Health Benefits of Coffee Consumption for Cancer and Other Diseases and Mechanisms of Action
Abstract
:1. Introduction
2. Coffee and Health Benefits: Non-Cancer
2.1. Mortality
2.2. Cardiovascular Diseases (CVDs)
2.3. Neurological Diseases
2.4. Metabolic Diseases including Diabetes
2.5. Sex-Dependent Effects of Coffee Consumption
3. Coffee and Cancer
3.1. Gastrointestinal Tract
3.1.1. Liver Cancer
3.1.2. Colon and Rectal Cancer
3.1.3. Other Gastrointestinal Cancers
3.2. Genitourinary Cancers
3.2.1. Prostate and Bladder Cancer
3.2.2. Renal Cancer
3.2.3. Endocrine Cancers
3.2.4. Breast Cancer
3.2.5. Endometrial Cancers
3.2.6. Other Cancers
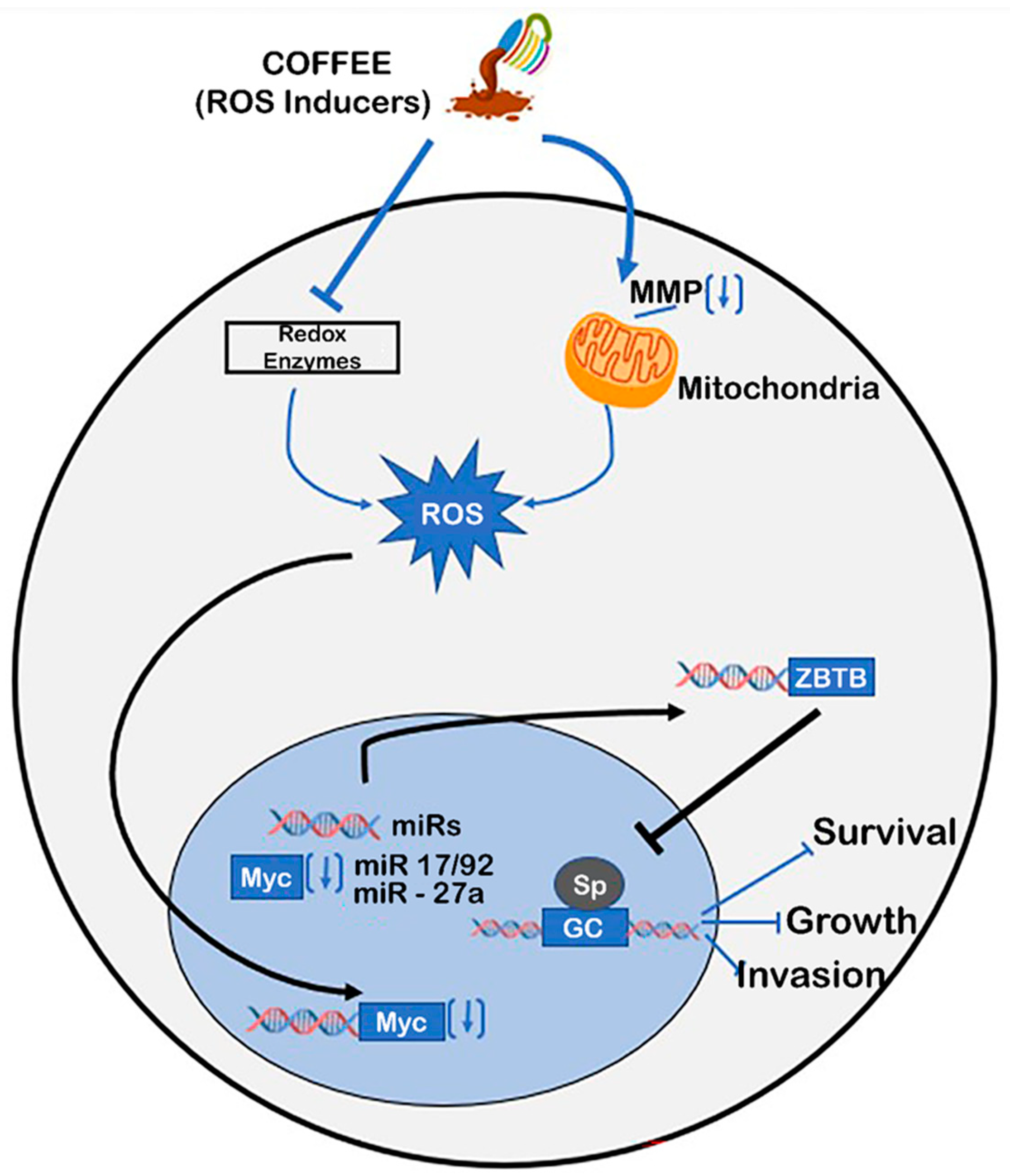
4. Chemopreventive and Chemotherapeutic Effects of Coffee
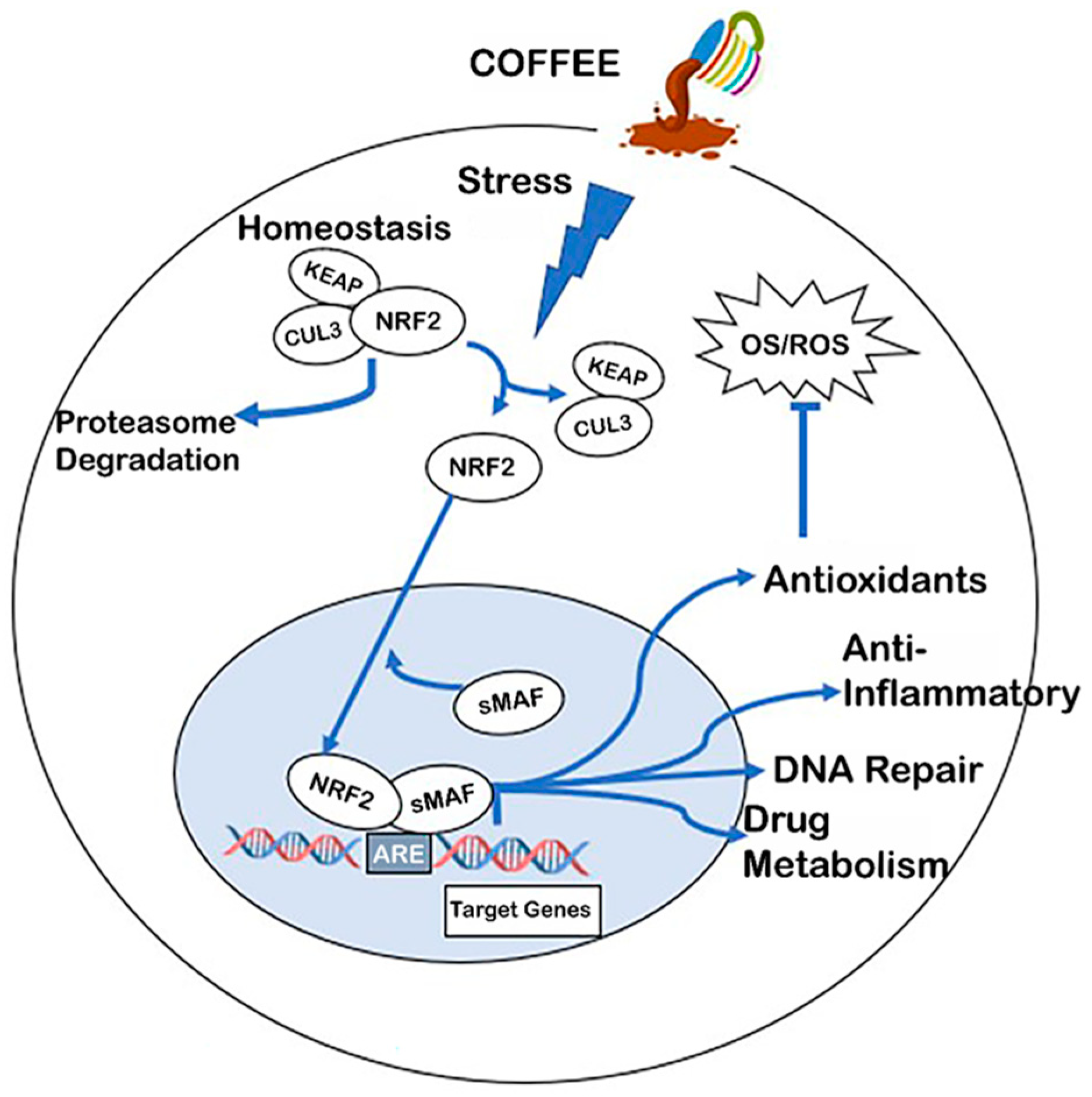
5. Mechanisms of Coffee-Mediated Anticancer Activities
Activation of Nrf2 by Coffee
6. Receptor-Mediated Responses
6.1. Aryl Hydrocarbon Receptor (AhR)
6.2. Nuclear Receptor 4A1 (NR4A1, Nur77)
7. Other Coffee-Induced Pathways
7.1. Changes in DNA Methylation
7.2. Coffee-Induced Microbiome Changes and Health
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chu, Y.F. Coffee: Emerging Health Effects and Disease Prevention. In Coffee: Emerging Health Effects and Disease Prevention; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Hou, C.; Zeng, Y.; Chen, W.; Han, X.; Yang, H.; Ying, Z.; Hu, Y.; Sun, Y.; Qu, Y.; Fang, F.; et al. Medical conditions associated with coffee consumption: Disease-trajectory and comorbidity network analyses of a prospective cohort study in UK Biobank. Am. J. Clin. Nutr. 2022, 116, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Tan, L.J.; Shin, S. Coffee Consumption and the Risk of All-Cause and Cause-Specific Mortality in the Korean Population. J. Acad. Nutr. Diet. 2021, 121, P2221–P2232.e4. [Google Scholar] [CrossRef]
- Di Maso, M.; Boffetta, P.; Negri, E.; la Vecchia, C.; Bravi, F. Caffeinated Coffee Consumption and Health Outcomes in the US Population: A Dose-Response Meta-Analysis and Estimation of Disease Cases and Deaths Avoided. Adv. Nutr. 2021, 12, 1160–1176. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, Z.H.; Shen, D.; Zhang, P.D.; Song, W.-Q.; Zhang, W.-T.; Huang, Q.-M.; Chen, P.L.; Zhang, X.R.; Mao, C. Association of Sugar-Sweetened, Artificially Sweetened, and Unsweetened Coffee Consumption With All-Cause and Cause-Specific Mortality: A Large Prospective Cohort Study. Ann. Intern. Med. 2022, 175, 909–917. [Google Scholar] [CrossRef]
- Lin, P.; Liang, Z.; Wang, M. Caffeine consumption and mortality in populations with different weight statuses: An analysis of NHANES 1999–2014. Nutrition 2022, 102, 111731. [Google Scholar] [CrossRef]
- Wang, T.; Wu, Y.; Wang, W.; Zhang, D. Association between coffee consumption and functional disability in older US adults. Br. J. Nutr. 2021, 125, 695–702. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Jose, M.; Seo, Y.; Feng, L.; Ge, S. Association between Caffeine Intake and All-Cause and Cause-Specific Mortality: An Analysis of the National Health and Nutrition Examination Survey (NHANES) 1999–2014 Database. Nurs. Rep. 2021, 11, 901–912. [Google Scholar] [CrossRef]
- Doepker, C.; Movva, N.; Cohen, S.S.; Wikoff, D.S. Benefit-risk of coffee consumption and all-cause mortality: A systematic review and disability adjusted life year analysis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2022, 170, 113472. [Google Scholar] [CrossRef]
- Shin, S.; Lee, J.E.; Loftfield, E.; Shu, X.O.; Abe, S.K.; Rahman, M.S.; Saito, E.; Islam, M.R.; Tsugane, S.; Sawada, N.; et al. Coffee and tea consumption and mortality from all causes, cardiovascular disease and cancer: A pooled analysis of prospective studies from the Asia Cohort Consortium. Int. J. Epidemiol. 2022, 51, 626–640. [Google Scholar] [CrossRef]
- Torres-Collado, L.; Compañ-Gabucio, L.M.; González-Palacios, S.; Notario-Barandiaran, L.; Oncina-Cánovas, A.; Vioque, J.; García-de la Hera, M. Coffee Consumption and All-Cause, Cardiovascular, and Cancer Mortality in an Adult Mediterranean Population. Nutrients 2021, 13, 1241. [Google Scholar] [CrossRef] [PubMed]
- Fraser, G.E.; Cosgrove, C.M.; Mashchak, A.D.; Orlich, M.J.; Altekruse, S.F. Lower rates of cancer and all-cause mortality in an Adventist cohort compared with a US Census population. Cancer 2020, 126, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Wang, F.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; et al. Healthy Eating Patterns and Risk of Total and Cause-Specific Mortality. JAMA Intern. Med. 2023; ahead of print. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, M.; Yang, H.; Wang, Y. Consumption of coffee and tea with all-cause and cause-specific mortality: A prospective cohort study. BMC Med. 2022, 20, 449. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Cho, H.; Myung, S.K. Effect of Coffee Consumption on Risk of Coronary Heart Disease in a Systematic Review and Meta-Analysis of Prospective Cohort Studies. Am. J. Cardiol. 2023, 186, 17–29. [Google Scholar] [CrossRef]
- Cornelis, M.C.; van Dam, R.M. Habitual coffee and tea consumption and cardiometabolic biomarkers in the UK biobank: The role of beverage types and genetic variation. J. Nutr. 2020, 150, 2772–2788. [Google Scholar] [CrossRef]
- Zhou, A.; Hyppönen, E. Long-term coffee consumption, caffeine metabolism genetics, and risk of cardiovascular disease: A prospective analysis of up to 347,077 individuals and 8368 cases. Am. J. Clin. Nutr. 2019, 109, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, M.; Yamagishi, K.; Muraki, I.; Tamakoshi, A.; Iso, H. Coffee and Green Tea Consumption and Cardiovascular Disease Mortality Among People With and Without Hypertension. J. Am. Heart Assoc. 2022, 12, e026477. [Google Scholar] [CrossRef]
- Borghi, C. Coffee and blood pressure: Exciting news! Blood Press. 2022, 31, 284–287. [Google Scholar] [CrossRef]
