A Comprehensive Review of mRNA Vaccines
Abstract
1. Introduction
2. The Pharmacology of mRNA Vaccines
2.1. mRNA Structure
2.2. 5′ Cap
2.3. 5′ and 3′ UTRs
2.4. Poly(A) Tail
2.5. Modified Nucleotides
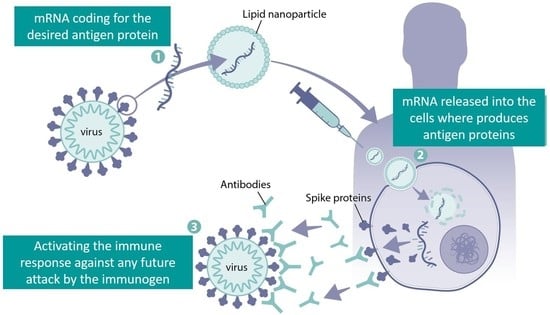
2.6. Innate and Adaptive Immune Stimulation by mRNA Vaccines
3. Drug Delivery Technologies for mRNA Vaccines
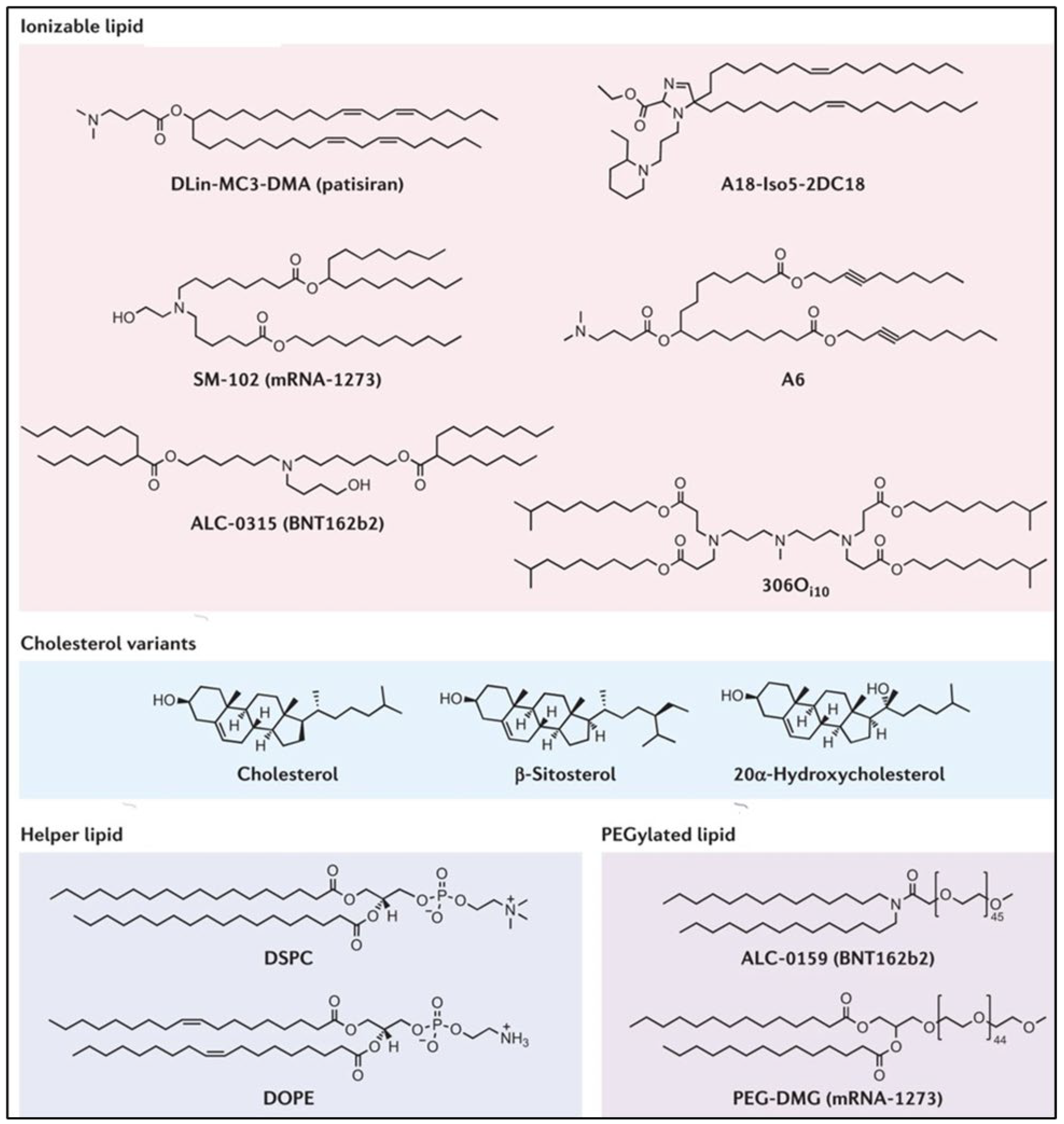
3.1. Lipid Nanoparticles (LNPs)
3.2. Cationic and Ionizable Lipids
3.3. PEG-Lipid
3.4. Helper Lipids
3.5. Physicochemical Properties Affecting mRNA-LNPs
4. mRNA Vaccines Manufacturing
4.1. Upstream Production
4.2. Downstream Purification
4.3. Formulation
5. mRNA Vaccines in Clinical Trials
5.1. mRNA-1345
5.2. mRNA-1010
5.3. mRNA-1647
5.4. Clinical Safety of mRNA-Based Vaccines
6. Secondgeneration mRNA Vaccines
6.1. Lyophilized mRNA Lipid Nanoparticles
6.2. Polymer Nanocarriers
6.3. Incorporation of Adjuvants to Lipid Nanoparticles
6.4. Antigen-Presenting Cells Targeting
6.5. Self-Amplifying mRNA Vaccines
7. Shortcomings of mRNA Vaccines
7.1. Duration of Antibody Response
7.2. Safety
7.3. Maternal/Neonatal Vaccination
7.4. Geriatric Vaccinations
7.5. Vaccine Acceptance
7.6. Access to Vaccines
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plotkin, S. History of Vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 12283–12287. [Google Scholar] [CrossRef]
- Bloom, D.E.; Fan, V.Y.; Sevilla, J.P. The Broad Socioeconomic Benefits of Vaccination. Sci. Transl. Med. 2018, 10, eaaj2345. [Google Scholar] [CrossRef] [PubMed]
- Zwerling, A.; Behr, M.A.; Verma, A.; Brewer, T.F.; Menzies, D.; Pai, M. The BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices. PLoS Med. 2011, 8, e1001012. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J.-G.; Lévy, J.-P.; Meulien, P. Induction of Virus-Specific Cytotoxic T Lymphocytes in Vivo by Liposome-Entrapped MRNA. Eur. J. Immunol. 1993, 23, 1719–1722. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines—A New Era in Vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Jirikowski, G.F.; Sanna, P.P.; Maciejewski-Lenoir, D.; Bloom, F.E. Reversal of Diabetes Insipidus in Brattleboro Rats: Intrahypothalamic Injection of Vasopressin MRNA. Science 1992, 255, 996–998. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct Gene Transfer into Mouse Muscle in Vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef]
- Kis, Z.; Kontoravdi, C.; Dey, A.K.; Shattock, R.; Shah, N. Rapid Development and Deployment of High-Volume Vaccines for Pandemic Response. J. Adv. Manuf. Process. 2020, 2, e10060. [Google Scholar] [CrossRef] [PubMed]
- Freyn, A.W.; Ramos da Silva, J.; Rosado, V.C.; Bliss, C.M.; Pine, M.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; de Souza Ferreira, L.C.; Weissman, D.; et al. A Multi-Targeting, Nucleoside-Modified MRNA Influenza Virus Vaccine Provides Broad Protection in Mice. Mol. Ther. 2020, 28, 1569–1584. [Google Scholar] [CrossRef]
- Ramanathan, A.; Robb, G.B.; Chan, S.H. MRNA Capping: Biological Functions and Applications. Nucleic Acids Res. 2016, 44, 7511–7526. [Google Scholar] [CrossRef]
- Daffis, S.; Szretter, K.J.; Schriewer, J.; Li, J.; Youn, S.; Errett, J.; Lin, T.Y.; Schneller, S.; Zust, R.; Dong, H.; et al. 2’-O Methylation of the Viral MRNA Cap Evades Host Restriction by IFIT Family Members. Nature 2010, 468, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Cowling, V.H. Regulation of MRNA Cap Methylation. Biochem. J. 2009, 425, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Murai, R.; Hagiwara, H.; Hoshino, T.; Suyama, K. Preparation of Eukaryotic MRNA Having Differently Methylated Adenosine at the 5’-Terminus and the Effect of the Methyl Group in Translation. In Nucleic Acids Symposium Series; Oxford University Press: Oxford, UK, 2009; pp. 129–130. [Google Scholar] [CrossRef]
- Sikorski, P.J.; Warminski, M.; Kubacka, D.; Ratajczak, T.; Nowis, D.; Kowalska, J.; Jemielity, J. The Identity and Methylation Status of the First Transcribed Nucleotide in Eukaryotic MRNA 5′ Cap Modulates Protein Expression in Living Cells. Nucleic Acids Res. 2020, 48, 1607–1626. [Google Scholar] [CrossRef]
- Chatterjee, S.; Pal, J.K. Role of 5’- and 3’-Untranslated Regions of MRNAs in Human Diseases. Biol. Cell 2009, 101, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Carralot, J.P.; Probst, J.; Hoerr, I.; Scheel, B.; Teufel, R.; Jung, G.; Rammensee, H.G.; Pascolo, S. Polarization of Immunity Induced by Direct Injection of Naked Sequence-Stabilized MRNA Vaccines. Cell. Mol. Life Sci. 2004, 61, 2418–2424. [Google Scholar] [CrossRef]
- Babendure, J.R.; Babendure, J.L.; Ding, J.H.; Tsien, R.Y. Control of Mammalian Translation by MRNA Structure near Caps. RNA 2006, 12, 851–861. [Google Scholar] [CrossRef]
- Zeng, C.; Hou, X.; Yan, J.; Zhang, C.; Li, W.; Zhao, W.; Du, S.; Dong, Y. Leveraging MRNA Sequences and Nanoparticles to Deliver SARS-CoV-2 Antigens In Vivo. Adv. Mater. 2020, 32, 2004452. [Google Scholar] [CrossRef]
- Eckmann, C.R.; Rammelt, C.; Wahle, E. Control of Poly(A) Tail Length. Wiley Interdiscip. Rev. RNA 2011, 2, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Godiska, R.; Mead, D.; Dhodda, V.; Wu, C.; Hochstein, R.; Karsi, A.; Usdin, K.; Entezam, A.; Ravin, N. Linear Plasmid Vector for Cloning of Repetitive or Unstable Sequences in Escherichia coli. Nucleic Acids Res. 2010, 38, e88. [Google Scholar] [CrossRef]
- Karikó, K.; Weissman, D. Naturally Occurring Nucleoside Modifications Suppress the Immunostimulatory Activity of RNA: Implication for Therapeutic RNA Development. Curr. Opin. Drug Discov. Dev. 2007, 10, 523–532. [Google Scholar]
- Oberli, M.A.; Reichmuth, A.M.; Dorkin, J.R.; Mitchell, M.J.; Fenton, O.S.; Jaklenec, A.; Anderson, D.G.; Langer, R.; Blankschtein, D. Lipid Nanoparticle Assisted MRNA Delivery for Potent Cancer Immunotherapy. Nano Lett. 2017, 17, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Karikó, K. Incorporation of Pseudouridine into MRNA Enhances Translation by Diminishing PKR Activation. Nucleic Acids Res. 2010, 38, 5884. [Google Scholar] [CrossRef]
