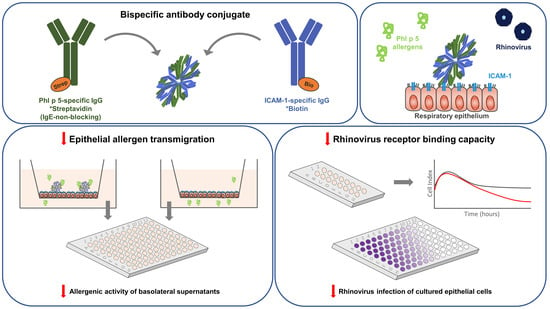
Antibody Conjugates Bispecific for Pollen Allergens and ICAM-1 with Potential to Prevent Epithelial Allergen Transmigration and Rhinovirus Infection
Abstract
1. Introduction
2. Results
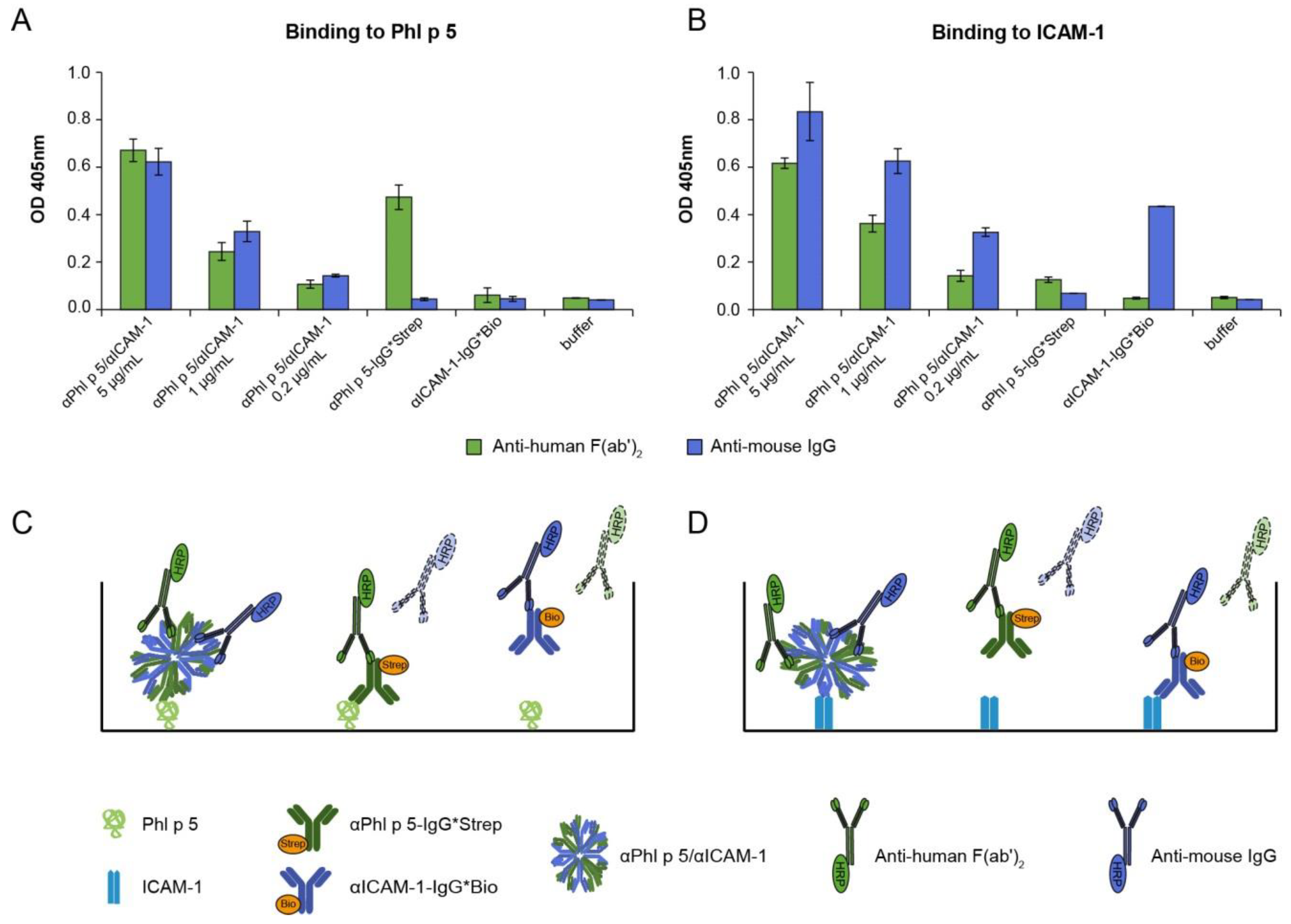
2.1. αPhl p 5/αICAM-1 Antibody Conjugates Specifically Bound to Phl p 5 and Human ICAM-1
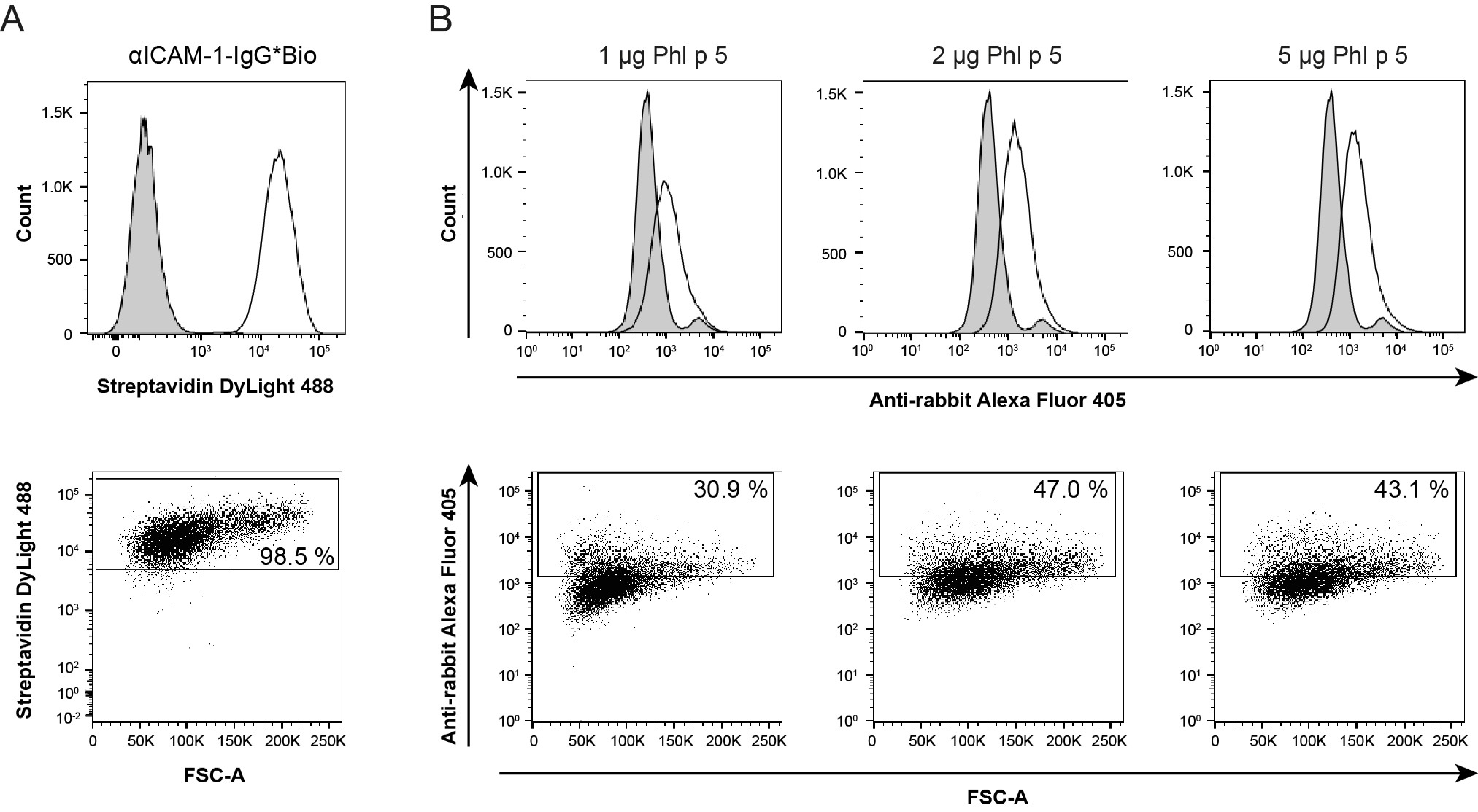
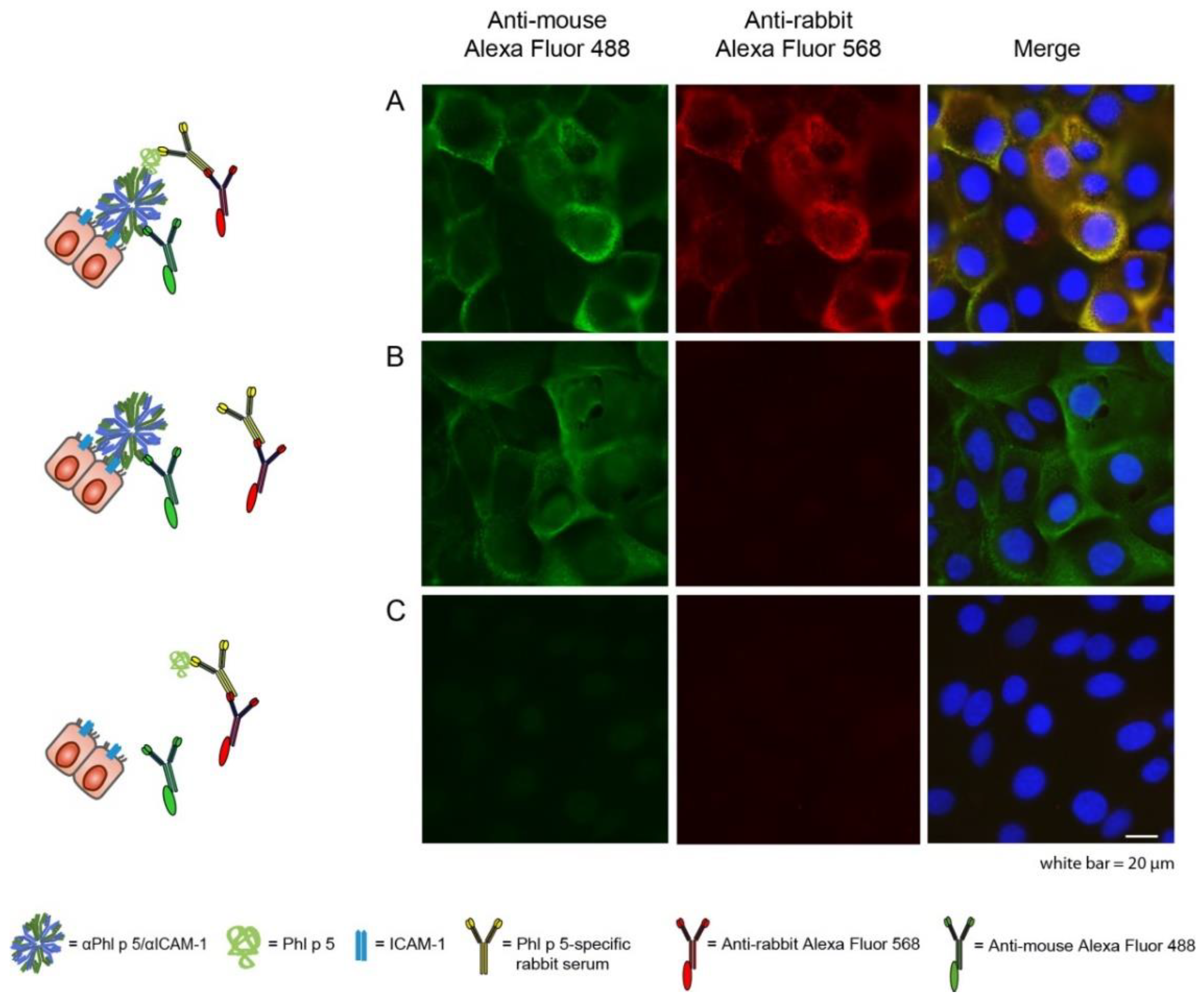
2.2. Phl p 5 Was Immobilized on the Surface of Bronchial Epithelial Cells via αPhl p 5/αICAM-1 Antibody Conjugates
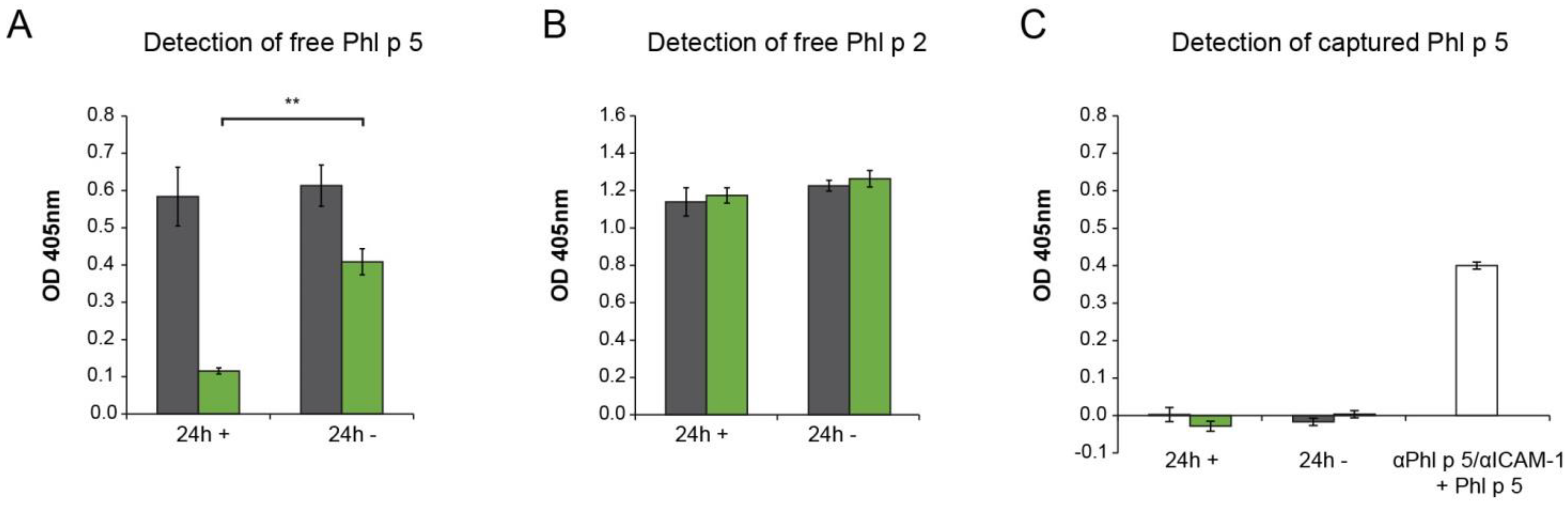
2.3. αPhl p 5/αICAM-1 Conjugates Prevented Transmigration of Phl p 5 through Bronchial Epithelial Cell Layers, Leading to Decreased Basophil Activation
2.4. The Ability to Inhibit Basolateral Basophil Activation Did Not Depend on the Capacity of Allergen-Specific IgG Antibodies to Block Allergen–IgE Interaction
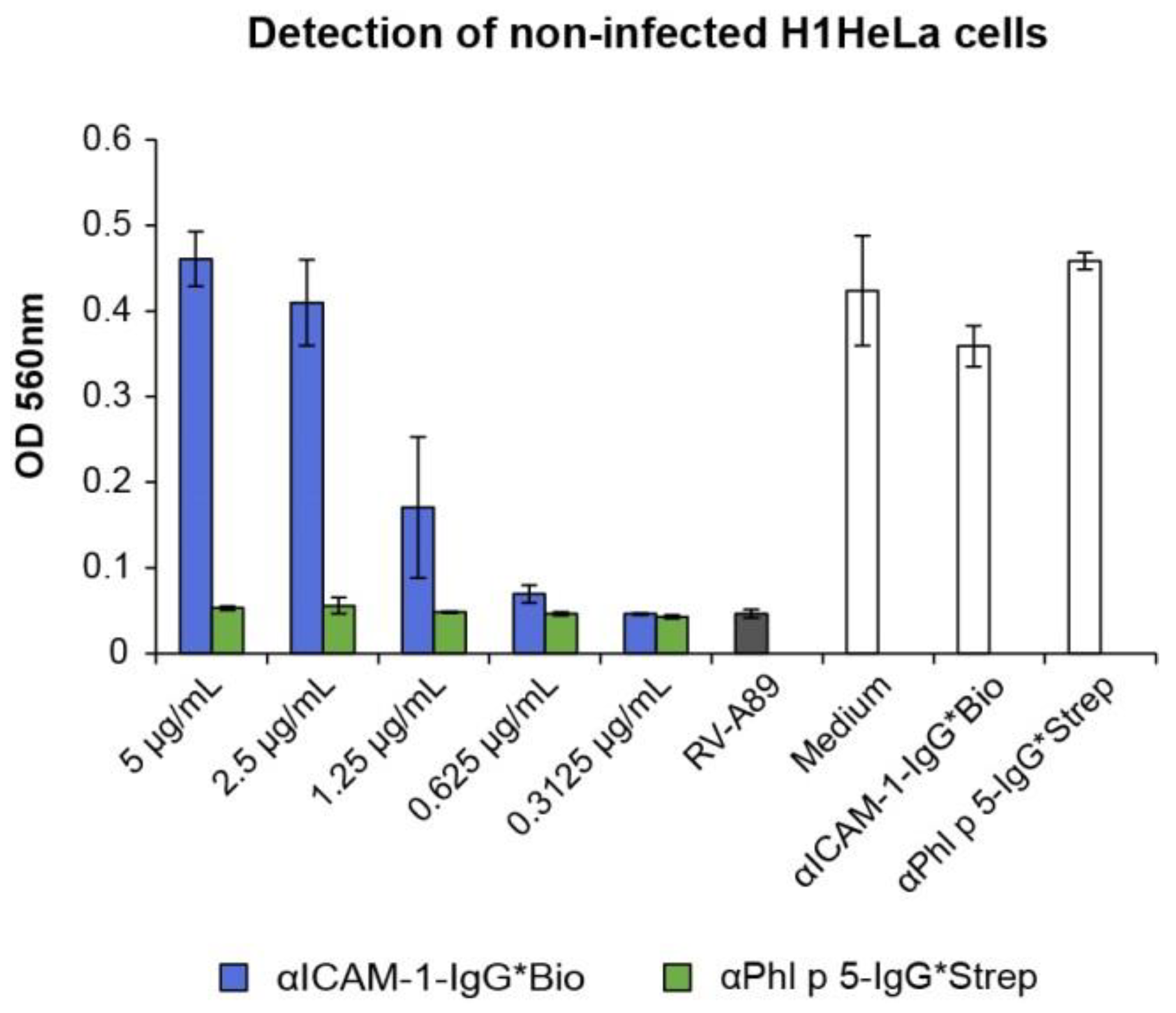
2.5. αPhl p 5/αICAM-1 Conjugates Protected16HBE14o- and H1HeLa Cells from RV Infection
3. Discussion
4. Materials and Methods
4.1. Antigens, Rabbit Immune Sera, Human Sera, Antibodies and Antibody Conjugation
4.2. ELISA Evaluation of the Binding Specificities of αPhl p 5/αICAM-1 Antibody Conjugates
4.3. Cultivation of the Human Epithelial Cell Lines 16HBE14o- and H1HeLa
4.4. Flow Cytometry
4.5. Immunofluorescence Microscopy
4.6. Transwell Assay
4.7. ELISA Measuring Free Phl p 5 in Apical and Basolateral Media
4.8. ELISA Measuring Phl p 5 Bound to αPhl p 5/αICAM-1 in Apical and Basolateral Media
4.9. Rat Basophilic Leukemia Cell-Based Mediator-Release Assay
4.10. ELISA Determining the Capacity of Phl p 2- and Phl p 5-Specific IgG1 Antibodies to Inhibit Patients’ IgE Binding
4.11. Preparation of RV Strains
4.12. xCELLigence Real-Time Cell Analysis DP System
4.13. Cell-Culture-Based RV Neutralization Assay
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valenta, R.; Karaulov, A.; Niederberger, V.; Gattinger, P.; van Hage, M.; Flicker, S.; Linhart, B.; Campana, R.; Focke-Tejkl, M.; Curin, M.; et al. Molecular Aspects of Allergens and Allergy. Adv. Immunol. 2018, 138, 195–256. [Google Scholar] [PubMed]