- Ruggiero, E.; di Castelnuovo, A.; Costanzo, S.; Persichillo, M.; de Curtis, A.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L.; Bonaccio, M. Daily Coffee Drinking Is Associated with Lower Risks of Cardiovascular and Total Mortality in a General Italian Population: Results from the Moli-sani Study. J. Nutr. 2021, 151, 395–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.D.; Wang, Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: A cohort study in the UK Biobank. PLoS Med. 2021, 18, e1003830. [Google Scholar] [CrossRef]
- Tran, A.; Zhang, C.Y.; Cao, C. The Role of Coffee in the Therapy of Parkinson’s Disease. J. Alzheimer’s Dis. Park. 2016, 5, 203. [Google Scholar] [CrossRef]
- Herden, L.; Weissert, R. The Impact of Coffee and Caffeine on Multiple Sclerosis Compared to Other Neurodegenerative Diseases. Front. Nutr. 2018, 5, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Cornelis, M.C.; Zhang, Z.; Liu, D.; Lian, X. Mendelian randomization study of coffee consumption and age at onset of Huntington’s disease. Clin. Nutr. 2021, 40, 5615–5618. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, F.; Lanza, G.; Pennisi, M.; Vagli, C.; Cantone, M.; Pennisi, G.; Ferri, R.; Bella, R. Moderate Mocha Coffee Consumption Is Associated with Higher Cognitive and Mood Status in a Non-Demented Elderly Population with Subcortical Ischemic Vascular Disease. Nutrients 2021, 13, 536. [Google Scholar] [CrossRef]
- Yahalom, G.; Rigbi, A.; Israeli-Korn, S.; Krohn, L.; Rudakou, U.; Ruskey, J.A.; Benshimol, L.; Tsafnat, T.; Gan-Or, Z.; Hassin-Baer, S.; et al. Age at Onset of Parkinson’s Disease Among Ashkenazi Jewish Patients: Contribution of Environmental Factors, LRRK2 p.G2019S and GBA p.N370S Mutations. J. Park. Dis. 2010, 10, 1123–1132. [Google Scholar] [CrossRef]
- Chan, L.; Hong, C.T.; Bai, C.H. Coffee consumption and the risk of cerebrovascular disease: A meta-analysis of prospective cohort studies. BMC Neurol. 2021, 21, 380. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S.; Kempf, K. Coffee and lower risk of type 2 diabetes: Arguments for a causal relationship. Nutrients 2021, 13, 1144. [Google Scholar] [CrossRef]
- Imamura, F.; Schulze, M.B.; Sharp, S.J.; Guevara, M.; Romaguera, D.; Bendinelli, B.; Salamanca-Fernández, E.; Ardanaz, E.; Arriola, L.; Aune, D.; et al. Estimated Substitution of Tea or Coffee for Sugar-Sweetened Beverages Was Associated with Lower Type 2 Diabetes Incidence in Case-Cohort Analysis across 8 European Countries in the EPIC-InterAct Study. J. Nutr. 2019, 149, 1985–1993. [Google Scholar] [CrossRef] [Green Version]
- Hang, D.; Kværner, A.S.; Ma, W.; Hu, Y.; Tabung, F.K.; Nan, H.; Hu, Z.; Shen, H.; Mucci, L.A.; Chan, A.T.; et al. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. Am. J. Clin. Nutr. 2019, 109, 635–647. [Google Scholar] [CrossRef]
- Komorita, Y.; Iwase, M.; Fujii, H.; Ohkuma, T.; Ide, H.; Jodai-Kitamura, T.; Yoshinari, M.; Oku, Y.; Higashi, T.; Nakamura, U.; et al. Additive effects of green tea and coffee on all-cause mortality in patients with type 2 diabetes mellitus: The Fukuoka Diabetes Registry. BMJ Open Diabetes Res. Care 2020, 8, e001252. [Google Scholar] [CrossRef]
- Wang, S.; Han, Y.; Zhao, H.; Han, X.; Yin, Y.; Wu, J.; Zhang, Y.; Zeng, X. Association between Coffee Consumption, Caffeine Intake, and Metabolic Syndrome Severity in Patients with Self-Reported Rheumatoid Arthritis: National Health and Nutrition Examination Survey 2003–2018. Nutrients 2022, 15, 107. [Google Scholar] [CrossRef]
- Kennedy, O.J.; Fallowfield, J.A.; Poole, R.; Hayes, P.C.; Parkes, J.; Roderick, P.J. All coffee types decrease the risk of adverse clinical outcomes in chronic liver disease: A UK Biobank study. BMC Public Health 2021, 21, 970. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Patarrão, R.S.; Sousa-Lima, I.; Ribeiro, R.T.; Meneses, M.J.; Andrade, R.; Mendes, V.M.; Manadas, B.; Raposo, J.F.; Macedo, M.P.; et al. Increased Intake of Both Caffeine and Non-Caffeine Coffee Components Is Associated with Reduced NAFLD Severity in Subjects with Type 2 Diabetes. Nutrients 2022, 15, 4. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Chen, J.; Li, X.; Fan, R.; Arsenault, B.; Gill, D.; Giovannucci, E.L.; Zheng, J.S.; Larsson, S.C. Lifestyle and metabolic factors for nonalcoholic fatty liver disease: Mendelian randomization study. Eur. J. Epidemiol. 2022, 37, 723–733. [Google Scholar] [CrossRef]
- Yang, J.; Tobias, D.K.; Li, S.; Bhupathiraju, S.N.; Ley, S.H.; Hinkle, S.N.; Qian, F.; Chen, Z.; Zhu, Y.; Bao, W.; et al. Habitual coffee consumption and subsequent risk of type 2 diabetes in individuals with a history of gestational diabetes—A prospective study. Am. J. Clin. Nutr. 2022, 116, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Park, J.I.; Kwon, S.O.; Hwang, D.D. Coffee consumption and diabetic retinopathy in adults with diabetes mellitus. Sci. Rep. 2022, 12, 3547. [Google Scholar] [CrossRef]
- Barré, T.; Fontaine, H.; Pol, S.; Ramier, C.; Di Beo, V.; Protopopescu, C.; Marcellin, F.; Bureau, M.; Bourlière, M.; Dorival, C.; et al. Metabolic Disorders in Patients with Chronic Hepatitis B Virus Infection: Coffee as a Panacea? (ANRS CO22 Hepather Cohort). Antioxidants 2022, 11, 379. [Google Scholar] [CrossRef]
- Ng, S.C.; Tang, W.; Leong, R.W.; Chen, M.; Ko, Y.; Studd, C.; Niewiadomski, O.; Bell, S.; Kamm, M.A.; de Silva, H.J.; et al. Environmental risk factors in inflammatory bowel disease: A population-based case-control study in Asia-Pacific. Gut 2015, 64, 1063–1071. [Google Scholar] [CrossRef] [Green Version]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef]
- Güngördük, K.; Özdemir, İ.A.; Güngördük, Ö.; Gülseren, V.; Gokçü, M.; Sancı, M. Effects of coffee consumption on gut recovery after surgery of gynecological cancer patients: A randomized controlled trial. Am. J. Obstet. Gynecol. 2017, 216, 145.e1–145.e7. [Google Scholar] [CrossRef] [Green Version]
- Jee, H.J.; Lee, S.G.; Bormate, K.J.; Jung, Y.S. Effect of Caffeine Consumption on the Risk for Neurological and Psychiatric Disorders: Sex Differences in Human. Nutrients 2020, 12, 3080. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Cheng, H.Y.; Lai, J.C.; Chen, S.J. The Relationship between Habitual Coffee Drinking and the Prevalence of Metabolic Syndrome in Taiwanese Adults: Evidence from the Taiwan Biobank Database. Nutrients 2022, 14, 1867. [Google Scholar] [CrossRef] [PubMed]