- Andries, O.; Mc Cafferty, S.; De Smedt, S.C.; Weiss, R.; Sanders, N.N.; Kitada, T. N(1)-Methylpseudouridine-Incorporated MRNA Outperforms Pseudouridine-Incorporated MRNA by Providing Enhanced Protein Expression and Reduced Immunogenicity in Mammalian Cell Lines and Mice. J. Control. Release 2015, 217, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Eygeris, Y.; Gupta, M.; Sahay, G. Self-Assembled MRNA Vaccines. Adv. Drug Deliv. Rev. 2021, 170, 83–112. [Google Scholar] [CrossRef] [PubMed]
- Raeven, R.H.M.; van Riet, E.; Meiring, H.D.; Metz, B.; Kersten, G.F.A. Systems Vaccinology and Big Data in the Vaccine Development Chain. Immunology 2019, 156, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, K.E.; Bhosle, S.M.; Zurla, C.; Beyersdorf, J.; Rogers, K.A.; Vanover, D.; Xiao, P.; Araínga, M.; Shirreff, L.M.; Pitard, B.; et al. Visualization of Early Events in MRNA Vaccine Delivery in Non-Human Primates via PET–CT and near-Infrared Imaging. Nat. Biomed. Eng. 2019, 3, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, S.; Giovani, C.; Mangiavacchi, S.; Magini, D.; Maione, D.; Baudner, B.; Geall, A.J.; De Gregorio, E.; D’Oro, U.; Buonsanti, C. CD8 T-Cell Priming upon MRNA Vaccination Is Restricted to Bone-Marrow-Derived Antigen-Presenting Cells and May Involve Antigen Transfer from Myocytes. Immunology 2015, 146, 312–326. [Google Scholar] [CrossRef]
- Alberer, M.; Gnad-Vogt, U.; Hong, H.S.; Mehr, K.T.; Backert, L.; Finak, G.; Gottardo, R.; Bica, M.A.; Garofano, A.; Koch, S.D.; et al. Safety and Immunogenicity of a MRNA Rabies Vaccine in Healthy Adults: An Open-Label, Non-Randomised, Prospective, First-in-Human Phase 1 Clinical Trial. Lancet 2017, 390, 1511–1520. [Google Scholar] [CrossRef]
- Firdessa-Fite, R.; Creusot, R.J. Nanoparticles versus Dendritic Cells as Vehicles to Deliver MRNA Encoding Multiple Epitopes for Immunotherapy. Mol. Ther. Methods Clin. Dev. 2019, 16, 50–62. [Google Scholar] [CrossRef]
- Heine, A.; Juranek, S.; Brossart, P. Clinical and Immunological Effects of MRNA Vaccines in Malignant Diseases. Mol. Cancer 2021, 20, 52. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for Translation: Non-Viral Materials for Therapeutic MRNA Delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef] [PubMed]
- Rizk, M.; Tüzmen, Ş. Update on the Clinical Utility of an RNA Interference-Based Treatment: Focus on Patisiran. Pharmgenomics Pers. Med. 2017, 10, 267–278.e16. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. MRNA Vaccines for Infectious Diseases: Principles, Delivery and Clinical Translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A Highly Efficient, Lipid-Mediated DNA-Transfection Procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef]
- O’Sullivan, J.; Muñoz-Muñoz, J.; Turnbull, G.; Sim, N.; Penny, S.; Moschos, S. Beyond GalNAc! Drug Delivery Systems Comprising Complex Oligosaccharides for Targeted Use of Nucleic Acid Therapeutics. RSC Adv. 2022, 12, 20432. [Google Scholar] [CrossRef]
- Malone, R.W.; Felgner, P.L.; Verma, I.M. Cationic Liposome-Mediated RNA Transfection. Proc. Natl. Acad. Sci. USA 1989, 86, 6077. [Google Scholar] [CrossRef]
- Pardi, N.; Tuyishime, S.; Muramatsu, H.; Kariko, K.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Weissman, D. Expression Kinetics of Nucleoside-Modified MRNA Delivered in Lipid Nanoparticles to Mice by Various Routes. J. Control. Release 2015, 217, 345–351. [Google Scholar] [CrossRef]
- Granot, Y.; Peer, D. Delivering the Right Message: Challenges and Opportunities in Lipid Nanoparticles-Mediated Modified MRNA Therapeutics-An Innate Immune System Standpoint. Semin. Immunol. 2017, 34, 68–77. [Google Scholar] [CrossRef]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro Story and the Clinical Translation of Nanomedicines Containing Nucleic Acid-Based Drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Heyes, J.; Palmer, L.; Bremner, K.; MacLachlan, I. Cationic Lipid Saturation Influences Intracellular Delivery of Encapsulated Nucleic Acids. J. Control. Release 2005, 107, 276–287. [Google Scholar] [CrossRef]
- Zimmermann, T.S.; Lee, A.C.H.; Akinc, A.; Bramlage, B.; Bumcrot, D.; Fedoruk, M.N.; Harborth, J.; Heyes, J.A.; Jeffs, L.B.; John, M.; et al. RNAi-Mediated Gene Silencing in Non-Human Primates. Nature 2006, 441, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Bloom, K.; van den Berg, F.; Arbuthnot, P. Self-Amplifying RNA Vaccines for Infectious Diseases. Gene Ther. 2020, 28, 117–129. [Google Scholar] [CrossRef]
- Lin, P.J.C.; Tam, Y.Y.C.; Hafez, I.; Sandhu, A.; Chen, S.; Ciufolini, M.A.; Nabi, I.R.; Cullis, P.R. Influence of Cationic Lipid Composition on Uptake and Intracellular Processing of Lipid Nanoparticle Formulations of SiRNA. Nanomedicine 2013, 9, 233–246. [Google Scholar] [CrossRef]
- Semple, S.C.; Akinc, A.; Chen, J.; Sandhu, A.P.; Mui, B.L.; Cho, C.K.; Sah, D.W.Y.; Stebbing, D.; Crosley, E.J.; Yaworski, E.; et al. Rational Design of Cationic Lipids for SiRNA Delivery. Nat. Biotechnol. 2010, 28, 172–176. [Google Scholar] [CrossRef]
- Maier, M.A.; Jayaraman, M.; Matsuda, S.; Liu, J.; Barros, S.; Querbes, W.; Tam, Y.K.; Ansell, S.M.; Kumar, V.; Qin, J.; et al. Biodegradable Lipids Enabling Rapidly Eliminated Lipid Nanoparticles for Systemic Delivery of RNAi Therapeutics. Mol. Ther. 2013, 21, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jozic, A.; Sahay, G. Naturally Derived Membrane Lipids Impact Nanoparticle-Based Messenger RNA Delivery. Cell. Mol. Bioeng. 2020, 13, 463. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Ryals, R.C.; Weller, K.K.; Pennesi, M.E.; Sahay, G. Lipid Nanoparticles for Delivery of Messenger RNA to the Back of the Eye. J. Control. Release 2019, 303, 91–100. [Google Scholar] [CrossRef]
- Robinson, E.; MacDonald, K.D.; Slaughter, K.; McKinney, M.; Patel, S.; Sun, C.; Sahay, G. Lipid Nanoparticle-Delivered Chemically Modified MRNA Restores Chloride Secretion in Cystic Fibrosis. Mol. Ther. 2018, 26, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Sedic, M.; Senn, J.J.; Lynn, A.; Laska, M.; Smith, M.; Platz, S.J.; Bolen, J.; Hoge, S.; Bulychev, A.; Jacquinet, E.; et al. Safety Evaluation of Lipid Nanoparticle-Formulated Modified MRNA in the Sprague-Dawley Rat and Cynomolgus Monkey. Vet. Pathol. 2018, 55, 341–354. [Google Scholar] [CrossRef]