- García-Mozo, H. Poaceae pollen as the leading aeroallergen worldwide: A review. Allergy 2017, 72, 1849–1858. [Google Scholar] [CrossRef]
- Holgate, S.T. Rhinoviruses in the pathogenesis of asthma: The bronchial epithelium as a major disease target. J. Allergy Clin. Immunol. 2006, 118, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Niespodziana, K.; Stenberg-Hammar, K.; Papadopoulos, N.G.; Focke-Tejkl, M.; Errhalt, P.; Konradsen, J.R.; Söderhäll, C.; van Hage, M.; Hedlin, G.; Valenta, R. Microarray Technology May Reveal the Contribution of Allergen Exposure and Rhinovirus Infections as Possible Triggers for Acute Wheezing Attacks in Preschool Children. Viruses 2021, 13, 915. [Google Scholar] [CrossRef] [PubMed]
- Gilles, S.; Blume, C.; Wimmer, M.; Damialis, A.; Meulenbroek, L.; Gökkaya, M.; Bergougnan, C.; Eisenbart, S.; Sundell, N.; Lindh, M.; et al. Pollen exposure weakens innate defense against respiratory viruses. Allergy 2020, 75, 576–587. [Google Scholar] [CrossRef]
- Edwards, M.R.; Strong, K.; Cameron, A.; Walton, R.P.; Jackson, D.J.; Johnston, S.L. Viral infections in allergy and immunology: How allergic inflammation influences viral infections and illness. J. Allergy Clin. Immunol. 2017, 140, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Heymann, P.W.; Kennedy, J.L. Rhinovirus-induced asthma exacerbations during childhood: The importance of understanding the atopic status of the host. J. Allergy Clin. Immunol. 2012, 130, 1315–1316. [Google Scholar] [CrossRef]
- Greve, J.M.; Davis, G.; Meyer, A.M.; Forte, C.P.; Yost, S.C.; Marlor, C.W.; Kamarck, M.E.; McClelland, A. The major human rhinovirus receptor is ICAM-1. Cell 1989, 56, 839–847. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Contoli, M.; Ito, K.; Padovani, A.; Poletti, D.; Marku, B.; Edwards, M.R.; Stanciu, L.A.; Gnesini, G.; Pastore, A.; Spanevello, A.; et al. Th2 cytokines impair innate immune responses to rhinovirus in respiratory epithelial cells. Allergy 2015, 70, 910–920. [Google Scholar] [CrossRef]