- Carter, P.; Yuan, S.; Kar, S.; Vithayathil, M.; Mason, A.M.; Burgess, S.; Larsson, S.C. Coffee consumption and cancer risk: A Mendelian randomization study. Clin. Nutr. 2022, 41, 2113–2123. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yoo, D.M.; Min, C.; Choi, H.G. Association between coffee consumption/physical exercise and gastric, hepatic, colon, breast, uterine cervix, lung, thyroid, prostate, and bladder cancer. Nutrients 2021, 13, 3927. [Google Scholar] [CrossRef]
- Nehlig, A. Effects of Coffee on the Gastro-Intestinal Tract: A Narrative Review and Literature Update. Nutrients 2022, 14, 399. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.; Coleman, H.G.; McMenamin, Ú.C.; Cardwell, C.R. Coffee consumption by type and risk of digestive cancer: A large prospective cohort study. Br. J. Cancer 2019, 120, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Tamakoshi, A.; Sugawara, Y.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; et al. Coffee, green tea and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2019, 49, 972–984. [Google Scholar] [CrossRef]
- Bhurwal, A.; Rattan, P.; Yoshitake, S.; Pioppo, L.; Reja, D.; Dellatore, P.; Rustgi, V. Inverse association of coffee with liver cancer development an updated systematic review and meta-analysis. J. Gastrointest. Liver Dis. 2020, 29, 421–428. [Google Scholar] [CrossRef]
- Loftfield, E.; Rothwell, J.A.; Sinha, R.; Keski-Rahkonen, P.; Robinot, N.; Albanes, D.; Weinstein, S.J.; Derkach, A.; Sampson, J.; Scalbert, A.; et al. Prospective investigation of serum metabolites, coffee drinking, liver cancer incidence, and liver disease mortality. J. Natl. Cancer Inst. 2020, 112, 286–294. [Google Scholar] [CrossRef]
- Ugai, T.; Haruki, K.; Vayrynen, J.P.; Zhong, R.; Borowsky, J.; Fujiyoshi, K.; Lau, M.C.; Zhao, M.; Akimoto, N.; Chang, T.W.; et al. Coffee intake and colorectal cancer incidence according to T-cell response. JNCI Cancer Spectr. 2020, 4, pkaa068. [Google Scholar] [CrossRef]
- Rosato, V.; Guercio, V.; Bosetti, C.; Gracia-Lavedan, E.; Villanueva, C.M.; Polesel, J.; Toffoluti, F.; Moreno, V.; Martin, V.; Aragonés, N.; et al. Coffee consumption and colorectal cancer risk: A multicentre case-control study from Italy and Spain. Eur. J. Cancer Prev. Off. J. Eur. Cancer Prev. Organ. ECP 2021, 30, 204–210. [Google Scholar] [CrossRef]
- Martimianaki, G.; Bertuccio, P.; Alicandro, G.; Pelucchi, C.; Bravi, F.; Carioli, G.; Bonzi, R.; Rabkin, C.S.; Liao, L.M.; Sinha, R.; et al. Coffee consumption and gastric cancer: A pooled analysis from the Stomach cancer Pooling Project consortium. Eur. J. Cancer Prev. 2022, 31, 117–127. [Google Scholar] [CrossRef]
- Um, C.Y.; McCullough, M.L.; Guinter, M.A.; Campbell, P.T.; Jacobs, E.J.; Gapstur, S.M. Coffee consumption and risk of colorectal cancer in the Cancer Prevention Study-II Nutrition Cohort. Cancer Epidemiol. 2020, 67, 101730. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, J.; Wong, M.C.S. Associations of alcohol and coffee with colorectal cancer risk in East Asian populations: A Mendelian randomization study. Eur. J. Nutr. 2022; ahead of print. [Google Scholar] [CrossRef]
- Lu, Y.T.; Gunathilake, M.; Lee, J.; Kim, Y.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Shin, A.; Kim, J. Coffee consumption and its interaction with the genetic variant AhR rs2066853 in colorectal cancer risk: A case-control study in Korea. Carcinogenesis 2022, 43, 203–216. [Google Scholar] [CrossRef]
- Bae, J.M.; Shim, S.R. Coffee consumption and pancreatic cancer risk: A meta-epidemiological study of population-based cohort studies. Asian Pac. J. Cancer Prev. 2020, 21, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.D.; Kuan, A.S.; Reeves, G.K.; Green, J.; Floud, S.; Beral, V.; Yang, T.Y.O. Coffee and pancreatic cancer risk among never-smokers in the UK prospective Million Women Study. Int. J. Cancer 2019, 145, 1484–1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.; Shen, X.; Chu, Q.; Zheng, X. Coffee consumption is not associated with the risk of gastric cancer: An updated systematic review and meta-analysis of prospective cohort studies. Nutr. Res. 2022, 102, 35–44. [Google Scholar] [CrossRef]
- Zeng, S.B.; Weng, H.; Zhou, M.; Duan, X.L.; Shen, X.F.; Zeng, X.T. Long-Term Coffee Consumption and Risk of Gastric Cancer: A PRISMA-Compliant Dose-Response Meta-Analysis of Prospective Cohort Studies. Medicine 2015, 94, e1640. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Luján-Barroso, L.; Bueno-De-Mesquita, H.B.; Dik, V.K.; Boeing, H.; Steffen, A.; Tjønneland, A.; Olsen, A.; Bech, B.H.; Overvad, K.; et al. Tea and coffee consumption and risk of esophageal cancer: The European prospective investigation into cancer and nutrition study. Int. J. Cancer 2014, 135, 1470–1479. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, B.; Hao, C. Coffee consumption and risk of esophageal cancer incidence: A meta-analysis of epidemiologic studies. Medicine 2018, 97, e0514. [Google Scholar] [CrossRef]
- Park, C.H.; Myung, S.K.; Kim, T.Y.; Seo, H.G.; Jeon, Y.J.; Kim, Y. Coffee consumption and risk of prostate cancer: A meta-analysis of epidemiological studies. BJU Int. 2010, 106, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhai, L.; Zeng, J.; Peng, Q.; Wang, J.; Deng, Y.; Xie, L.; Mo, C.; Yang, S.; Li, S.; et al. Coffee consumption and prostate cancer risk: An updated meta-analysis. Cancer Causes Control 2014, 25, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.D.; Chen, J.; Xue, J.X.; Yang, J.; Wang, Z.J. An up-to-date meta-analysis of coffee consumption and risk of prostate cancer. Urol. J. 2017, 14, 4079–4088. [Google Scholar]