- Veiga, N.; Goldsmith, M.; Granot, Y.; Rosenblum, D.; Dammes, N.; Kedmi, R.; Ramishetti, S.; Peer, D. Cell Specific Delivery of Modified MRNA Expressing Therapeutic Proteins to Leukocytes. Nat. Commun. 2018, 9, 4493. [Google Scholar] [CrossRef] [PubMed]
- Arteta, M.Y.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful Reprogramming of Cellular Protein Production through MRNA Delivered by Functionalized Lipid Nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, J.; Li, M.; Jin, X. Modified MRNA-LNP Vaccines Confer Protection against Experimental DENV-2 Infection in Mice. Mol. Ther. Methods Clin. Dev. 2020, 18, 702. [Google Scholar] [CrossRef]
- Gilham, D.; Lehner, R. Techniques to Measure Lipase and Esterase Activity in Vitro. Methods 2005, 36, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Sabnis, S.; Kumarasinghe, E.S.; Salerno, T.; Mihai, C.; Ketova, T.; Senn, J.J.; Lynn, A.; Bulychev, A.; McFadyen, I.; Chan, J.; et al. A Novel Amino Lipid Series for MRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-Human Primates. Mol. Ther. 2018, 26, 1509–1519. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakurai, Y.; Anindita, J.; Akita, H. Development of Lipid-like Materials for RNA Delivery Based on Intracellular Environment-Responsive Membrane Destabilization and Spontaneous Collapse. Adv. Drug Deliv. Rev. 2020, 154–155, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Hashiba, K.; Sasaki, K.; Maeki, M.; Tokeshi, M.; Harashima, H. Understanding Structure-Activity Relationships of PH-Sensitive Cationic Lipids Facilitates the Rational Identification of Promising Lipid Nanoparticles for Delivering SiRNAs in Vivo. J. Control. Release 2019, 295, 140–152. [Google Scholar] [CrossRef]
- Shobaki, N.; Sato, Y.; Suzuki, Y.; Okabe, N.; Harashima, H. Manipulating the Function of Tumor-Associated Macrophages by SiRNA-Loaded Lipid Nanoparticles for Cancer Immunotherapy. J. Control. Release 2020, 325, 235–248. [Google Scholar] [CrossRef]
- Mahon, K.P.; Love, K.T.; Whitehead, K.A.; Qin, J.; Akinc, A.; Leshchiner, E.; Leshchiner, I.; Langer, R.; Anderson, D.G. A Combinatorial Approach to Determine Functional Group Effects on Lipidoid-Mediated SiRNA Delivery. Bioconjug. Chem. 2010, 21, 1448. [Google Scholar] [CrossRef]
- Akinc, A.; Zumbuehl, A.; Goldberg, M.; Leshchiner, E.S.; Busini, V.; Hossain, N.; Bacallado, S.A.; Nguyen, D.N.; Fuller, J.; Alvarez, R.; et al. A Combinatorial Library of Lipid-like Materials for Delivery of RNAi Therapeutics. Nat. Biotechnol. 2008, 26, 561–569. [Google Scholar] [CrossRef]
- Love, K.T.; Mahon, K.P.; Levins, C.G.; Whitehead, K.A.; Querbes, W.; Dorkin, J.R.; Qin, J.; Cantley, W.; Qin, L.L.; Racie, T.; et al. Lipid-like Materials for Low-Dose, in Vivo Gene Silencing. Proc. Natl. Acad. Sci. USA 2010, 107, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, I.C.; Eltoukhy, A.A.; Fish, K.M.; Nonnenmacher, M.; Ishikawa, K.; Chen, J.; Hajjar, R.J.; Anderson, D.G.; Costa, K.D. Myocardial Delivery of Lipidoid Nanoparticle Carrying ModRNA Induces Rapid and Transient Expression. Mol. Ther. 2016, 24, 66. [Google Scholar] [CrossRef]
- Jiang, C.; Mei, M.; Li, B.; Zhu, X.; Zu, W.; Tian, Y.; Wang, Q.; Guo, Y.; Dong, Y.; Tan, X. A Non-Viral CRISPR/Cas9 Delivery System for Therapeutically Targeting HBV DNA and Pcsk9 in Vivo. Cell Res. 2017, 27, 440–443. [Google Scholar] [CrossRef]
- Li, B.; Luo, X.; Deng, B.; Wang, J.; McComb, D.W.; Shi, Y.; Gaensler, K.M.L.; Tan, X.; Dunn, A.L.; Kerlin, B.A.; et al. An Orthogonal Array Optimization of Lipid-like Nanoparticles for MRNA Delivery in Vivo. Nano Lett. 2015, 15, 8099–8107. [Google Scholar] [CrossRef] [PubMed]
- Lokugamage, M.P.; Sago, C.D.; Gan, Z.; Krupczak, B.R.; Dahlman, J.E.; Lokugamage, M.P.; Sago, C.D.; Gan, Z.; Krupczak, B.R.; Dahlman, J.E.; et al. Constrained Nanoparticles Deliver SiRNA and SgRNA to T Cells In Vivo without Targeting Ligands. Adv. Mater. 2019, 31, 1902251. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of MRNA Vaccines with Heterocyclic Lipids Increases Anti-Tumor Efficacy by STING-Mediated Immune Cell Activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, X.; Zhao, W.; Zeng, C.; Deng, B.; McComb, D.W.; Du, S.; Zhang, C.; Li, W.; Dong, Y. Vitamin Lipid Nanoparticles Enable Adoptive Macrophage Transfer for the Treatment of Multidrug-Resistant Bacterial Sepsis. Nat. Nanotechnol. 2020, 15, 41–46. [Google Scholar] [CrossRef]
- Ho, W.; Gao, M.; Li, F.; Li, Z.; Zhang, X.Q.; Xu, X. Next-Generation Vaccines: Nanoparticle-Mediated DNA and MRNA Delivery. Adv. Healthc. Mater. 2021, 10, 2001812. [Google Scholar] [CrossRef] [PubMed]
- Heyes, J.; Hall, K.; Tailor, V.; Lenz, R.; MacLachlan, I. Synthesis and Characterization of Novel Poly(Ethylene Glycol)-Lipid Conjugates Suitable for Use in Drug Delivery. J. Control. Release 2006, 112, 280–290. [Google Scholar] [CrossRef]
- Mui, B.L.; Tam, Y.K.; Jayaraman, M.; Ansell, S.M.; Du, X.; Tam, Y.Y.; Lin, P.J.; Chen, S.; Narayanannair, J.K.; Rajeev, K.G.; et al. Influence of Polyethylene Glycol Lipid Desorption Rates on Pharmacokinetics and Pharmacodynamics of SiRNA Lipid Nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, e139. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; He, Y.; Shi, K. Cleavable PEGylation: A Strategy for Overcoming the “PEG Dilemma” in Efficient Drug Delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef]
- Leung, A.K.K.; Tam, Y.Y.C.; Cullis, P.R. Lipid Nanoparticles for Short Interfering RNA Delivery. Adv. Genet. 2014, 88, 71–110. [Google Scholar] [CrossRef] [PubMed]
- Paunovska, K.; Da Silva Sanchez, A.J.; Sago, C.D.; Gan, Z.; Lokugamage, M.P.; Islam, F.Z.; Kalathoor, S.; Krupczak, B.R.; Dahlman, J.E. Nanoparticles Containing Oxidized Cholesterol Deliver MRNA to the Liver Microenvironment at Clinically Relevant Doses. Adv. Mater. 2019, 31, 1807748. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Xia, Y.; Mihai, C.; Griffith, J.P.; Hou, S.; Esposito, A.A.; Ketova, T.; Welsher, K.; et al. Naturally-Occurring Cholesterol Analogues in Lipid Nanoparticles Induce Polymorphic Shape and Enhance Intracellular Delivery of MRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28. [Google Scholar] [CrossRef]