- Nikonova, A.; Khaitov, M.; Jackson, D.J.; Traub, S.; Trujillo-Torralbo, M.-B.; Kudlay, D.A.; Dvornikov, A.S.; del-Rosario, A.; Valenta, R.; Stanciu, L.A.; et al. M1-like macrophages are potent producers of anti-viral interferons and M1-associated marker-positive lung macrophages are decreased during rhinovirus-induced asthma exacerbations. EBioMedicine 2020, 54, 102734. [Google Scholar] [CrossRef] [PubMed]
- Gangl, K.; Waltl, E.E.; Vetr, H.; Cabauatan, C.R.; Niespodziana, K.; Valenta, R.; Niederberger, V. Infection with Rhinovirus Facilitates Allergen Penetration Across a Respiratory Epithelial Cell Layer. Int. Arch. Allergy Immunol. 2015, 166, 291–296. [Google Scholar] [CrossRef] [PubMed]
- De Schryver, E.; Devuyst, L.; Derycke, L.; Dullaers, M.; van Zele, T.; Bachert, C.; Gevaert, P. Local immunoglobulin e in the nasal mucosa: Clinical implications. Allergy Asthma Immunol. Res. 2015, 7, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Niederberger, V.; Ring, J.; Rakoski, J.; Jager, S.; Spitzauer, S.; Valent, P.; Horak, F.; Kundi, M.; Valenta, R. Antigens drive memory IgE responses in human allergy via the nasal mucosa. Int. Arch. Allergy Immunol. 2007, 142, 133–144. [Google Scholar] [CrossRef]
- Eckl-Dorna, J.; Fröschl, R.; Lupinek, C.; Kiss, R.; Gattinger, P.; Marth, K.; Campana, R.; Mittermann, I.; Blatt, K.; Valent, P.; et al. Intranasal administration of allergen increases specific IgE whereas intranasal omalizumab does not increase serum IgE levels-A pilot study. Allergy 2018, 73, 1003–1012. [Google Scholar] [CrossRef]
- Gökkaya, M.; Schwierzeck, V.; Thölken, K.; Knoch, S.; Gerstlauer, M.; Hammel, G.; Traidl-Hoffmann, C.; Gilles, S. Nasal specific IgE correlates to serum specific IgE: First steps towards nasal molecular allergy diagnostic. Allergy 2020, 75, 1802–1805. [Google Scholar] [CrossRef]
- Kasman, L.M. Engineering the common cold to be a live-attenuated SARS-CoV-2 vaccine. Front. Immunol. 2022, 13, 871463. [Google Scholar] [CrossRef] [PubMed]
- Edlmayr, J.; Niespodziana, K.; Focke-Tejkl, M.; Linhart, B.; Valenta, R. Allergen-specific immunotherapy: Towards combination vaccines for allergic and infectious diseases. Curr. Top Microbiol. Immunol. 2011, 352, 121–140. [Google Scholar] [PubMed]