- Yu, X.; Bao, Z.; Zou, J.; Dong, J. Coffee consumption and risk of cancers: A meta-analysis of cohort studies. BMC Cancer 2011, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, S.; Chen, W.; Yu, X.; Chen, Z.; Hu, Q.; Zhao, J. Coffee consumption and risk of prostate cancer: An up-to-date meta-analysis. Eur. J. Clin. Nutr. 2014, 68, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liu, L.; Yin, X.; Wang, Y.; Liu, J.; Lu, Z. Coffee consumption and risk of prostate cancer: A meta-analysis of prospective cohort studies. Carcinogenesis 2014, 35, 256–261. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Hu, G.H.; Wang, X.C.; Huang, T.B.; Xu, L.; Lai, P.; Guo, Z.F.; Xu, Y.F. Coffee consumption and prostate cancer risk: A meta-analysis of cohort studies. Nutr. Cancer 2015, 67, 392–400. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Tao, Z.; Wang, K. Coffee consumption and risk of prostate cancer: A systematic review and meta-analysis. BMJ Open 2021, 11, e038902. [Google Scholar] [CrossRef]
- Imatoh, T.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Tsugane, S. Association between Coffee Consumption and Risk of Prostate Cancer in Japanese Men: A Population-Based Cohort Study in Japan. Cancer Epidemiol. Biomark. Prev. 2022, 31, 471–478. [Google Scholar] [CrossRef]
- Dai, Z.-W.; Cai, K.-D.; Li, F.-R.; Wu, X.-B.; Chen, G.-C. Association between coffee consumption and risk of bladder cancer in a meta-analysis of 16 prospective studies. Nutr. Metab. 2019, 16, 66. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Wu, T.; Luo, G.; Chen, L. Exploring the casual association between coffee intake and bladder cancer risk using Mendelian Randomization. Front. Genet. 2022, 13, 992599. [Google Scholar] [CrossRef] [PubMed]
- Li, B.H.; Yan, S.Y.; Li, X.H.; Huang, Q.; Luo, L.S.; Wang, Y.Y.; Huang, J.; Jin, Y.H.; Wang, Y.B. Coffee and caffeine consumption and risk of renal cell carcinoma: A Mendelian randomization study. Front. Nutr. 2022, 9, 898279. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.; Lim, R.K.; Purdue, M.P. Coffee consumption and risk of renal cancer: A meta-analysis of cohort evidence. Cancer Causes Control 2022, 33, 101–108. [Google Scholar] [CrossRef]
- Rhee, J.; Loftfield, E.; Freedman, N.D.; Liao, L.M.; Sinha, R.; Purdue, M.P. Coffee consumption and risk of renal cell carcinoma in the NIH-AARP Diet and Health Study. Int. J. Epidemiol. 2021, 50, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Makino, T.; Izumi, K.; Hiratsuka, K.; Kano, H.; Shimada, T.; Nakano, T.; Kadomoto, S.; Naito, R.; Iwamoto, H.; Yaegashi, H.; et al. Anti-proliferative and anti-migratory properties of coffee diterpenes kahweol acetate and cafestol in human renal cancer cells. Sci. Rep. 2021, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Nehlig, A.; Reix, N.; Arbogast, P.; Mathelin, C. Coffee consumption and breast cancer risk: A narrative review in the general population and in different subtypes of breast cancer. Eur. J. Nutr. 2021, 60, 1197–1235. [Google Scholar] [CrossRef]
- Zheng, K.H.; Zhu, K.; Wactawski-Wende, J.; Freudenheim, J.L.; LaMonte, M.J.; Hovey, K.M.; Mu, L. Caffeine intake from coffee and tea and invasive breast cancer incidence among postmenopausal women in the Women’s Health Initiative. Int. J. Cancer 2021, 149, 2032–2044. [Google Scholar] [CrossRef]
- Yaghjyan, L.; McLaughlin, E.; Lehman, A.; Neuhouser, M.L.; Rohan, T.; Lane, D.S.; Snetselaar, L.; Paskett, E. Associations of coffee/caffeine consumption with postmenopausal breast cancer risk and their interactions with postmenopausal hormone use. Eur. J. Nutr. 2022, 61, 3449–3459. [Google Scholar] [CrossRef]
- Gapstur, S.M.; Gaudet, M.M.; Wang, Y.; Hodge, R.A.; Um, C.Y.; Hartman, T.J.; McCullough, M.L. Coffee Consumption and Invasive Breast Cancer Incidence among Postmenopausal Women in the Cancer Prevention Study-II Nutrition Cohort. Cancer Epidemiol. Biomark. Prev. 2020, 29, 2383–2386. [Google Scholar] [CrossRef]
- Li, Y.; Ma, L. The association between coffee intake and breast cancer risk: A meta-analysis and dose-response analysis using recent evidence. Ann. Palliat. Med. 2021, 10, 3804–3816. [Google Scholar] [CrossRef]
- Sánchez-Quesada, C.; Romanos-Nanclares, A.; Navarro, A.M.; Gea, A.; Cervantes, S.; Martínez-González, M.Á.; Toledo, E. Coffee consumption and breast cancer risk in the SUN project. Eur. J. Nutr. 2020, 59, 3461–3471. [Google Scholar] [CrossRef]
- Liu, M.; Song, S.S.; Park, S. Coffee Intake Interacted with the Bcl-2 rs1944420, rs7236090, and rs2849382 Haplotype to Influence Breast Cancer Risk in Middle-Aged Women. Nutr. Cancer 2022, 74, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhai, P.; Jiang, F.; Zhou, F.; Wang, X. Association between coffee drinking and endometrial cancer risk: A meta-analysis. J. Obstet. Gynaecol. Res. 2022, 48, 774–795. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Du, M.; Gunter, M.J.; Setiawan, V.W.; Schouten, L.J.; Shu, X.O.; Wentzensen, N.; Bertrand, K.A.; Cook, L.S.; Friedenreich, C.M.; et al. Coffee consumption and risk of endometrial cancer: A pooled analysis of individual participant data in the Epidemiology of Endometrial Cancer Consortium (E2C2). Am. J. Clin. Nutr. 2022, 116, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Smith-Warner, S.A.; Yu, D.; Zhang, X.; Blot, W.J.; Xiang, Y.B.; Sinha, R.; Park, Y.; Tsugane, S.; White, E.; et al. Associations of coffee and tea consumption with lung cancer risk. Int. J. Cancer 2021, 148, 2457–2470. [Google Scholar] [CrossRef]
- Seow, W.J.; Koh, W.P.; Jin, A.; Wang, R.; Yuan, J.M. Associations between tea and coffee beverage consumption and the risk of lung cancer in the Singaporean Chinese population. Eur. J. Nutr. 2020, 59, 3083–3091. [Google Scholar] [CrossRef]
- Kudwongsa, W.; Promthet, S.; Suwanrungruang, K.; Phunmanee, A.; Vatanasapt, P. Coffee consumption and lung cancer risk: A prospective cohort study in Khon Kaen Thailand. Asian Pac. J. Cancer Prev. 2020, 21, 2367–2371. [Google Scholar] [CrossRef]
- Cote, D.J.; Bever, A.M.; Wilson, K.M.; Smith, T.R.; Smith-Warner, S.A.; Stampfer, M.J. A prospective study of tea and coffee intake and risk of glioma. Int. J. Cancer 2020, 146, 2442–2449. [Google Scholar] [CrossRef]