- Tusup, M.; French, L.E.; De Matos, M.; Gatfield, D.; Kundig, T.; Pascolo, S. Design of in Vitro Transcribed MRNA Vectors for Research and Therapy. Chimia 2019, 73, 391–394. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, M.; Seo, Y.; Moon, Y.S.; Lee, H.J.; Lee, K.; Lee, H. Emergence of Synthetic MRNA: In Vitro Synthesis of MRNA and Its Applications in Regenerative Medicine. Biomaterials 2018, 156, 172–193. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Ludwig, J.; Weissman, D. Generating the Optimal MRNA for Therapy: HPLC Purification Eliminates Immune Activation and Improves Translation of Nucleoside-Modified, Protein-Encoding MRNA. Nucleic Acids Res. 2011, 39, e142. [Google Scholar] [CrossRef]
- Pascolo, S. Messenger RNA-Based Vaccines. Expert Opin. Biol. Ther. 2004, 4, 1285–1294. [Google Scholar] [CrossRef]
- Lukavsky, P.J.; Puglisi, J.D. Large-Scale Preparation and Purification of Polyacrylamide-Free RNA Oligonucleotides. RNA 2004, 10, 889–893. [Google Scholar] [CrossRef]
- McKenna, S.A.; Kim, I.; Puglisi, E.V.; Lindhout, D.A.; Aitken, C.E.; Marshall, R.A.; Puglisi, J.D. Purification and Characterization of Transcribed RNAs Using Gel Filtration Chromatography. Nat. Protoc. 2007, 2, 3270–3277. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D.; Pardi, N.; Muramatsu, H.; Karikó, K. HPLC Purification of in Vitro Transcribed Long RNA. Methods Mol. Biol. 2013, 969, 43–54. [Google Scholar] [CrossRef]
- Henninger, H.P.; Hoffmann, R.; Grewe, M.; Schulze-Specking, A.; Decker, K. Purification and Quantitative Analysis of Nucleic Acids by Anion-Exchange High-Performance Liquid Chromatography. Biol. Chem. Hoppe. Seyler. 1993, 374, 625–634. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Isolation of Poly(A)+ Messenger RNA Using Magnetic Oligo(DT) Beads. Cold Spring Harb. Protoc. 2019, 2019, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Rosa, S.S.; Prazeres, D.M.F.; Azevedo, A.M.; Marques, M.P.C. MRNA Vaccines Manufacturing: Challenges and Bottlenecks. Vaccine 2021, 39, 2190. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Issadore, D.; Mitchell, M.J. Microfluidic Formulation of Nanoparticles for Biomedical Applications. Biomaterials 2021, 274, 120826. [Google Scholar] [CrossRef]
- Zhang, N.N.; Li, X.F.; Deng, Y.Q.; Zhao, H.; Huang, Y.J.; Yang, G.; Huang, W.J.; Gao, P.; Zhou, C.; Zhang, R.R.; et al. A Thermostable MRNA Vaccine against COVID-19. Cell 2020, 182, 1271–1283.e16. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Carrasco, M.J.; Alishetty, S.; Paige, M.; Alameh, M.G.; Weissman, D. Nanomaterial Delivery Systems for MRNA Vaccines. Vaccines 2021, 9, 65. [Google Scholar] [CrossRef]
- Pilkington, E.H.; Suys, E.J.A.; Trevaskis, N.L.; Wheatley, A.K.; Zukancic, D.; Algarni, A.; Al-Wassiti, H.; Davis, T.P.; Pouton, C.W.; Kent, S.J.; et al. From Influenza to COVID-19: Lipid Nanoparticle MRNA Vaccines at the Frontiers of Infectious Diseases. Acta Biomater. 2021, 131, 16–40. [Google Scholar] [CrossRef]
- Knezevic, I.; Liu, M.A.; Peden, K.; Zhou, T.; Kang, H.N. Development of MRNA Vaccines: Scientific and Regulatory Issues. Vaccines 2021, 9, 81. [Google Scholar] [CrossRef]
- Barbier, A.J.; Jiang, A.Y.; Zhang, P.; Wooster, R.; Anderson, D.G. The Clinical Progress of MRNA Vaccines and Immunotherapies. Nat. Biotechnol. 2022, 40, 840–854. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid Nanoparticles for MRNA Delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.S. From COVID-19 to Cancer MRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape. Front. Immunol. 2021, 12, 2648. [Google Scholar] [CrossRef] [PubMed]
- Nitika; Wei, J.; Hui, A.M. The Development of MRNA Vaccines for Infectious Diseases: Recent Updates. Infect. Drug Resist. 2021, 14, 5271. [Google Scholar] [CrossRef]
- Bilotta, C.; Perrone, G.; Adelfio, V.; Spatola, G.F.; Uzzo, M.L.; Argo, A.; Zerbo, S. COVID-19 Vaccine-Related Thrombosis: A Systematic Review and Exploratory Analysis. Front. Immunol. 2021, 12, 729251. [Google Scholar] [CrossRef]
- Mahase, E. COVID-19: Pfizer and BioNTech Submit Vaccine for US Authorisation. BMJ 2020, 371, m4552. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Machhi, J.; Shahjin, F.; Das, S.; Patel, M.; Abdelmoaty, M.M.; Cohen, J.D.; Singh, P.A.; Baldi, A.; Bajwa, N.; Kumar, R.; et al. Nanocarrier Vaccines for SARS-CoV-2. Adv. Drug Deliv. Rev. 2021, 171, 215–239. [Google Scholar] [CrossRef]
- Abdelwahed, W.; Degobert, G.; Stainmesse, S.; Fessi, H. Freeze-Drying of Nanoparticles: Formulation, Process and Storage Considerations. Adv. Drug Deliv. Rev. 2006, 58, 1688–1713. [Google Scholar] [CrossRef]
- Yan, L.L.; Zaher, H.S. How Do Cells Cope with RNA Damage and Its Consequences? J. Biol. Chem. 2019, 294, 15158. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. MRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Rehman, M.U.; Khan, M.A.; Khan, W.S.; Shafique, M.; Khan, M. Fabrication of Niclosamide Loaded Solid Lipid Nanoparticles: In Vitro Characterization and Comparative in Vivo Evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1926–1934. [Google Scholar] [CrossRef]
- Butreddy, A.; Dudhipala, N.; Janga, K.Y.; Gaddam, R.P. Lyophilization of Small-Molecule Injectables: An Industry Perspective on Formulation Development, Process Optimization, Scale-Up Challenges, and Drug Product Quality Attributes. AAPS PharmSciTech 2020, 21, 252. [Google Scholar] [CrossRef]
- Wenzel, T.; Gieseler, H. Evaluation of Packaging Materials in Freeze-Drying: Use of Polymer Caps and Nested Vials and Their Impact on Process and Product Attributes. AAPS PharmSciTech 2021, 22, 82. [Google Scholar] [CrossRef]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef]
- Shirane, D.; Tanaka, H.; Nakai, Y.; Yoshioka, H.; Akita, H. Development of an Alcohol Dilution-Lyophilization Method for Preparing Lipid Nanoparticles Containing Encapsulated SiRNA. Biol. Pharm. Bull. 2018, 41, 1291–1294. [Google Scholar] [CrossRef]