- Edlmayr, J.; Niespodziana, K.; Linhart, B.; Focke-Tejkl, M.; Westritschnig, K.; Scheiblhofer, S.; Stoecklinger, A.; Kneidinger, M.; Valent, P.; Campana, R.; et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus-derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen Phl p 1. J. Immunol. 2009, 182, 6298–6306. [Google Scholar] [CrossRef] [PubMed]
- Madritsch, C.; Eckl-Dorna, J.; Blatt, K.; Ellinger, I.; Kundi, M.; Niederberger, V.; Valent, P.; Valenta, R.; Flicker, S. Antibody conjugates bispecific for intercellular adhesion molecule 1 and allergen prevent migration of allergens through respiratory epithelial cell layers. J. Allergy Clin. Immunol. 2015, 136, 490–493.e11. [Google Scholar] [CrossRef]
- Ciprandi, G.; Pronzato, C.; Ricca, V.; Bagnasco, M.; Canonica, G.W. Evidence of intercellular adhesion molecule-1 expression on nasal epithelial cells in acute rhinoconjunctivitis caused by pollen exposure. J. Allergy Clin. Immunol. 1994, 94, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Traub, S.; Nikonova, A.; Carruthers, A.; Dunmore, R.; Vousden, K.A.; Gogsadze, L.; Hao, W.; Zhu, Q.; Bernard, K.; Zhu, J.; et al. An anti-human ICAM-1 antibody inhibits rhinovirus-induced exacerbations of lung inflammation. PLoS Pathog. 2013, 9, e1003520. [Google Scholar] [CrossRef] [PubMed]
- Flicker, S.; Vrtala, S.; Steinberger, P.; Vangelista, L.; Bufe, A.; Petersen, A.; Ghannadan, M.; Sperr, W.R.; Valent, P.; Norderhaug, L.; et al. A human monoclonal IgE antibody defines a highly allergenic fragment of the major timothy grass pollen allergen, Phl p 5: Molecular, immunological, and structural characterization of the epitope-containing domain. J. Immunol. 2000, 165, 3849–3859. [Google Scholar] [CrossRef]
- Flicker, S.; Steinberger, P.; Norderhaug, L.; Sperr, W.R.; Majlesi, Y.; Valent, P.; Kraft, D.; Valenta, R. Conversion of grass pollen allergen-specific human IgE into a protective IgG1 antibody. Eur. J. Immunol. 2002, 32, 2156–2162. [Google Scholar] [CrossRef] [PubMed]