- Pranata, R.; Feraldho, A.; Lim, M.A.; Henrina, J.; Vania, R.; Golden, N.; July, J. Coffee and tea consumption and the risk of glioma: A systematic review and dose-response meta-analysis. Br. J. Nutr. 2022, 127, 78–86. [Google Scholar] [CrossRef]
- Creed, J.H.; Smith-Warner, S.A.; Gerke, T.A.; Egan, K.M. A prospective study of coffee and tea consumption and the risk of glioma in the UK Biobank. Eur. J. Cancer 2020, 129, 123–131. [Google Scholar] [CrossRef]
- Salaroli, L.B.; Ferreira, J.R.S.; Prado, C.B.D.; De Podestá, O.P.G.; Carvalho, A.L.; Mercante, A.M.D.C.; Toporcov, T.N. Cumulative Coffee Consumption as a Protective Factor for Head and Neck Cancer in Brazil. Nutr. Cancer 2022, 75, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Karalexi, M.A.; Dessypris, N.; Clavel, J.; Metayer, C.; Erdmann, F.; Orsi, L.; Kang, A.Y.; Schüz, J.; Bonaventure, A.; Greenop, K.R.; et al. Coffee and tea consumption during pregnancy and risk of childhood acute myeloid leukemia: A Childhood Leukemia International Consortium (CLIC) study. Cancer Epidemiol. 2019, 62, 101581. [Google Scholar] [CrossRef] [PubMed]
- Madsen, C.N.; Henriksen, T.B.; Ramlau-Hansen, C.H.; Parner, E.T.; Olsen, J.; Bech, B.H. Coffee intake during pregnancy and childhood acute leukemia—A cohort study. Cancer Epidemiol. 2020, 67, 101747. [Google Scholar] [CrossRef] [PubMed]
- Soldato, D.; Havas, J.; Presti, D.; Lapidari, P.; Rassy, N.; Pistilli, B.; Martin, E.; del Mastro, L.; Martin, A.-L.; Jacquet, A.; et al. 1694P Coffee and tea consumption (CTC), patient-reported (PRO), and clinical outcomes in a longitudinal study of patients (pts) with breast cancer (BC). Ann. Oncol. 2021, 32, S1184. [Google Scholar] [CrossRef]
- Farvid, M.S.; Spence, N.D.; Rosner, B.A.; Willett, W.C.; Eliassen, A.H.; Holmes, M.D. Post-diagnostic coffee and tea consumption and breast cancer survival. Br. J. Cancer 2021, 124, 1873–1881. [Google Scholar] [CrossRef]
- Mackintosh, C.; Yuan, C.; Ou, F.S.; Zhang, S.; Niedzwiecki, D.; Chang, I.W.; O’Neil, B.H.; Mullen, B.C.; Lenz, H.J.; Blanke, C.D.; et al. Association of Coffee Intake with Survival in Patients with Advanced or Metastatic Colorectal Cancer. JAMA Oncol. 2020, 6, 1713. [Google Scholar] [CrossRef]
- Rubino, R.; Iuliucci, M.R.; Gatani, S.; Piscosquito, A.; D’Ambrosio, B.; Ingenito, C.; Scafuri, L.; Buonerba, C.; Di Lorenzo, G. Mediterranean Diet as a Supportive Intervention in Cancer Patients: Current Evidence and Future Directions. Curr. Oncol. 2022, 29, 7579–7582. [Google Scholar] [CrossRef]
- Mokhtari, E.; Jamshidi, S.; Farhadnejad, H.; Teymoori, F.; Rashidkhani, B.; Mirmiran, P.; Tehrani, F.R.; Heidari, Z. The relationship between Mediterranean-DASH diet intervention for the neurodegenerative delay (MIND) Diet and risk of breast Cancer: A case-control study among iranian adult women. BMC Nutr. 2022, 8, 123. [Google Scholar] [CrossRef]
- Kolb, H.; Kempf, K.; Martin, S. Health effects of coffee: Mechanism unraveled? Nutrients 2020, 12, 1842. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Hassmann, U.; Haupt, L.M.; Smith, R.A.; Winkler, S.; Bytof, G.; Lantz, I.; Griffiths, L.R.; Marko, D. Potential antioxidant response to coffee—A matter of genotype? Meta Gene 2014, 2, 525–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional Regulation of NF-E2 p45-related Factor (NRF2) Expression by the Aryl Hydrocarbon Receptor-Xenobiotic Response Element Signaling Pathway. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Mcmahon, M. Cross-talk between transcription factors AhR and Nrf2: Lessons for cancer chemoprevention from dioxin. Toxicol. Sci. 2009, 111, 199–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Atanasov, A.G.; Li, Y.; Kumar, N.; Bishayee, A. Chlorogenic acid for cancer prevention and therapy: Current status on efficacy and mechanisms of action. Pharmacol. Res. 2022, 186, 106505. [Google Scholar] [CrossRef]
- Chen, X.Q.; Yang, J.H.; Cho, S.S.; Kim, J.H.; Xu, J.Q.; Seo, K.; Ki, S.H. 5-Caffeoylquinic acid ameliorates oxidative stress-mediated cell death via Nrf2 activation in hepatocytes. Pharm. Biol. 2020, 58, 999–1005. [Google Scholar] [CrossRef]
- Lonati, E.; Carrozzini, T.; Bruni, I.; Mena, P.; Botto, L.; Cazzaniga, E.; del Rio, D.; Labra, M.; Palestini, P.; Bulbarelli, A. Coffee-Derived Phenolic Compounds Activate Nrf2 Antioxidant Pathway in I/R Injury In Vitro Model: A Nutritional Approach Preventing Age Related-Damages. Molecules 2022, 27, 1049. [Google Scholar] [CrossRef]
- Sun, W.; Xie, W.; Huang, D.; Cui, Y.; Yue, J.; He, Q.; Jiang, L.; Xiong, J.; Sun, W.; Yi, Q. Caffeic acid phenethyl ester attenuates osteoarthritis progression by activating NRF2/HO 1 and inhibiting the NF κB signaling pathway. Int. J. Mol. Med. 2022, 50, 134. [Google Scholar] [CrossRef]
- Volz, N.; Boettler, U.; Winkler, S.; Teller, N.; Schwarz, C.; Bakuradze, T.; Eisenbrand, G.; Haupt, L.; Griffiths, L.R.; Stiebitz, H.; et al. Effect of coffee combining green coffee bean constituents with typical roasting products on the Nrf2/ARE pathway in vitro and in vivo. J. Agric. Food Chem. 2012, 60, 9631–9641. [Google Scholar] [CrossRef]
- Kweon, M.H.; In Park, Y.; Sung, H.C.; Mukhtar, H. The novel antioxidant 3-O-caffeoyl-1-methylquinic acid induces Nrf2-dependent phase II detoxifying genes and alters intracellular glutathione redox. Free Radic. Biol. Med. 2006, 40, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Stähli, A.; Maheen, C.U.; Strauss, F.J.; Eick, S.; Sculean, A.; Gruber, R. Caffeic acid phenethyl ester protects against oxidative stress and dampens inflammation via heme oxygenase 1. Int. J. Oral Sci. 2019, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.; Cagide, F.; Tavares, L.C.; Simões, R.F.; Soares, P.; Benfeito, S.; Baldeiras, I.; Jones, J.G.; Borges, F.; Oliveira, P.J.; et al. Mitochondriotropic antioxidant based on caffeic acid AntiOxCIN4 activates Nrf2-dependent antioxidant defenses and quality control mechanisms to antagonize oxidative stress-induced cell damage. Free Radic. Biol. Med. 2022, 179, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Peng, S.; Xu, J.; Fang, J. Reversing ROS-mediated neurotoxicity by chlorogenic acid involves its direct antioxidant activity and activation of Nrf2-ARE signaling pathway. BioFactors 2019, 45, 616–626. [Google Scholar] [CrossRef]