- Chen, C.; Han, D.; Cai, C.; Tang, X. An Overview of Liposome Lyophilization and Its Future Potential. J. Control. Release 2010, 142, 299–311. [Google Scholar] [CrossRef]
- Zhao, P.; Hou, X.; Yan, J.; Du, S.; Xue, Y.; Li, W.; Xiang, G.; Dong, Y. Long-Term Storage of Lipid-like Nanoparticles for MRNA Delivery. Bioact. Mater. 2020, 5, 358–363. [Google Scholar] [CrossRef]
- Hong, H.C.; Kim, K.S.; Park, S.A.; Chun, M.J.; Hong, E.Y.; Chung, S.W.; Kim, H.J.; Shin, B.G.; Braka, A.; Thanappan, J.; et al. An MRNA Vaccine against SARS-CoV-2: Lyophilized, Liposome-Based Vaccine Candidate EG-COVID Induces High Levels of Virus Neutralizing Antibodies. bioRxiv 2021. [Google Scholar] [CrossRef]
- Bhatnagar, B.S.; Bogner, R.H.; Pikal, M.J. Protein Stability during Freezing: Separation of Stresses and Mechanisms of Protein Stabilization. Pharm. Dev. Technol. 2007, 12, 505–523. [Google Scholar] [CrossRef]
- Muldrew, K.; McGann, L.E. The Osmotic Rupture Hypothesis of Intracellular Freezing Injury. Biophys. J. 1994, 66, 532–541. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Friess, W. Freeze-Drying of Nanoparticles: How to Overcome Colloidal Instability by Formulation and Process Optimization. Eur. J. Pharm. Biopharm. 2021, 165, 345–360. [Google Scholar] [CrossRef]
- Luthra, S.; Obert, J.P.; Kalonia, D.S.; Pikal, M.J. Investigation of Drying Stresses on Proteins during Lyophilization: Differentiation between Primary and Secondary-Drying Stresses on Lactate Dehydrogenase Using a Humidity Controlled Mini Freeze-Dryer. J. Pharm. Sci. 2007, 96, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Janga, K.Y.; Ajjarapu, S.; Sarabu, S.; Dudhipala, N. Instability of Therapeutic Proteins—An Overview of Stresses, Stabilization Mechanisms and Analytical Techniques Involved in Lyophilized Proteins. Int. J. Biol. Macromol. 2021, 167, 309–325. [Google Scholar] [CrossRef]
- Jones, K.L.; Drane, D.; Gowans, E.J. Long-Term Storage of DNA-Free RNA for Use in Vaccine Studies. Biotechniques 2007, 43, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, H.; Lam, K.; Bajusz, C.; Laczkó, D.; Karikó, K.; Schreiner, P.; Martin, A.; Lutwyche, P.; Heyes, J.; Pardi, N. Lyophilization Provides Long-Term Stability for a Lipid Nanoparticle-Formulated, Nucleoside-Modified MRNA Vaccine. Mol. Ther. 2022, 30, 1941–1951. [Google Scholar] [CrossRef] [PubMed]
- Crowe, L.M.; Crowe, J.H. Trehalose and Dry Dipalmitoylphosphatidylcholine Revisited. Biochim. Biophys. Acta 1988, 946, 193–201. [Google Scholar] [CrossRef]
- Crowe, J.H.; Hoekstra, F.A.; Nguyen, K.H.N.; Crowe, L.M. Is Vitrification Involved in Depression of the Phase Transition Temperature in Dry Phospholipids? Biochim. Biophys. Acta 1996, 1280, 187–196. [Google Scholar] [CrossRef]
- Koster, K.L.; Webb, M.S.; Bryant, G.; Lynch, D.V. Interactions between Soluble Sugars and POPC (1-Palmitoyl-2-Oleoylphosphatidylcholine) during Dehydration: Vitrification of Sugars Alters the Phase Behavior of the Phospholipid. Biochim. Biophys. Acta 1994, 1193, 143–150. [Google Scholar] [CrossRef]
- Wolfe, J.; Bryant, G. Freezing, Drying, and/or Vitrification of Membrane- Solute-Water Systems. Cryobiology 1999, 39, 103–129. [Google Scholar] [CrossRef]
- Yoon, Y.H.; Pope, J.M.; Wolfe, J. The Effects of Solutes on the Freezing Properties of and Hydration Forces in Lipid Lamellar Phases. Biophys. J. 1998, 74, 1949–1965. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Blenner, M.; Alexander-Bryant, A.; Larsen, J. Polymersomes for Therapeutic Delivery of Protein and Nucleic Acid Macromolecules: From Design to Therapeutic Applications. Biomacromolecules 2020, 21, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Palamà, I.E.; Cortese, B.; D’Amone, S.; Gigli, G. MRNA Delivery Using Non-Viral PCL Nanoparticles. Biomater. Sci. 2015, 3, 144–151. [Google Scholar] [CrossRef]
- Paloncýová, M.; Čechová, P.; Šrejber, M.; Kührová, P.; Otyepka, M. Role of Ionizable Lipids in SARS-CoV-2 Vaccines As Revealed by Molecular Dynamics Simulations: From Membrane Structure to Interaction with MRNA Fragments. J. Phys. Chem. Lett. 2021, 12, 11199–11205. [Google Scholar] [CrossRef]
- Yan, J.; Chen, R.; Zhang, H.; Bryers, J.D. Injectable Biodegradable Chitosan-Alginate 3D Porous Gel Scaffold for MRNA Vaccine Delivery. Macromol. Biosci. 2019, 19, e1800242. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.X.; Szoka, F.C. The Influence of Polymer Structure on the Interactions of Cationic Polymers with DNA and Morphology of the Resulting Complexes. Gene Ther. 1997, 4, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhao, M.; Fu, Y.; Li, Y.; Gong, T.; Zhang, Z.; Sun, X. Enhanced Intranasal Delivery of MRNA Vaccine by Overcoming the Nasal Epithelial Barrier via Intra- and Paracellular Pathways. J. Control. Release 2016, 228, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Zheng, T.; Li, M.; Zhong, X.; Tang, Y.; Qin, M.; Sun, X. Optimization of an MRNA Vaccine Assisted with Cyclodextrin-Polyethyleneimine Conjugates. Drug Deliv. Transl. Res. 2020, 10, 678–689. [Google Scholar] [CrossRef] [PubMed]
- Ulkoski, D.; Bak, A.; Wilson, J.T.; Krishnamurthy, V.R. Recent Advances in Polymeric Materials for the Delivery of RNA Therapeutics. Expert Opin. Drug Deliv. 2019, 16, 1149–1167. [Google Scholar] [CrossRef]
- Alameh, M.; Lavertu, M.; Tran-Khanh, N.; Chang, C.Y.; Lesage, F.; Bail, M.; Darras, V.; Chevrier, A.; Buschmann, M.D. SiRNA Delivery with Chitosan: Influence of Chitosan Molecular Weight, Degree of Deacetylation, and Amine to Phosphate Ratio on in Vitro Silencing Efficiency, Hemocompatibility, Biodistribution, and in Vivo Efficacy. Biomacromolecules 2018, 19, 112–131. [Google Scholar] [CrossRef]
- Lallana, E.; Rios De La Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef] [PubMed]