- Waltl, E.E.; Selb, R.; Eckl-Dorna, J.; Mueller, C.A.; Cabauatan, C.R.; Eiwegger, T.; Resch-Marat, Y.; Niespodziana, K.; Vrtala, S.; Valenta, R.; et al. Betamethasone prevents human rhinovirus- and cigarette smoke- induced loss of respiratory epithelial barrier function. Sci. Rep. 2018, 8, 9688. [Google Scholar] [CrossRef]
- Conant, R.M.; Hamparian, V.V. Rhinoviruses: Basis for a numbering system. 1. HeLa cells for propagationand serologic procedures. J. Immunol. 1968, 100, 107–113. [Google Scholar] [CrossRef]
- Vrtala, S.; Sperr, W.R.; Reimitzer, I.; van Ree, R.; Laffer, S.; Müller, W.D.; Valent, P.; Lechner, K.; Rumpold, H.; Kraft, D. cDNA cloning of a major allergen from timothy grass (Phleum pratense) pollen; characterization of the recombinant Phl pV allergen. J. Immunol. 1993, 151, 4773–4781. [Google Scholar] [CrossRef] [PubMed]
- Mastrobattista, E.; Storm, G.; van Bloois, L.; Reszka, R.; Bloemen, P.G.M.; Crommelin, D.J.A.; Henricks, P.A.J. Cellular uptake of liposomes targeted to intercellular adhesion molecule-1 (ICAM-1) on bronchial epithelial cells. Biochim. Biophys. Acta (BBA)–Biomembr. 1999, 1419, 353–363. [Google Scholar] [CrossRef]
- Muro, S.; Wiewrodt, R.; Thomas, A.; Koniaris, L.; Albelda, S.M.; Muzykantov, V.R.; Koval, M. A novel endocytic pathway induced by clustering endothelial ICAM-1 or PECAM-1. J. Cell Sci. 2003, 116, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Muro, S.; Gajewski, C.; Koval, M.; Muzykantov, V.R. ICAM-1 recycling in endothelial cells: A novel pathway for sustained intracellular delivery and prolonged effects of drugs. Blood 2005, 105, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarian, R.; Muro, S. Distinct subcellular trafficking resulting from monomeric vs. multimeric targeting to endothelial ICAM-1: Implications for drug delivery. Mol. Pharm. 2014, 11, 4350–4362. [Google Scholar] [CrossRef] [PubMed]
- Ghaffarian, R.; Roki, N.; Abouzeid, A.; Vreeland, W.; Muro, S. Intra- and trans-cellular delivery of enzymes by direct conjugation with non-multivalent anti-ICAM molecules. J. Control. Release 2016, 238, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Hua, S. Targeting sites of inflammation: Intercellular adhesion molecule-1 as a target for novel inflammatory therapies. Front. Pharmacol. 2013, 4, 127. [Google Scholar] [CrossRef] [PubMed]
