [CrossRef]
- Spray, D.C.; Harris, A.L.; Bennett, M.V. Gap junctional conductance is a simple and sensitive function of intracellular pH. Science 1981, 211, 712–715. [Google Scholar] [CrossRef]
- Huang, T.Y.; Cherkas, P.S.; Rosenthal, D.W.; Hanani, M. Dye coupling among satellite glial cells in mammalian dorsal root ganglia. Brain Res. 2005, 1036, 42–49. [Google Scholar] [CrossRef]
- Dublin, P.; Hanani, M. Satellite glial cells in sensory ganglia: Their possible contribution to inflammatory pain. Brain Behav. Immun. 2007, 21, 592–598. [Google Scholar] [CrossRef]
- Cabanes, C.; de Armentia, M.L.; Viana, F.; Belmonte, C. Postnatal changes in membrane properties of mice trigeminal ganglion neurons. J. Neurophysiol. 2002, 87, 2398–2407. [Google Scholar] [CrossRef]
- Cherkas, P.S.; Huang, T.Y.; Pannicke, T.; Tal, M.; Reichenbach, A.; Hanani, M. The effects of axotomy on neurons and satellite glial cells in mouse trigeminal ganglion. Pain 2004, 110, 290–298. [Google Scholar] [CrossRef]
- Amir, R.; Michaelis, M.; Devor, M. Burst discharge in primary sensory neurons: Triggered by subthreshold oscillations, maintained by depolarizing afterpotentials. J. Neurosci. 2002, 22, 1187–1198. [Google Scholar] [CrossRef] [Green Version]
- El Tumi, H.; Johnson, M.I.; Dantas, P.B.F.; Maynard, M.J.; Tashani, O.A. Age-related changes in pain sensitivity in healthy humans: A systematic review with meta-analysis. Eur. J. Pain 2017, 21, 955–964. [Google Scholar] [CrossRef] [Green Version]
- Millecamps, M.; Shi, X.Q.; Piltonen, M.; Echeverry, S.; Diatchenko, L.; Zhang, J.; Stone, L.S. The geriatric pain experience in mice: Intact cutaneous thresholds but altered responses to tonic and chronic pain. Neurobiol. Aging 2020, 89, 1–11. [Google Scholar] [CrossRef]
- Mogil, J.S.; Wilson, S.G.; Bon, K.; Lee, S.E.; Chung, K.; Raber, P.; Pieper, J.O.; Hain, H.S.; Belknap, J.K.; Hubert, L.; et al. Heritability of nociception I: Responses of 11 inbred mouse strains on 12 measures of nociception. Pain 1999, 80, 67–82. [Google Scholar] [CrossRef]
- Martinelli, C.; Sartori, P.; De Palo, S.; Ledda, M.; Pannese, E. Increase in number of the gap junctions between satellite neuroglial cells during lifetime: An ultrastructural study in rabbit spinal ganglia from youth to extremely advanced age. Brain Res. Bull. 2005, 67, 19–23. [Google Scholar] [CrossRef]
- Martinelli, C.; Sartori, P.; Ledda, M.; Pannese, E. Age-related quantitative changes in mitochondria of satellite cell sheaths enveloping spinal ganglion neurons in the rabbit. Brain Res. Bull. 2003, 61, 147–151. [Google Scholar] [CrossRef]
- Pannese, E. Quantitative, structural and molecular changes in neuroglia of aging mammals: A review. Eur. J. Histochem. 2021, 65, 3249. [Google Scholar] [CrossRef] [PubMed]