- Chahal, J.S.; Khan, O.F.; Cooper, C.L.; McPartlan, J.S.; Tsosie, J.K.; Tilley, L.D.; Sidik, S.M.; Lourido, S.; Langer, R.; Bavari, S.; et al. Dendrimer-RNA Nanoparticles Generate Protective Immunity against Lethal Ebola, H1N1 Influenza, and Toxoplasma Gondii Challenges with a Single Dose. Proc. Natl. Acad. Sci. USA 2016, 113, E4133–E4142. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, M.D.; Amiji, M.M. Development of Novel Biodegradable Polymeric Nanoparticles-in-Microsphere Formulation for Local Plasmid DNA Delivery in the Gastrointestinal Tract. AAPS PharmSciTech 2008, 9, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Karpenko, L.I.; Rudometov, A.P.; Sharabrin, S.V.; Shcherbakov, D.N.; Borgoyakova, M.B.; Bazhan, S.I.; Volosnikova, E.A.; Rudometova, N.B.; Orlova, L.A.; Pyshnaya, I.A.; et al. Delivery of MRNA Vaccine against SARS-CoV-2 Using a Polyglucin:Spermidine Conjugate. Vaccines 2021, 9, 76. [Google Scholar] [CrossRef]
- Jeandupeux, E.; Alameh, M.G.; Ghattas, M.; De Crescenzo, G.; Lavertu, M. Poly(2-Propylacrylic Acid) Increases In Vitro Bioactivity of Chitosan/MRNA Nanoparticles. J. Pharm. Sci. 2021, 110, 3439–3449. [Google Scholar] [CrossRef]
- Chatzikleanthous, D.; O’Hagan, D.T.; Adamo, R. Lipid-Based Nanoparticles for Delivery of Vaccine Adjuvants and Antigens: Toward Multicomponent Vaccines. Mol. Pharm. 2021, 18, 2867–2888. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like Receptors and Their Crosstalk with Other Innate Receptors in Infection and Immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Yu, X.; Dai, Y.; Zhao, Y.; Qi, S.; Liu, L.; Lu, L.; Luo, Q.; Zhang, Z. Melittin-Lipid Nanoparticles Target to Lymph Nodes and Elicit a Systemic Anti-Tumor Immune Response. Nat. Commun. 2020, 11, 1110. [Google Scholar] [CrossRef]
- Ravindran, R.; Maji, M.; Ali, N. Vaccination with Liposomal Leishmanial Antigens Adjuvanted with Monophosphoryl Lipid-Trehalose Dicorynomycolate (MPL-TDM) Confers Long-Term Protection against Visceral Leishmaniasis through a Human Administrable Route. Mol. Pharm. 2012, 9, 59–70. [Google Scholar] [CrossRef]
- Chikh, G.; De Jong, S.D.; Sekirov, L.; Raney, S.G.; Kazem, M.; Wilson, K.D.; Cullis, P.R.; Dutz, J.P.; Tam, Y.K. Synthetic Methylated CpG ODNs Are Potent in Vivo Adjuvants When Delivered in Liposomal Nanoparticles. Int. Immunol. 2009, 21, 757–767. [Google Scholar] [CrossRef]
- Lee, K.; Kim, S.Y.; Seo, Y.; Kim, M.H.; Chang, J.; Lee, H. Adjuvant Incorporated Lipid Nanoparticles for Enhanced MRNA-Mediated Cancer Immunotherapy. Biomater. Sci. 2020, 8, 1101–1105. [Google Scholar] [CrossRef]
- Alameh, M.G.; Tombácz, I.; Bettini, E.; Lederer, K.; Sittplangkoon, C.; Wilmore, J.R.; Gaudette, B.T.; Soliman, O.Y.; Pine, M.; Hicks, P.; et al. Lipid Nanoparticles Enhance the Efficacy of MRNA and Protein Subunit Vaccines by Inducing Robust T Follicular Helper Cell and Humoral Responses. Immunity 2021, 54, 2877–2892.e7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; You, X.; Wang, X.; Cui, L.; Wang, Z.; Xu, F.; Li, M.; Yang, Z.; Liu, J.; Huang, P.; et al. Delivery of MRNA Vaccine with a Lipid-like Material Potentiates Antitumor Efficacy through Toll-like Receptor 4 Signaling. Proc. Natl. Acad. Sci. USA 2021, 118, e2005191118. [Google Scholar] [CrossRef] [PubMed]
- Saunders, K.O.; Pardi, N.; Parks, R.; Santra, S.; Mu, Z.; Sutherland, L.; Scearce, R.; Barr, M.; Eaton, A.; Hernandez, G.; et al. Lipid Nanoparticle Encapsulated Nucleoside-Modified MRNA Vaccines Elicit Polyfunctional HIV-1 Antibodies Comparable to Proteins in Nonhuman Primates. npj Vaccines 2021, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Hald Albertsen, C.; Kulkarni, J.A.; Witzigmann, D.; Lind, M.; Petersson, K.; Simonsen, J.B. The Role of Lipid Components in Lipid Nanoparticles for Vaccines and Gene Therapy. Adv. Drug Deliv. Rev. 2022, 188, 114416. [Google Scholar] [CrossRef]
- Singh, A. Eliciting B Cell Immunity against Infectious Diseases Using Nanovaccines. Nat. Nanotechnol. 2020, 16, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.; Chatzikleanthous, D.; Lou, G.; Giusti, F.; Bonci, A.; Taccone, M.; Brazzoli, M.; Gallorini, S.; Ferlenghi, I.; Berti, F.; et al. Mannosylation of LNP Results in Improved Potency for Self-Amplifying RNA (SAM) Vaccines. ACS Infect. Dis. 2019, 5, 1546–1558. [Google Scholar] [CrossRef]
- Zhuang, X.; Qi, Y.; Wang, M.; Yu, N.; Nan, F.; Zhang, H.; Tian, M.; Li, C.; Lu, H.; Jin, N. MRNA Vaccines Encoding the HA Protein of Influenza A H1N1 Virus Delivered by Cationic Lipid Nanoparticles Induce Protective Immune Responses in Mice. Vaccines 2020, 8, 123. [Google Scholar] [CrossRef]
- Irvine, D.J.; Aung, A.; Silva, M. Controlling Timing and Location in Vaccines. Adv. Drug Deliv. Rev. 2020, 158, 91. [Google Scholar] [CrossRef]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the MRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Laczkó, D.; Hogan, M.J.; Toulmin, S.A.; Hicks, P.; Lederer, K.; Gaudette, B.T.; Castaño, D.; Amanat, F.; Muramatsu, H.; Oguin, T.H.; et al. A Single Immunization with Nucleoside-Modified MRNA Vaccines Elicits Strong Cellular and Humoral Immune Responses against SARS-CoV-2 in Mice. Immunity 2020, 53, 724–732.e7. [Google Scholar] [CrossRef] [PubMed]
- Lederer, K.; Castaño, D.; Gómez Atria, D.; Oguin, T.H.; Wang, S.; Manzoni, T.B.; Muramatsu, H.; Hogan, M.J.; Amanat, F.; Cherubin, P.; et al. SARS-CoV-2 MRNA Vaccines Foster Potent Antigen-Specific Germinal Center Responses Associated with Neutralizing Antibody Generation. Immunity 2020, 53, 1281–1295.e5. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Naradikian, M.S.; Parkhouse, K.; Cain, D.W.; Jones, L.; Moody, M.A.; Verkerke, H.P.; Myles, A.; Willis, E.; et al. Nucleoside-Modified MRNA Vaccines Induce Potent T Follicular Helper and Germinal Center B Cell Responses. J. Exp. Med. 2018, 215, 1571–1588. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.; Lazzaro, S.; Habbeddine, M.; Schmidt, K.E.; Baumhof, P.; Mui, B.L.; Tam, Y.K.; Madden, T.D.; Hope, M.J.; Heidenreich, R.; et al. Unmodified MRNA in LNPs Constitutes a Competitive Technology for Prophylactic Vaccines. npj Vaccines 2017, 2, 29. [Google Scholar] [CrossRef]