- Balstad, T.R.; Carlsen, H.; Myhrstad, M.C.W.; Kolberg, M.; Reiersen, H.; Gilen, L.; Ebihara, K.; Paur, I.; Blomhoff, R. Coffee, broccoli and spices are strong inducers of electrophile response element-dependent transcription in vitro and in vivo—Studies in electrophile response element transgenic mice. Mol. Nutr. Food Res. 2011, 55, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Higgins, L.G.; Cavin, C.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Induction of cancer chemopreventive enzymes by coffee is mediated by transcription factor Nrf2. Evidence that the coffee-specific diterpenes cafestol and kahweol confer protection against acrolein. Toxicol. Appl. Pharmacol. 2008, 226, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Toydemir, G.; Loonen, L.M.P.; Venkatasubramanian, P.B.; Mes, J.J.; Wells, J.M.; de Wit, N. Coffee induces AHR- and Nrf2-mediated transcription in intestinal epithelial cells. Food Chem. 2021, 341, 128261. [Google Scholar] [CrossRef] [PubMed]
- Yazheng, L.; Kitts, D.D. Activation of antioxidant response element (ARE)-dependent genes by roasted coffee extracts. Food Funct. 2012, 3, 950–954. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takahashi, S.; Morita, K.; Okinaga, H.; Teramoto, T. Induction of AhR-mediated gene transcription by coffee. PLoS ONE 2014, 9, e102152. [Google Scholar] [CrossRef] [Green Version]
- Kalthoff, S.; Ehmer, U.; Freiberg, N.; Manns, M.P.; Strassburg, C.P. Coffee induces expression of glucuronosyl transferases by the aryl hydrocarbon receptor and Nrf2 in liver and stomach. Gastroenterology 2010, 139, 1699–1710.e2. [Google Scholar] [CrossRef]
- Nigra, A.D.; Teodoro, A.J.; Gil, G.A. A Decade of ReseArch. on Coffee as an Anticarcinogenic Beverage. Oxidative Med. Cell. Longev. 2021, 2021, 4420479. [Google Scholar] [CrossRef]
- Yang, J.S.; Liu, C.W.; Ma, Y.S.; Weng, S.W.; Tang, N.Y.; Wu, S.H.; Ji, B.C.; Ma, C.Y.; Ko, Y.C.; Funayama, S.; et al. Chlorogenic acid induces apoptotic cell death in U937 leukemia cells through caspase-and mitochondria-dependent pathways. In Vivo 2012, 26, 971–978. [Google Scholar] [PubMed]
- Rakshit, S.; Mandal, L.; Pal, B.C.; Bagchi, J.; Biswas, N.; Chaudhuri, J.; Chowdhury, A.A.; Manna, A.; Chaudhuri, U.; Konar, A.; et al. Involvement of ROS in chlorogenic acid-induced apoptosis of Bcr-Abl+ CML cells. Biochem. Pharmacol. 2010, 80, 1662–1675. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; Yu, C.; Xiang, L.; Li, L.; Shi, D.; Lin, F. Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ros production. OncoTargets Ther. 2020, 13, 513–523. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Needs, P.W.; Lu, Y.; Kroon, P.A.; Ren, D.; Yang, X. Different antitumor effects of quercetin, quercetin-3′-sulfate and quercetin-3-glucuronide in human breast cancer MCF-7 cells. Food Funct. 2018, 9, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zongyuan, Y.; Cheng, G.; Lingyun, Z.; Guilian, Y.; Wei, G. Quercetin enhances apoptotic effect of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in ovarian cancer cells through reactive oxygen species (ROS) mediated CCAAT enhancer-binding protein homologous protein (CHOP)-death receptor 5 pathway. Cancer Sci. 2014, 105, 520–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, I.K.; Lin-Shiau, S.Y.; Lin, J.K. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. Eur. J. Cancer 1999, 35, 1517–1525. [Google Scholar] [CrossRef]
- Safe, S.; Abbruzzese, J.; Abdelrahim, M.; Hedrick, E. Specificity Protein Transcription Factors and Cancer: Opportunities for Drug Development. Cancer Prev. Res. 2018, 11, 371–382. [Google Scholar] [CrossRef]
- Pouremamali, F.; Pouremamali, A.; Dadashpour, M.; Soozangar, N.; Jeddi, F. An update of Nrf2 activators and inhibitors in cancer prevention/promotion. Cell Commun. Signal. CCS 2022, 20, 100. [Google Scholar] [CrossRef]
- Jenkins, T.; Gouge, J. Nrf2 in Cancer, Detoxifying Enzymes and Cell Death Programs. Antioxidants 2021, 10, 1030. [Google Scholar] [CrossRef]
- Islam, S.S.; Qassem, K.; Islam, S.; Parag, R.R.; Rahman, M.Z.; Farhat, W.A.; Yeger, H.; Aboussekhra, A.; Karakas, B.; Noman, A.S.M. Genetic alterations of Keap1 confers chemotherapeutic resistance through functional activation of Nrf2 and Notch pathway in head and neck squamous cell carcinoma. Cell Death Dis. 2022, 13, 696. [Google Scholar] [CrossRef] [PubMed]
- Fragoulis, A.; Schenkel, J.; Schröder, N.; Brandt, E.F.; Weiand, M.; Neu, T.; Ramadori, P.; Caspers, T.; Kant, S.; Pufe, T.; et al. Nrf2 induces malignant transformation of hepatic progenitor cells by inducing β-catenin expression. Redox Biol. 2022, 57, 102453. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Hu, B.; Ma, J.; Zhang, C.; Xie, H.; Wei, T.; Yang, Y.; Ni, B. Histone lysine methyltransferase SETDB2 suppresses NRF2 to restrict tumor progression and modulates chemotherapy sensitivity in lung adenocarcinoma. Cancer Med. 2022; ahead of print. [Google Scholar] [CrossRef]
- Mancini, M.C.S.; Morelli, A.P.; Severino, M.B.; Pavan, I.C.B.; Zambalde, É.P.; Góis, M.M.; Silva, L.G.S.D.; Quintero-Ruiz, N.; Romeiro, C.F.; Dos Santos, D.F.G.; et al. Knockout of NRF2 triggers prostate cancer cells death through ROS modulation and sensitizes to cisplatin. J. Cell Biochem. 2022, 123, 2079–2092. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chen, S.; Huang, K.; Lin, G. Triptolide promotes ferroptosis by suppressing Nrf2 to overcome leukemia cell resistance to doxorubicin. Mol. Med. Rep. 2023, 27, 17. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H. Oxaliplatin induces ferroptosis and oxidative stress in HT29 colorectal cancer cells by inhibiting the Nrf2 signaling pathway. Exp. Med. 2022, 23, 394. [Google Scholar] [CrossRef]
- Wu, S.; Liao, X.; Zhu, Z.; Huang, R.; Chen, M.; Huang, A.; Zhang, J.; Wu, Q.; Wang, J.; Ding, Y. Antioxidant and anti-inflammation effects of dietary phytochemicals: The Nrf2/NF-κB signalling pathway and upstream factors of Nrf2. Phytochemistry 2022, 204, 113429. [Google Scholar] [CrossRef]