- Armendariz, E.M.P.; Norcini, M.; Hernández-Tellez, B.; Castell-Rodríguez, A.; Coronel-Cruz, C.; Alquicira, R.G.; Sideris, A.; Recio-Pinto, E. Neurons and satellite glial cells in adult rat lumbar dorsal root ganglia express connexin 36. Acta Histochem. 2018, 120, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, R.; Cherkas, P.S.; Hanani, M. Peripheral Inflammation Upregulates P2X Receptor Expression in Satellite Glial Cells of Mouse Trigeminal Ganglia: A Calcium Imaging Study. Neuropharmacology 2011, 61, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Lalo, U.; Bogdanov, A.; Pankratov, Y. Age- and Experience-Related Plasticity of ATP-Mediated Signaling in the Neocortex. Front Cell Neurosci. 2019, 13, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021, 64, 1–14. [Google Scholar] [CrossRef]
- Nakamura, T.; Oh, C.K.; Zhang, X.; Tannenbaum, S.R.; Lipton, S.A. Antioxid. Redox Signal. Protein Transnitrosylation Signaling Networks Contribute to Inflammaging and Neurodegenerative Disorders. Antioxid. Redox Signal. 2021, 35, 531–550. [Google Scholar] [CrossRef]
- Belzer, V.; Hanani, M. Nitric oxide as a messenger between neurons and satellite glial cells in dorsal root ganglia. Glia 2019, 67, 1296–1307. [Google Scholar] [CrossRef]

| A-like Type Neurons | |||||
| Age (months) | 3 | 3+CBX | 12 | 12+CBX | 17 |
| n | 63 | 34 | 59 | 45 | 47 |
| RMP (mV) | 49.9 ± 1.1 | 50.1 ± 1.3 | 44.7 ± 0.9 * | 49.7 ± 1.1 | 46.5 ± 0.8 * |
| APA (mV) | 55.8 ± 1.6 | 58.1 ± 1.7 | 54.5 ± 1.7 | 59.1 ± 1.4 | 57.6 ± 1.5 |
| APD (ms) | 5.8 ± 0.2 | 6.1 ± 0.3 | 6.5 ± 0.2 * | 6.1 ± 0.3 | 6.3 ± 0.3 |
| Threshold current (nA) | 0.42 ± 0.03 | 0.43 ± 0.04 | 0.34 ± 0.03 * | 0.45 ± 0.03 | 0.30 ± 0.03 * |
| Ri (MΩ) | 42.1 ± 0.7 | 43.2 ± 1.1 | 46.2 ± 0.8 * | 41.1 ± 0.8 | 46.0 ± 0.8 * |
| SPS | 2 (3.2%) | 1(2.9%) | 2 (3.4%) | 0 | 3 (6.4%) |
| SPO | 12 (19%) | 6 (17.6%) | 22 (38.6%) * | 8 (17.8%) | 18 (38.3%) * |
| C-like Type Neurons | |||||
| n | 38 | 28 | 23 | 37 | 35 |
| RMP (mV) | 51.7 ± 1.4 | 52.0 ± 1.6 | 47.0 ± 1.6 * | 53.1 ± 1.2 | 48.4 ± 0.9 * |
| APA (mV) | 65.1 ± 1.8 | 64.6 ± 2.3 | 60.2 ± 2.5 | 66.2 ± 1.6 | 61.7 ± 1.4 |
| APD (ms) | 11.7 ± 0.4 | 12.1 ± 0.4 | 13.2 ± 0.6 * | 13.1 ± 0.4* | 12.9 ± 0.4 * |
| Threshold current (nA) | 0.38 ± 0.04 | 0.39 ± 0.04 | 0.25 ± 0.04 ** | 0.36 ± 0.02 | 0.25 ± 0.03 ** |
| Ri (MΩ) | 46.6 ± 0.09 | 45.9 ± 1.1 | 49.9 ± 1.2 * | 43.5 ± 1.0 | 49.8 ± 1.1 * |
| SPS | 1 (2.6%) | 0 | 1 (4.3%) | 0 | 3 (8.6%) |
| SPO | 7 (18.4%) | 5 (17.8%) | 10 (41.7%) * | 6 (16.2%) | 14 (40%) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanani, M.; Spray, D.C.; Huang, T.-Y. Age-Related Changes in Neurons and Satellite Glial Cells in Mouse Dorsal Root Ganglia. Int. J. Mol. Sci. 2023, 24, 2677. https://doi.org/10.3390/ijms24032677
Hanani M, Spray DC, Huang T-Y. Age-Related Changes in Neurons and Satellite Glial Cells in Mouse Dorsal Root Ganglia. International Journal of Molecular Sciences. 2023; 24(3):2677. https://doi.org/10.3390/ijms24032677
Chicago/Turabian StyleHanani, Menachem, David C. Spray, and Tian-Ying Huang. 2023. "Age-Related Changes in Neurons and Satellite Glial Cells in Mouse Dorsal Root Ganglia" International Journal of Molecular Sciences 24, no. 3: 2677. https://doi.org/10.3390/ijms24032677
APA StyleHanani, M., Spray, D. C., & Huang, T. -Y. (2023). Age-Related Changes in Neurons and Satellite Glial Cells in Mouse Dorsal Root Ganglia. International Journal of Molecular Sciences, 24(3), 2677. https://doi.org/10.3390/ijms24032677