- Feldman, R.A.; Fuhr, R.; Smolenov, I.; Ribeiro, A.M.; Panther, L.; Watson, M.; Senn, J.J.; Smith, M.; Almarsson, Ö.; Pujar, H.S.; et al. MRNA Vaccines against H10N8 and H7N9 Influenza Viruses of Pandemic Potential Are Immunogenic and Well Tolerated in Healthy Adults in Phase 1 Randomized Clinical Trials. Vaccine 2019, 37, 3326–3334. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, T.T.; Cole, M.; Su, J.R. Reports of Anaphylaxis After Receipt of MRNA COVID-19 Vaccines in the US—December 14, 2020–January 18, 2021. JAMA 2021, 325, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.M.; Weintraub, E.S.; Duffy, J.; Sukumaran, L.; Jacobsen, S.J.; Klein, N.P.; Hambidge, S.J.; Lee, G.M.; Jackson, L.A.; Irving, S.A.; et al. Risk of Anaphylaxis after Vaccination in Children and Adults. J. Allergy Clin. Immunol. 2016, 137, 868–878. [Google Scholar] [CrossRef]
- Besin, G.; Milton, J.; Sabnis, S.; Howell, R.; Mihai, C.; Burke, K.; Benenato, K.E.; Stanton, M.; Smith, P.; Senn, J.; et al. Accelerated Blood Clearance of Lipid Nanoparticles Entails a Biphasic Humoral Response of B-1 Followed by B-2 Lymphocytes to Distinct Antigenic Moieties. ImmunoHorizons 2019, 3, 282–293. [Google Scholar] [CrossRef]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG Antibodies: Properties, Formation, Testing and Role in Adverse Immune Reactions to PEGylated Nano-Biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Yockey, L.J.; Lucas, C.; Iwasaki, A. Contributions of Maternal and Fetal Antiviral Immunity in Congenital Disease. Science 2020, 368, 608–612. [Google Scholar] [CrossRef]
- Barrero-Castillero, A.; Beam, K.S.; Bernardini, L.B.; Ramos, E.G.C.; Davenport, P.E.; Duncan, A.R.; Fraiman, Y.S.; Frazer, L.C.; Healy, H.; Herzberg, E.M.; et al. COVID-19: Neonatal-Perinatal Perspectives. J. Perinatol. 2021, 41, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 Vertical Transmission during Pregnancy. Nat. Commun. 2020, 11, 5128. [Google Scholar] [CrossRef] [PubMed]
- Jagger, B.W.; Dowd, K.A.; Chen, R.E.; Desai, P.; Foreman, B.; Burgomaster, K.E.; Himansu, S.; Kong, W.P.; Graham, B.S.; Pierson, T.C.; et al. Protective Efficacy of Nucleic Acid Vaccines Against Transmission of Zika Virus During Pregnancy in Mice. J. Infect. Dis. 2019, 220, 1577–1588. [Google Scholar] [CrossRef]
- LaTourette, P.C.; Awasthi, S.; Desmond, A.; Pardi, N.; Cohen, G.H.; Weissman, D.; Friedman, H.M. Protection against Herpes Simplex Virus Type 2 Infection in a Neonatal Murine Model Using a Trivalent Nucleoside-Modified MRNA in Lipid Nanoparticle Vaccine. Vaccine 2020, 38, 7409–7413. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Chiarot, E.; Giovani, C.; Buccato, S.; Bonacci, S.; Frigimelica, E.; Margarit, I.; Geall, A.; Bensi, G.; Maione, D. Immunogenicity and Protective Efficacy Induced by Self-Amplifying MRNA Vaccines Encoding Bacterial Antigens. Vaccine 2017, 35, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Richner, J.M.; Jagger, B.W.; Shan, C.; Fontes, C.R.; Dowd, K.A.; Cao, B.; Himansu, S.; Caine, E.A.; Nunes, B.T.D.; Medeiros, D.B.A.; et al. Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease. Cell 2017, 170, 273–283.e12. [Google Scholar] [CrossRef]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence and Human Vaccine Immune Responses. Immun. Ageing 2019, 16, 25. [Google Scholar] [CrossRef]
- Yanez, N.D.; Weiss, N.S.; Romand, J.A.; Treggiari, M.M. COVID-19 Mortality Risk for Older Men and Women. BMC Public Health 2020, 20, 1742. [Google Scholar] [CrossRef]
- Van Den Biggelaar, A.H.J.; Huizinga, T.W.J.; De Craen, A.J.M.; Gussekloo, J.; Heijmans, B.T.; Frölich, M.; Westendorp, R.G.J. Impaired Innate Immunity Predicts Frailty in Old Age. The Leiden 85-plus Study. Exp. Gerontol. 2004, 39, 1407–1414. [Google Scholar] [CrossRef]
- Boucher, N.; Dufeu-Duchesne, T.; Vicaut, E.; Farge, D.; Effros, R.B.; Schächter, F. CD28 Expression in T Cell Aging and Human Longevity. Exp. Gerontol. 1998, 33, 267–282. [Google Scholar] [CrossRef]
- Lazuardi, L.; Jenewein, B.; Wolf, A.M.; Pfister, G.; Tzankov, A.; Grubeck-Loebenstein, B. Age-Related Loss of Naïve T Cells and Dysregulation of T-Cell/B-Cell Interactions in Human Lymph Nodes. Immunology 2005, 114, 37. [Google Scholar] [CrossRef] [PubMed]
- Qi, Q.; Liu, Y.; Cheng, Y.; Glanville, J.; Zhang, D.; Lee, J.Y.; Olshen, R.A.; Weyand, C.M.; Boyd, S.D.; Goronzy, J.J. Diversity and Clonal Selection in the Human T-Cell Repertoire. Proc. Natl. Acad. Sci. USA 2014, 111, 13139–13144. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.F. Fluad®-MF59®-Adjuvanted Influenza Vaccine in Older Adults. Infect. Chemother. 2013, 45, 159–174. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Han, T.; Liu, C.; Li, X.; Yan, L.; Yang, B.; Yang, X. Effectiveness, Immunogenicity, and Safety of Influenza Vaccines with MF59 Adjuvant in Healthy People of Different Age Groups: A Systematic Review and Meta-Analysis. Medicine 2020, 99, e19095. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ratzan, S.C.; Palayew, A.; Gostin, L.O.; Larson, H.J.; Rabin, K.; Kimball, S.; El-Mohandes, A. A Global Survey of Potential Acceptance of a COVID-19 Vaccine. Nat. Med. 2021, 27, 225–228. [Google Scholar] [CrossRef]
- Lippi, G.; Henry, B.M. How Will Emerging SARS-CoV-2 Variants Impact Herd Immunity? Ann. Transl. Med. 2021, 9, 585. [Google Scholar] [CrossRef] [PubMed]
- Sallam, M. COVID-19 Vaccine Hesitancy Worldwide: A Concise Systematic Review of Vaccine Acceptance Rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef]
- Qi, Y.; Fox, C.B. Development of Thermostable Vaccine Adjuvants. Expert Rev. Vaccines 2021, 20, 497. [Google Scholar] [CrossRef] [PubMed]




| Vaccine | Formulation Type/Route of Administration | Indication | Clinical Trial Number | Phase | Sponsor | Status |
|---|---|---|---|---|---|---|
| eOD-GT8 60mer mRNA | Nanoparticle/Intraperitoneal | HIV | NCT05414786 | 1 | International AIDS Vaccine Initiative | Recruiting |
| Core-g28v2 60mer mRNA vaccine and eOD-GT8 60mer mRNA vaccine | Nanoparticle/Intramuscular injection | HIV | NCT05001373 | 1 | International AIDS Vaccine Initiative | Recruiting |
| BG505 MD39.3 mRNA, BG505 MD39.3 gp151 mRNA, and BG505 MD39.3 gp151 CD4KO mRNA | NA/Intramuscular injection | HIV | NCT05217641 | 1 | National Institute of Allergy and Infectious Diseases (NIAID) | Recruiting |