- Plath, F.; Ringler, P.; Graff-Meyer, A.; Stahlberg, H.; Lauer, M.E.; Rufer, A.C.; Graewert, M.A.; Svergun, D.; Gellermann, G.; Finkler, C.; et al. Characterization of mAb dimers reveals predominant dimer forms common in therapeutic mAbs. MAbs 2016, 8, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.H.; Liu, M.; Nolting, B.; Go, J.G.; Gervay-Hague, J.; Liu, G.-y. A nanoengineering approach for investigation and regulation of protein immobilization. ACS Nano 2008, 2, 2374–2384. [Google Scholar] [CrossRef] [PubMed]
- Orengo, J.M.; Radin, A.R.; Kamat, V.; Badithe, A.; Ben, L.H.; Bennett, B.L.; Zhong, S.; Birchard, D.; Limnander, A.; Rafique, A.; et al. Treating cat allergy with monoclonal IgG antibodies that bind allergen and prevent IgE engagement. Nat. Commun. 2018, 9, 1421. [Google Scholar] [CrossRef] [PubMed]
- Shamji, M.; Singh, I.; Layhadi, J.A.; Ito, C.; Karamani, A.; Kouser, L.; Sharif, H.; Tang, J.; Handijiev, S.; Parkin, R.V.; et al. Passive Prophylactic Administration with a Single Dose of Anti-Fel d 1 Monoclonal Antibodies REGN1908-1909 in Cat Allergen-induced Allergic Rhinitis: A Randomized, Double-Blind, Placebo-controlled Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 204, 23–33. [Google Scholar] [CrossRef]
- Atanasio, A.; Franklin, M.C.; Kamat, V.; Hernandez, A.R.; Badithe, A.; Ben, L.-H.; Jones, J.; Bautista, J.; Yancopoulos, G.D.; Olson, W.; et al. Targeting immunodominant Bet v 1 epitopes with monoclonal antibodies prevents the birch allergic response. J. Allergy Clin. Immunol. 2022, 149, 200–211. [Google Scholar] [CrossRef]
- Gevaert, P.; de Craemer, J.; de Ruyck, N.; Rottey, S.; de Hoon, J.; Hellings, P.W.; Volckaert, B.; Lesneuck, K.; Orengo, J.M.; Atanasio, A.; et al. Novel antibody cocktail targeting Bet v 1 rapidly and sustainably treats birch allergy symptoms in a phase 1 study. J. Allergy Clin. Immunol. 2022, 149, 189–199. [Google Scholar] [CrossRef]