- Liao, J.C.; Lee, K.T.; You, B.J.; Lee, C.L.; Chang, W.T.; Wu, Y.C.; Lee, H.Z. Raf/ERK/Nrf2 signaling pathway and MMP-7 expression involvement in the trigonelline-mediated inhibition of hepatocarcinoma cell migration. Food Nutr. Res. 2015, 59, 29884. [Google Scholar] [CrossRef] [Green Version]
- Pirpour Tazehkand, A.; Salehi, R.; Velaei, K.; Samadi, N. The potential impact of trigonelline loaded micelles on Nrf2 suppression to overcome oxaliplatin resistance in colon cancer cells. Mol. Biol. Rep. 2020, 47, 5817–5829. [Google Scholar] [CrossRef]
- Tanveer, M.A.; Rashid, H.; Nazir, L.A.; Archoo, S.; Shahid, N.H.; Ragni, G.; Umar, S.A.; Tasduq, S.A. Trigonelline, a plant derived alkaloid prevents ultraviolet-B-induced oxidative DNA damage in primary human dermal fibroblasts and BALB/c mice via modulation of phosphoinositide 3-kinase-Akt-Nrf2 signalling axis. Exp. Gerontol. 2022, 171, 112028. [Google Scholar] [CrossRef]
- Arlt, A.; Sebens, S.; Krebs, S.; Geismann, C.; Grossmann, M.; Kruse, M.L.; Schreiber, S.; Schäfer, H. Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 2013, 32, 4825–4835. [Google Scholar] [CrossRef]
- Fouzder, C.; Mukhuty, A.; Mukherjee, S.; Malick, C.; Kundu, R. Trigonelline inhibits Nrf2 via EGFR signalling pathway and augments efficacy of Cisplatin and Etoposide in NSCLC cells. Toxicol. In Vitro 2021, 70, 105038. [Google Scholar] [CrossRef] [PubMed]
- Chapkin, R.S.; Davidson, L.A.; Park, H.; Jin, U.H.; Fan, Y.Y.; Cheng, Y.; Hensel, M.E.; Landrock, K.K.; Allred, C.; Menon, R.; et al. Role of the Aryl Hydrocarbon Receptor (AhR) in Mediating the Effects of Coffee in the Colon. Mol. Nutr. Food Res. 2021, 65, 2100539. [Google Scholar] [CrossRef]
- Serna, E.; Cespedes, C.; Vina, J. Anti-aging physiological roles of aryl hydrocarbon receptor and its dietary regulators. Int. J. Mol. Sci. 2021, 22, 374. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Aryl hydrocarbon receptor (AhR) reveals evidence of antagonistic pleiotropy in the regulation of the aging process. Cell. Mol. Life Sci. CMLS 2022, 79, 489. [Google Scholar] [CrossRef] [PubMed]
- Ojo, E.S.; Tischkau, S.A. The role of ahr in the hallmarks of brain aging: Friend and foe. Cells 2021, 10, 2729. [Google Scholar] [CrossRef]
- Nacarino-Palma, A.; Rico-Leo, E.M.; Campisi, J.; Ramanathan, A.; González-Rico, F.J.; Rejano-Gordillo, C.M.; Ordiales-Talavero, A.; Merino, J.M.; Fernández-Salguero, P.M. Aryl hydrocarbon receptor blocks aging-induced senescence in the liver and fibroblast cells. Aging 2022, 14, 4281–4304. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.E.; Wang, Y.N.; Hua, M.R.; Miao, H.; Zhao, Y.Y.; Cao, G. Aryl hydrocarbon receptor: From pathogenesis to therapeutic targets in aging-related tissue fibrosis. Ageing Res. Rev. 2022, 79, 101662. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, K.; Kobayashi, Y.; Ohtake, F.; Ikuta, T.; Matsushima, Y.; Mimura, J.; Pettersson, S.; Pollenz, R.S.; Sakaki, T.; Hirokawa, T.; et al. Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc. Natl. Acad. Sci. USA 2009, 106, 13481–13486. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Díaz, C.J.; Ronnekleiv-Kelly, S.M.; Nukaya, M.; Geiger, P.G.; Balbo, S.; Dator, R.; Megna, B.W.; Carney, P.R.; Bradfield, C.A.; Kennedy, G.D. The Aryl Hydrocarbon Receptor is a Repressor of Inflammation-associated Colorectal Tumorigenesis in Mouse. Ann. Surg. 2016, 264, 429–436. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Davidson, L.A.; Hensel, M.; Yoon, G.; Landrock, K.; Allred, C.; Jayaraman, A.; Ivanov, I.; Safe, S.H.; Chapkin, R.S. Loss of aryl hydrocarbon receptor promotes colon tumorigenesis in ApcS580/þ; KrasG12D/þ mice. Mol. Cancer Res. 2021, 19, 771–783. [Google Scholar] [CrossRef]
- Han, H.; Davidson, L.A.; Fan, Y.; Goldsby, J.S.; Yoon, G.; Jin, U.; Wright, G.A.; Landrock, K.K.; Weeks, B.R.; Wright, R.C.; et al. Loss of aryl hydrocarbon receptor potentiates FoxM1 signaling to enhance self-renewal of colonic stem and progenitor cells. EMBO J. 2020, 39, e104319. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Osorio, D.; Davidson, L.A.; Han, H.; Mullens, D.A.; Jayaraman, A.; Safe, S.; Ivanov, I.; Cai, J.J.; Chapkin, R.S. Single-cell RNA Sequencing Reveals How the Aryl Hydrocarbon Receptor Shapes Cellular Differentiation Potency in the Mouse Colon. Cancer Prev. Res. 2022, 15, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Davidson, L.A.; Fan, Y.Y.; Landrock, K.K.; Jayaraman, A.; Safe, S.H.; Chapkin, R.S. Loss of aryl hydrocarbon receptor suppresses the response of colonic epithelial cells to IL22 signaling by upregulating SOCS3. Am. J. Physiol.-Gastrointest. Liver Physiol. 2022, 322, G93–G106. [Google Scholar] [CrossRef] [PubMed]
- Kolluri, S.K.; Jin, U.H.; Safe, S. Role of the aryl hydrocarbon receptor in carcinogenesis and potential as an anti-cancer drug target. Arch. Toxicol. 2017, 91, 2497–2513. [Google Scholar] [CrossRef]
- DiNatale, B.C.; Smith, K.; John, K.; Krishnegowda, G.; Amin, S.G.; Perdew, G.H. Ah receptor antagonism represses head and neck tumor cell aggressive phenotype. Mol. Cancer Res. 2012, 10, 1369–1379. [Google Scholar] [CrossRef] [Green Version]
- Pearen, M.A.; Muscat, G.E.O. Minireview: Nuclear hormone receptor 4A signaling: Implications for metabolic disease. Mol. Endocrinol. 2010, 24, 1891–1903. [Google Scholar] [CrossRef] [Green Version]
- Safe, S.; Jin, U.H.; Hedrick, E.; Reeder, A.; Lee, S.O. Minireview: Role of orphan nuclear receptors in cancer and potential as drug targets. Mol. Endocrinol. 2014, 28, 157–172. [Google Scholar] [CrossRef]
- Safe, S.; Karki, K. The paradoxical roles of orphan nuclear receptor 4A (NR4A) in cancer. Mol. Cancer Res. 2021, 19, 180–191. [Google Scholar] [CrossRef]
- Paillasse, M.R.; de Medina, P. The NR4A nuclear receptors as potential targets for anti-aging interventions. Med. Hypotheses 2015, 84, 135–140. [Google Scholar] [CrossRef]