| mRNA-1345 | Lipid nanoparticle/Intramuscular injection | Respiratory Syncytial Virus (RSV) | NCT05127434 | 2/3 | Moderna | Recruiting |
| RSV | NCT04528719 | 1 | Moderna | Recruiting | ||
| mRNA-1345 and mRNA-1273.214 | Lipid nanoparticle/Intramuscular injection | RSV | NCT05330975 | 3 | Moderna | Recruiting |
| Influenza vaccines (mRNA-1020, mRNA-1030, and mRNA-1010) | Lipid nanoparticle/Intramuscular injection | Influenza (A and B strains) | NCT05333289 | 1/2 | Moderna | Recruiting |
| Influenza (A and B strains) | NCT05375838 | 1/2 | Moderna | Recruiting | ||
| mRNA-1010 | Lipid nanoparticle/Intramuscular injection | Seasonal influenza | NCT04956575 | 1/2 | Moderna | Recruiting |
| Seasonal influenza | NCT05415462 | 3 | Moderna | Recruiting | ||
| Influenza vaccines {monovalent influenza modRNA vaccine (mIRV), bivalent influenza modRNA vaccine (bIRV AB, bIRV AA, and bIRV BB) quadrivalent influenza modRNA vaccine (qIRV)} | NA/Intramuscular injection | Influenza | NCT05052697 | 1/2 | Pfizer | Recruiting |
| Seasonal quadrivalent influenza mRNA vaccine CVSQIV | NA/Intramuscular injection | Influenza | NCT05252338 | 1 | CureVac AG | Recruiting |
| Self-amplifying ribonucleic acid (saRNA) vaccines (PF-07852352, PF-07836391, PF-07836394, PF-07836395, PF-07836396, and PF-07867246) | NA/Intramuscular injection | Influenza | NCT05227001 | 1 | Pfizer | Recruiting |
| mRNA NA vaccine | NA/Intramuscular injection | Influenza | NCT05426174 | 1 | Sanofi Pasteur | Recruiting |
| mRNA-1647 | NA/Intramuscular injection | Cytomegalovirus infection | NCT05085366 | 3 | Moderna | Recruiting |
| NCT04232280 | 2 | Moderna | Recruiting | |||
| NCT05105048 | 1 | Moderna | Recruiting | |||
| mRNA -1215 | Lipid nanoparticle/Intramuscular injection | Nipah virus | NCT05398796 | 1 | National Institute of Allergy and Infectious Diseases (NIAID) | Recruiting |
| W_ova1 vaccine | Liposome/Intravenous injection | Ovarian cancer | NCT04163094 | 1 | University Medical Center Groningen | Active, not recruiting |
| National Cancer Institute (NCI)-4650 | Lipid nanoparticle/Intramuscular injection | Cancer (Melanoma, Colon, Gastrointestinal, Genitourinary, and Hepatocellular) | NCT03480152 | 1/2 | National Cancer Institute (NCI) | Terminated |
| BNT113 | Liposome/Intradermal vaccine | Carcinoma, Squamous Cell, Head and Neck Neoplasm, Cervical Neoplasm, Penile Neoplasms Malignant | NCT03418480 | 1/2 | University of Southampton | Recruiting |
| Liposome/Intradermal vaccine | Unresectable Head and Neck Squamous Cell Carcinoma Metastatic Head and Neck Cancer Recurrent Head and Neck Cancer | NCT04534205 | 2 | BioNTech SE | Recruiting | |
| BNT111 | NA/Intravenous infusion | Melanoma Stage III Melanoma Stage IV Unresectable Melanoma | NCT04526899 | 2 | BioNTech SE | Recruiting |
| Individualized Cancer RNA Immunotherapy (IVAC®) vaccines: IVAC_W_bre1_uID and IVAC_W_bre1_uID/IVAC_M_uID | NA/Intravenous injection | Triple Negative Breast Cancer (TNBC) | NCT02316457 | 1 | BioNTech SE | Active, not recruiting |
| RNA tumor vaccine | NA/Intramuscular injection | Solid tumor | NCT05202561 | 1 | First Affiliated Hospital Bengbu Medical College | Recruiting |
| mRNA-1893 | Solution/Intramuscular injection | Zika virus | NCT04917861 | 1 | Moderna | Recruiting |
| DC-006 vaccine loaded with amplified cancer stem cell mRNA | NA/Intranodal injection | Recurrent Epithelial Ovarian Cancer | NCT01334047 | 1/2 | Steinar Aamdal | Terminated |
| Lipo-MERIT | NA/IV injection | Melanoma | NCT02410733 | 1 | BioNTech SE | Active, not recruiting |
| mRNA-4157 | Lipid nanoparticles/intramuscular injection | Melanoma | NCT03897881 | 2 | Moderna | Active, not recruiting |
| mRNA-1653 | NA/Intramuscular injection | Human Metapneumovirus and Human Parainfluenza Infection | NCT04144348 | 1 | Moderna | Recruiting |
| mRNA-1189 | Lipid nanoparticles/intramuscular injection | Epstein-Barr Virus Infection | NCT05164094 | 1 | Moderna | Recruiting |
| Dendritic cells loaded with mRNA | Dendritic cell vaccine/NA | Prostate cancer | NCT01197625 | 1/2 | Oslo University Hospital | Active, not recruiting |
| Langerhans-type dendritic cells with mRNA | Dendritic cell vaccine/Intradermal injection | Melanoma | NCT01456104 | 1 | Memorial Sloan Kettering Cancer Center | Active, not recruiting |
| RNA-lipid particle (RNA-LP) vaccines | Liposome/Intravenous infusion | Adult Glioblastoma | NCT04573140 | 1 | University of Florida | Recruiting |
| Autologous dendritic cells electroporated with WT1 mRNA | Dendritic cell vaccine/Intradermal injection | Acute Myeloid Leukemia | NCT01686334 | 2 | Zwi Berneman | Recruiting |
| Myelodysplastic Syndromes Acute Myeloid Leukemia | NCT03083054 | 1/2 | University of Campinas, Brazil | Active, not recruiting | ||
| WT1 mRNA-loaded autologous monocyte-derived dendritic cells | Dendritic cell vaccine/Intradermal injection | High Grade Glioma Diffuse Intrinsic Pontine Glioma | NCT04911621 | 1/2 | University Hospital, Antwerp | Recruiting |
| Human CMV pp65-LAMP mRNA-pulsed autologous dendritic cells | Dendritic cell vaccine/Intradermal injection | Glioblastoma | NCT03688178 | 2 | Gary Archer Ph.D and Celldex Therapeutics | Recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W. A Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. https://doi.org/10.3390/ijms24032700
Gote V, Bolla PK, Kommineni N, Butreddy A, Nukala PK, Palakurthi SS, Khan W. A Comprehensive Review of mRNA Vaccines. International Journal of Molecular Sciences. 2023; 24(3):2700. https://doi.org/10.3390/ijms24032700
Chicago/Turabian StyleGote, Vrinda, Pradeep Kumar Bolla, Nagavendra Kommineni, Arun Butreddy, Pavan Kumar Nukala, Sushesh Srivatsa Palakurthi, and Wahid Khan. 2023. "A Comprehensive Review of mRNA Vaccines" International Journal of Molecular Sciences 24, no. 3: 2700. https://doi.org/10.3390/ijms24032700
APA StyleGote, V., Bolla, P. K., Kommineni, N., Butreddy, A., Nukala, P. K., Palakurthi, S. S., & Khan, W. (2023). A Comprehensive Review of mRNA Vaccines. International Journal of Molecular Sciences, 24(3), 2700. https://doi.org/10.3390/ijms24032700