- Padayachee, Y.; Flicker, S.; Linton, S.; Cafferkey, J.; Kon, O.M.; Johnston, S.L.; Ellis, A.K.; Desrosiers, M.; Turner, P.; Valenta, R.; et al. Review: The Nose as a Route for Therapy. Part 2 Immunotherapy. Front. Allergy 2021, 2, 668781. [Google Scholar] [CrossRef]
- Tegart, L.J.; Johnston, F.H.; Borchers Arriagada, N.; Workman, A.; Dickinson, J.L.; Green, B.J.; Jones, P.J. ‘Pollen potency’: The relationship between atmospheric pollen counts and allergen exposure. Aerobiologia 2021, 37, 825–841. [Google Scholar] [CrossRef]
- Cabrera, M.; Subiza, J.; Fernández-Caldas, E.; Garzón García, B.; Moreno-Grau, S.; Subiza, J.L. Influence of environmental drivers on allergy to pollen grains in a case study in Spain (Madrid): Meteorological factors, pollutants, and airborne concentration of aeroallergens. Environ. Sci. Pollut. Res. Int. 2021, 28, 53614–53628. [Google Scholar] [CrossRef] [PubMed]
- Subiza, J.; Cabrera, M.; Cárdenas-Rebollo, J.M.; Craciunescu, J.C.; Narganes, M.J. Influence of climate change on airborne pollen concentrations in Madrid, 1979–2018. Clin. Exp. Allergy 2022, 52, 574–577. [Google Scholar] [CrossRef]
- Wan, H.; Winton, H.L.; Soeller, C.; Stewart, G.A.; Thompson, P.J.; Gruenert, D.C.; Cannell, M.B.; Garrod, D.R.; Robinson, C. Tight junction properties of the immortalized human bronchial epithelial cell lines Calu-3 and 16HBE14o-. Eur. Respir. J. 2000, 15, 1058–1068. [Google Scholar] [CrossRef]
- Reisinger, J.; Triendl, A.; Küchler, E.; Bohle, B.; Krauth, M.T.; Rauter, I.; Valent, P.; Koenig, F.; Valenta, R.; Niederberger, V. IFN-gamma-enhanced allergen penetration across respiratory epithelium augments allergic inflammation. J. Allergy Clin. Immunol. 2005, 115, 973–981. [Google Scholar] [CrossRef]
- Nakamura, R.; Uchida, Y.; Higuchi, M.; Nakamura, R.; Tsuge, I.; Urisu, A.; Teshima, R. A convenient and sensitive allergy test: IgE crosslinking-induced luciferase expression in cultured mast cells. Allergy 2010, 65, 1266–1273. [Google Scholar] [CrossRef] [PubMed]
- Pazderova, P.; Waltl, E.E.; Niederberger-Leppin, V.; Flicker, S.; Valenta, R.; Niespodziana, K. ELISA-Based Assay for Studying Major and Minor Group Rhinovirus-Receptor Interactions. Vaccines 2020, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-M.; Chen, Y.; Wang, W.; Mosser, A. Infectivity assays of human rhinovirus-A and -B serotypes. Methods Mol. Biol. 2015, 1221, 71–81. [Google Scholar]