- Asami, T.; Endo, K.; Matsui, R.; Sawa, T.; Tanaka, Y.; Saiki, T.; Tanba, N.; Haga, H.; Tanaka, S. Long-term caloric restriction ameliorates T cell immunosenescence in mice. Mech. Ageing Dev. 2022, 206, 111710. [Google Scholar] [CrossRef]
- Ma, G.; Chen, F.; Liu, Y.; Zheng, L.; Jiang, X.; Tian, H.; Wang, X.; Song, X.; Yu, Y.; Wang, D. Nur77 ameliorates age-related renal tubulointerstitial fibrosis by suppressing the TGF-β/Smads signaling pathway. FASEB J. 2022, 36, e22124. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Walsh, E.N.; Yan, A.L.; Giese, K.P.; Safe, S.; Abel, T. Pharmacological activation of Nr4a rescues age-associated memory decline. Neurobiol. Aging 2020, 85, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Kwapis, J.L.; Alaghband, Y.; López, A.J.; Long, J.M.; Li, X.; Shu, G.; Bodinayake, K.K.; Matheos, D.P.; Rapp, P.R.; Wood, M.A. HDAC3-mediated repression of the Nr4a family contributes to age-related impairments in long-term memory. J. Neurosci. 2019, 39, 4999–5009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, R.; Mohankumar, K.; Martin, G.; Hailemariam, A.; Lee, S.O.; Jin, U.H.; Burghardt, R.; Safe, S. Flavonoids kaempferol and quercetin are nuclear receptor 4A1 (NR4A1, Nur77) ligands and inhibit rhabdomyosarcoma cell and tumor growth. J. Exp. Clin. Cancer Res. 2021, 40, 392. [Google Scholar] [CrossRef]
- Karabegović, I.; Portilla-Fernandez, E.; Li, Y.; Ma, J.; Maas, S.C.E.; Sun, D.; Hu, E.A.; Kühnel, B.; Zhang, Y.; Ambatipudi, S.; et al. Epigenome-wide association meta-analysis of DNA methylation with coffee and tea consumption. Nat. Commun. 2021, 12, 2830. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Izard, J.; Walsh, E.; Batich, K.; Chongsathidkiet, P.; Clarke, G.; Sela, D.A.; Muller, A.J.; Mullin, J.M.; Albert, K.; et al. The host microbiome regulates and maintains human health: A primer and perspective for non-microbiologists. Cancer Res. 2017, 77, 1783–1812. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.R.; Falcone, J.N.; Cantley, L.C.; Goncalves, M.D. Developing dietary interventions as therapy for cancer. Nat. Rev. Cancer 2022, 22, 452–466. [Google Scholar] [CrossRef]
- Chen, L.; Wang, D.; Garmaeva, S.; Kurilshikov, A.; Vich Vila, A.; Gacesa, R.; Sinha, T.; Segal, E.; Weersma, R.K.; Wijmenga, C.; et al. The long-term genetic stability and individual specificity of the human gut microbiome. Cell 2021, 184, 2302–2315.e12. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Song, Z.; Liu, L.; Xu, Y.; Cao, R.; Lan, X.; Pan, C.; Zhang, S.; Zhao, H. Caffeine-Induced Sleep Restriction Alters the Gut Microbiome and Fecal Metabolic Profiles in Mice. Int. J. Mol. Sci. 2022, 23, 14837. [Google Scholar] [CrossRef] [PubMed]
- Gniechwitz, D.; Reichardt, N.; Blaut, M.; Steinhart, H.; Bunzel, M. Dietary fiber from coffee beverage: Degradation by human fecal microbiota. J. Agric. Food Chem. 2007, 55, 6989–6996. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, N.; Gniechwitz, D.; Steinhart, H.; Bunzel, M.; Blaut, M. Characterization of high molecular weight coffee fractions and their fermentation by human intestinal microbiota. Mol. Nutr. Food Res. 2009, 53, 287–299. [Google Scholar] [CrossRef] [PubMed]
- González, S.; Salazar, N.; Ruiz-Saavedra, S.; Gómez-Martín, M.; de Los Reyes-Gavilán, C.G.; Gueimonde, M. Long-Term Coffee Consumption is Associated with Fecal Microbial Composition in Humans. Nutrients 2020, 12, 1287. [Google Scholar] [CrossRef] [PubMed]
- van der Beek, C.M.; Dejong, C.H.C.; Troost, F.J.; Masclee, A.A.M.; Lenaerts, K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr. Rev. 2017, 75, 286–305. [Google Scholar] [CrossRef] [Green Version]
- Nowotny, B.; Zahiragic, L.; Bierwagen, A.; Kabisch, S.; Groener, J.B.; Nowotny, P.J.; Fleitmann, A.K.; Herder, C.; Pacini, G.; Erlund, I.; et al. Low-energy diets differing in fibre, red meat and coffee intake equally improve insulin sensitivity in type 2 diabetes: A randomised feasibility trial. Diabetologia 2015, 58, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Iljazovic, A.; Roy, U.; Gálvez, E.J.C.; Lesker, T.R.; Zhao, B.; Gronow, A.; Amend, L.; Will, S.E.; Hofmann, J.D.; Pils, M.C.; et al. Perturbation of the gut microbiome by Prevotella spp. enhances host susceptibility to mucosal inflammation. Mucosal Immunol. 2021, 14, 113–124. [Google Scholar] [CrossRef]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of coffee consumption on the gut microbiota: A human volunteer study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef]
- Mills, C.E.; Tzounis, X.; Oruna-Concha, M.J.; Mottram, D.S.; Gibson, G.R.; Spencer, J.P.E. In vitro colonic metabolism of coffee and chlorogenic acid results in selective changes in human faecal microbiota growth. Br. J. Nutr. 2015, 113, 1220–1227. [Google Scholar] [CrossRef] [Green Version]
- Moco, S.; Martin, F.P.J.; Rezzi, S. Metabolomics view on gut microbiome modulation by polyphenol-rich foods. J. Proteome Res. 2012, 11, 4781–4790. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Safe, S.; Kothari, J.; Hailemariam, A.; Upadhyay, S.; Davidson, L.A.; Chapkin, R.S. Health Benefits of Coffee Consumption for Cancer and Other Diseases and Mechanisms of Action. Int. J. Mol. Sci. 2023, 24, 2706. https://doi.org/10.3390/ijms24032706
Safe S, Kothari J, Hailemariam A, Upadhyay S, Davidson LA, Chapkin RS. Health Benefits of Coffee Consumption for Cancer and Other Diseases and Mechanisms of Action. International Journal of Molecular Sciences. 2023; 24(3):2706. https://doi.org/10.3390/ijms24032706
Chicago/Turabian StyleSafe, Stephen, Jainish Kothari, Amanuel Hailemariam, Srijana Upadhyay, Laurie A. Davidson, and Robert S. Chapkin. 2023. "Health Benefits of Coffee Consumption for Cancer and Other Diseases and Mechanisms of Action" International Journal of Molecular Sciences 24, no. 3: 2706. https://doi.org/10.3390/ijms24032706
APA StyleSafe, S., Kothari, J., Hailemariam, A., Upadhyay, S., Davidson, L. A., & Chapkin, R. S. (2023). Health Benefits of Coffee Consumption for Cancer and Other Diseases and Mechanisms of Action. International Journal of Molecular Sciences, 24(3), 2706. https://doi.org/10.3390/ijms24032706