- Edlmayr, J.; Niespodziana, K.; Popow-Kraupp, T.; Krzyzanek, V.; Focke-Tejkl, M.; Blaas, D.; Grote, M.; Valenta, R. Antibodies induced with recombinant VP1 from human rhinovirus exhibit cross-neutralisation. Eur. Respir. J. 2011, 37, 44–52. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weichwald, C.; Zettl, I.; Ellinger, I.; Niespodziana, K.; Waltl, E.E.; Villazala-Merino, S.; Ivanov, D.; Eckl-Dorna, J.; Niederberger-Leppin, V.; Valenta, R.; et al. Antibody Conjugates Bispecific for Pollen Allergens and ICAM-1 with Potential to Prevent Epithelial Allergen Transmigration and Rhinovirus Infection. Int. J. Mol. Sci. 2023, 24, 2725. https://doi.org/10.3390/ijms24032725
Weichwald C, Zettl I, Ellinger I, Niespodziana K, Waltl EE, Villazala-Merino S, Ivanov D, Eckl-Dorna J, Niederberger-Leppin V, Valenta R, et al. Antibody Conjugates Bispecific for Pollen Allergens and ICAM-1 with Potential to Prevent Epithelial Allergen Transmigration and Rhinovirus Infection. International Journal of Molecular Sciences. 2023; 24(3):2725. https://doi.org/10.3390/ijms24032725
Chicago/Turabian StyleWeichwald, Christina, Ines Zettl, Isabella Ellinger, Katarzyna Niespodziana, Eva E. Waltl, Sergio Villazala-Merino, Daniel Ivanov, Julia Eckl-Dorna, Verena Niederberger-Leppin, Rudolf Valenta, and et al. 2023. "Antibody Conjugates Bispecific for Pollen Allergens and ICAM-1 with Potential to Prevent Epithelial Allergen Transmigration and Rhinovirus Infection" International Journal of Molecular Sciences 24, no. 3: 2725. https://doi.org/10.3390/ijms24032725
APA StyleWeichwald, C., Zettl, I., Ellinger, I., Niespodziana, K., Waltl, E. E., Villazala-Merino, S., Ivanov, D., Eckl-Dorna, J., Niederberger-Leppin, V., Valenta, R., & Flicker, S. (2023). Antibody Conjugates Bispecific for Pollen Allergens and ICAM-1 with Potential to Prevent Epithelial Allergen Transmigration and Rhinovirus Infection. International Journal of Molecular Sciences, 24(3), 2725. https://doi.org/10.3390/ijms24032725